Abstract
Background
Splanchnic perfusion following hypovolemic shock is an important marker of adequate resuscitation. We tested whether the gap between esophageal partial carbon dioxide tension (PeCO2) and arterial partial carbon dioxide tension (PaCO2) is increased during graded hemorrhagic hypotension and reversed after blood reinfusion, using a fiberoptic carbon dioxide sensor.
Materials and method
Ten Sprague–Dawley rats were anesthetized, tracheotomized, and cannulated in one femoral artery and vein. A calibrated fiberoptic PCO2 probe was inserted into the distal third of the esophagus for determination of luminal PeCO2 during maintained anesthesia (pentobarbital 15 mg/kg per hour), normothermia (38 ± 0.5°C), and fluid balance (saline 5 ml/kg per hour). Three out of 10 rats were used to determine the limits of hemodynamic stability during gradual hemorrhage. Seven of the 10 rats were then subjected to mild and severe hemorrhage (15 and 20–25 ml/kg, respectively). Thirty minutes after severe hemorrhage, these rats were resuscitated by reinfusion of the shed blood. Arterial gas exchange, hemodynamic variables, and PeCO2 were recorded at each steady-state level of hemorrhage (at 30 and 60 min) and after resuscitation.
Results
The PeCO2–PaCO2 gap was significantly increased after mild and severe hemorrhage and returned to baseline (prehemorrhagic) values following blood reinfusion. Base deficit increased significantly following severe hemorrhage and remained significantly elevated after blood reinfusion. Significant correlations were found between base deficit and PeCO2–PaCO2 (P < 0.002) and PeCO2 (P < 0.022). Blood bicarbonate concentration decreased significantly following mild and severe hemorrhage, but its recovery was not complete at 60 min after blood reinfusion.
Conclusion
Esophageal–arterial PCO2 gap increases during graded hemorrhagic hypotension and returns to baseline value after resuscitation without complete reversal of the base deficit. These data suggest that esophageal capnometry could be used as an alternative for gastric tonometry during management of hypovolemic shock.
Similar content being viewed by others
Introduction
The intestinal tract is highly susceptible to hypoperfusion because of its greater level of critical oxygen delivery and countercurrent microcirculation of the villi [1]. There is increasing evidence that gastrointestinal hypoperfusion plays an important role in development of systemic inflammatory response and multiple organ failure [1,2]. Decreased splanchnic perfusion precedes the appearance of the usual indicators of hypovolemic shock, such as hypotension and lactic acidosis [3,4,5]. Gastric intramucosal acidosis and hypercapnia are observed during inadequate organ perfusion [6,7,8] and are predictive of poor clinical outcome [9,10,11]. Therefore, early detection of gastrointestinal hypoperfusion and effective treatment may improve clinical outcome. Because gastric intubation is done in most critically ill patients, gastric tonometry has traditionally been used to evaluate intramucosal pH or partial carbon dioxide tension (PCO2) indirectly during the management of critically ill patients [9,10,11,12,13,14,15]. However, gastric tonometers have some limitations. For example, air and saline tonometers may require 10–90 min for equilibration [16,17,18,19]. Reliable gastric tonome-try requires suppression of gastric acid [20], whereas gastric feedings can influence its outcome [21,22,23]. Therefore, several other sites, including esophagus, have been used for tonometric measurements [8,24,25,26,27].
Studies have demonstrated that an increase in veno–arterial PCO2 gradient could be a reliable marker of tissue hypoperfusion [28,29,30,31,32]. Knichwitz and coworkers [25] demonstrated that continuous intramucosal PCO2 measurement allows early detection of regional intestinal ischemia before the onset of changes in global hemodynamic or metabolic variables. Furthermore, measurement of tissue PCO2 in several organs has been shown to correlate with gastrointestinal perfusion [8,26,27,33]. Sato and coworkers [8] studied the relationship between gastric wall PCO2 and esophageal PCO2 (PeCO2) before, during, and after reversal of hemorrhagic shock in five spontaneously breathing rats, using an ion-sensitive field-effect transistor. They found a high correlation (r = 0.9) between the gastric wall PCO2 and PeCO2 during hemorrhagic hypotension induced reduction in splanchnic blood flow. The use of tissue PCO2 and arterial PCO2 (PaCO2) difference is a better marker of ischemia than is either gastric intra-mucosal pH or intramucosal PCO2 [34] because the gap is not influenced by alveolar ventilation [35]. Therefore, in the present study we measured intraluminal PeCO2 using a rapidly responsive fiberoptic sensor [25,35,36]. The arterial blood gases were periodically measured for determination of the PeCO2–PaCO2 gap. Our hypothesis is that the PeCO2–PaCO2 gap could be significantly increased during graded hemorrhagic hypotension and will return to baseline shortly after resuscitation.
Materials and method
Surgical procedures
The experimental protocol for the present study was approved by the Institutional Animal Care and Use Committee of Miami Children's Hospital. Ten young, albino Sprague–Dawley rats (250–350 g) were initially anesthetized with 60 mg/kg pentobarbital intraperitoneally. In a supine position, a tracheostomy was performed and an endotracheal tube (3.5 cm of a polyethylene tube, 2.4 mm diameter) was advanced to a position approximately 1 cm above the carina. Subsequently, a femoral vein and a femoral artery were exposed and cannulated. Each rat then was placed over an electric heating blanket. Rectal temperature (TH-5; Physitemp Thermalert, Clifton, NJ, USA; with a rat size thermal probe), mean arterial blood pressure (MABP), and heart rate (HR; 2001A, Datascope Corp, Paramus, NJ, USA) were continuously monitored. Normothermia (38 ± 0.5°C) was established while anesthesia (pentobarbital 15 mg/kg per hour) and fluid balance (saline 5 ml/kg per hour) were strictly maintained (Medfusion pump 2010; Medex, Duluth, CA, USA). Rats breathed room air, spontaneously, during the experiments.
Esophageal capnometry
The esophagus was intubated orally with a 22-gauge, 1.5-inch-long catheter. A fiberoptic carbon dioxide sensor (Paratrend7; Diametrics Medical Inc, Roseville, MN, USA) was introduced through the oral catheter up to 8–10 cm from the incisor teeth into lower third of the esophagus (at 2–3 cm above the gastroesophageal junction). The fiberoptic sensor consisted of two optical fibers for the measurement of PCO2 and pH, a miniature Clark electrode for determination of partial oxygen tension, and a thermocouple for measuring temperature. The sensor was automatically calibrated with precision gases under microprocessor control, as per the manufacturer's recommendations, before insertion into the esophagus.
Baseline measurements
Within 30–60 min after the insertion of the sensor, baseline values for PeCO2, core temperature, HR, and MABP were recorded. The rats then were heparinized with 200 U/kg per hour heparin and an arterial blood sample was taken for baseline (time0) gas analysis (ABL-30 Blood Gas Analyzer; Radiometer, Copenhagen, Denmark), hemoglobin, and arterial oxygen saturation (OSM3 Hemoxymeter; Radiometer). Measurements of PaCO2 and PeCO2, as well as partial arterial oxygen tension, were corrected for each animal's body temperature. Values for bicarbonate and base excess were automatically calculated by the blood gas analyzer's program.
Hemorrhagic hypotension
Three out of the 10 rats were used to test the limits of hemodynamic stability during hemorrhagic hypotension in this model. Gradual bleeding up to 15 ml/kg in these three rats led to a 30–40% reduction in MABP. Additional bleeding up to 25 ml/kg was tolerated as long as the MABP did not drop below 30 mmHg. Lower blood pressures, caused by removal of 25 ml/kg blood, created a deteriorating and irreversible systemic hypotension, accompanied by severe tachycardia. Therefore, in the actual experiments (n = 7) we considered 15 ml/kg bleeding over a 30-min period as mild hemorrhagic hypotension. Removal of 20–25 ml/kg blood, while maintaining a MABP equal to or higher than 35 mmHg, was considered severe hemorrhagic hypotension. The blood was collected in a heparinized (400 U) tube and incubated at 38°C. Thirty minutes after mild hemorrhagic hypotension, all the baseline variables were again measured. This procedure was repeated after removal of another 5–10 ml/kg blood (for generation of severe but reversible hemorrhage). All variables were recorded during severe hemorrhagic hypotension, and then the shed blood was reinfused over 20–30 min. All variables were measured again at 30 and 60 min following termination of blood reinfusion. At the end of the experiment, the animals were killed with intravenous pentobarbital and the exact position of the esophageal probe was verified.
Statistical analysis
Statistical evaluation was performed in the seven rats that completed mild and severe hemorrhage with resuscitation. All variables are presented as mean ± SD. The data were computed by repeated measures of analysis of variance followed by Dunnett multiple comparisons test, using the baseline values as controls. A linear regression analysis was also performed to evaluate association between PeCO2–PaCO2 gap and the base deficit. P < 0.05 was considered statistically significant.
Results
Hemodynamic and gas exchange variables
Mild and severe homorrhagic hypotension created average reductions of 33% and 53% in MABP, respectively. Reinfusion of the blood restored MABP to the normal range. Blood hemoglobin concentration followed a pattern similar to that of blood pressure (Table 1). The HR was significantly increased following severe hemorrhage (29%). After blood reinfusion, the HR remained significantly higher than its prehemorrhagic baseline value (Table 1). The partial arterial oxygen tension was increased significantly during both mild and severe hemorrhagic hypotension, apparently caused by hyperventilation. The latter also reduced the PaCO2 significantly (Fig. 1). Arterial saturation following blood reinfusion was not significantly different from baseline. Blood bicarbonate concentrations decreased significantly following hemorrhage, but recovery was not complete at 60 min after blood reinfusion (Table 1).
Changes in partial arterial carbon dioxide tension (PaCO2), partial esophageal carbon dioxide tension (PeCO2) and esophageal–arterial PCO2 gap in seven anesthetized, spontaneously breathing rats subjected to mild and severe hemorrhagic hypotension followed by blood reinfusion. *P < 0.05, by repeated measures of analysis of variance followed by Dunnett multiple comparison test, using baseline as controls.
Esophageal–arterial partial carbon dioxide tension gap and base deficit
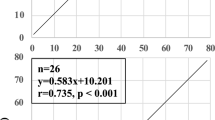
The PeCO2–PaPCO2 was significantly increased after mild and severe hemorrhage, and returned to baseline values following blood reinfusion (Fig. 1). The base deficit became slightly more negative after mild hemorrhage but was significantly reduced after severe hemorrhage (-5.5 mmol/l and -14.4 mmol/l, respectively). The base deficit remained significantly high after blood reinfusion (-7.2 mmol/l after 60 min). After blood reinfusion, unlike base deficit, the PaCO2 rapidly normalized (Table 1). A significant correlation was found between base deficit and PeCO2–PaCO2 gap during hemorrhagic hypotension (Fig. 2; r2 = 0.39, P < 0.002). At the same time, there was also a significant correlation between base deficit and PeCO2 (Fig. 3; r2 = 0.24, P < 0.022).
Linear regression analysis of the association between partial esophageal carbon dioxide tension (PeCO2) minus partial arterial carbon dioxide tension (PaCO2; i.e. PeCO2–PaCO2 gap) and base deficit in seven anesthetized, spontaneously breathing rats during mild and severe hemorrhagic hypotension. Broken lines represent the upper and lower limits of 95% confidence interval.
Discussion
A correlation between PeCO2 and gastric PCO2 during hemorrhagic shock was previously demonstrated in spontaneously breathing rats [8]. Our results, using a fiberoptic carbon dioxide sensor, are generally in agreement with those of Sato and coworkers [8], who used an ion-sensitive field-effect transistor sensor. In the present study, unlike that of Sato and coworkers, PeCO2 did not significantly increase during hemorrhage, whereas the PeCO2–PaCO2 gap was significantly increased. The PeCO2–PaCO2 gap returned to baseline immediately after resuscitation (Fig. 1). Our data also demonstrate a significant association between the PeCO2–PaCO2 gap and the corresponding base deficit that occurred during hemorrhagic hypotension (Fig. 2). Whereas the PeCO2–PaCO2 gap rapidly recovered after resuscitation (Fig. 1), the base deficit did not completely return to baseline after restoration of blood volume (Table 1).
The animals in our study hyperventilated because of metabolic acidosis, presumably secondary to hypoperfusion. Arterial hypocapnia can impact on the expected rise in tissue PCO2 that occurs as a result of decreased tissue perfusion. Therefore, intramucosal PCO2 as an indicator of tissue hypoperfusion is not as accurate as PeCO2–PaCO2 [34]. Moreover, the tissue PCO2 and PaCO2 gap is not influenced by alveolar ventilation [37]. However, when ventilation is controlled, the change in tissue PCO2 by itself could become a reliable indicator of tissue perfusion. In our spontaneously breathing rats the PeCO2 was lower after severe hemorrhage. We reason that the PeCO2 would have been higher if the rats were mechanically ventilated to maintain a relative arterial normo-capnia. In ventilated subjects, change in tissue PCO2 is an indicator of changes in tissue perfusion before any other global parameters of perfusion are changed [25,38]. In spontaneously breathing subjects, continuous measurements of tissue PCO2 and PaCO2 gap can be used as an early indicator of tissue hypoperfusion.
Gastric tonometry versus esophageal and sublingual capnometry
Traditionally, stomach has been used as the organ to measure intramucosal pH or PCO2 in both animal and human studies [6,7,8,9,10,11,12,13,14,15]. The low pH of stomach may interfere with tonometry, and therefore gastric acid suppression may be needed for reliable measurements [20]. Other limiting factors in gastric tonometry are related to feeding [22,23] and the large lumen of the stomach, requiring longer time for intralu-minal contents to equilibrate with intramucosal PCO2. Moreover, in the presence of low gastric pH, secretion of bicarbonate leads to intraluminal production of carbon dioxide [39]. The above factors may prevent rapid detection of changes in intramucosal PCO2. Therefore, several other sites have been used for tonometry. In animals, ileum has been used to assess splanchnic perfusion [25,36] – a clinically impractical procedure. Studies have demonstrated that sublingual capnometry, a relatively noninvasive procedure, correlates with gastric tonometry [26,40,41,42]. Practically, it may be difficult to lodge the sensor securely under the tongue in uncooperative patients, thereby preventing equilibration with tissue PCO2 [27]. Esophageal intubation, which is commonly used in critically ill patients, can be utilized to secure placement of the esophageal sensor. Similar to the gastric environment, bicarbonate is secreted in the esophagus and may enter the esophagus from salivary secretions. However, a relative alkaline pH in the esophagus, in the absence of acid reflux, may not lead to generation of a significant amount of carbon dioxide. Currently available tonometers have equilibration periods ranging between 10 and 90 min [16,17,18,19] and are therefore not efficient for rapid detection of changes in tissue perfusion on a continuous basis. Fiberoptic sensors that are used in clinical medicine for automatic and continuous measurements of blood gases [43,44] have a rapid response time [45]. Experimental evaluation of a fiberoptic PCO2 sensor, similar to that used in the present study, has shown a high degree of precision in detecting short-term changes in intramucosal PCO2 [35].
Capnometry and end-points of resuscitation
An interesting observation in the present study was the delayed recovery of base deficit after resuscitation (Table 1), whereas PeCO2, PaCO2, and the gap between them were actually recovered (Fig. 1). Porter and Ivatury [46] demonstrated that the use of base deficit, lactate, and/or gastric intramucosal pH are appropriate end-points of resuscitation for trauma patients. They also recommended that one or all of the above markers of tissue perfusion be corrected to normal range within 24 hours after injury. Povoas and coworkers [42] reported persistently elevated blood lactate level after reinfusion of blood when all other parameters of tissue perfusion, such as sublingual PCO2, gastric PCO2, and veno–arterial PCO2 gradient, were normalized. In the present study, the delay in normalization of the base deficit in the face of a rapid normalization of the PeCO2–PaCO2 gap may suggest that the PeCO2–PaCO2 gap can serve as an early indicator for resuscitation end-point rather than base deficit. Physiologically, it takes time for liver and kidneys to correct metabolic acidosis following tissue dysoxia. It is therefore anticipated that there will be a lag phase between restoration of blood volume and return of base deficit to normal.
Studies indicate that PeCO2–PaCO2 gap can continue to increase or remain abnormally high after resuscitation [25,47,48]. In those experiments [47,48], severe hemorrhage (45–47 ml/kg versus 30 ml/kg) might have contributed to ischemia/reperfusion injury, leading to persistent mucosal hypoperfusion and elevated tissue PCO2–PaCO2 gap. In the presence of ischemia/reperfusion mucosal injury, the PeCO2–PaCO2 gap may not return to normal even after restoration of circulatory volume. In such instances, base deficit (or other global parameters of tissue perfusion) may be a better index for the end-point of resuscitation.
Conclusion
The data presented here demonstrate that PeCO2–PaCO2 gap increases during hemorrhagic hypotension and reverses after resuscitation, without complete recovery of base deficit. We suggest that esophageal capnometry could be used as an alternative to gastric tonometry for assessing splanchnic hypoperfusion.
Key messages
-
Esophageal capnometry could be used as an alternative for gastric tonometry during the management of hypovolemic shock
-
PeCO2–PaCO2 gap increases during graded hemorrhagic hypotension and returns to baseline value after resuscitation, without complete reversal of the base deficit
Abbreviations
- HR:
-
= heart rate
- MABP:
-
= mean arterial blood pressure
- Pa:
-
CO2 = arterial partial carbon dioxide tension
- P:
-
CO2 = partial carbon dioxide tension
- Pe:
-
CO2 = esophageal partial carbon dioxide tension.
References
Desai VS, Weil MH, Tang W, Yang G, Bisera J: Gastric intramural PCO 2 during peritonitis and shock. Chest 1993, 104: 1254-1258.
Tang W, Weil MH, Sun S, Noc M, Gazmuri RJ, Bisera J: Gastric intramural PCO 2 as a monitor of perfusion failure during hemorrhagic and anaphylactic shock. J Appl Physiol 1994, 76: 572-577.
Pastores SM, Katz DP, Kvetan V: Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol 1996, 91: 1697-1710.
Nielsen VG, Tan S, Baird MS, McCammon AT, Parks DA: Gastric intramucosal pH and multiple organ injury: impact of ischemia-reperfusion and xanthine oxidase. Crit Care Med 1996, 24: 1339-1344. 10.1097/00003246-199608000-00012
Antonsson JB, Boyle CCd, Kruithoff KL, Wang HL, Sacristan E, Rothschild HR, Fink MP: Validation of tonometric measurement of gut intramural pH during endotoxemia and mesenteric occlusion in pigs. Am J Physiol 1990, 259: G519-G523.
Fink MP: Adequacy of gut oxygenation in endotoxemia and sepsis. Crit Care Med 1993,21(suppl):S4-S8.
Guzman JA, Lacoma FJ, Kruse JA: Relationship between systemic oxygen supply dependency and gastric intramucosal PCO2 during progressive hemorrhage. J Trauma 1998, 44: 696-700.
Sato Y, Wei MH, Tanf W, Sun S, Xie J, Bisera J, Hosaka H: Esophageal PCO 2 as a monitor of perfusion failure during Esophageal PCO 2 hemorrhagic shock. J Appl Physiol 1997, 82: 558-562.
Doglio GR, Pusajo JF, Egurrola MA, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Fernandez S, Palizas F, Gutierrez G: Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med 1991, 19: 1037-1040.
Maynard N, Bihari D, Beale R, Smithies M, Baldock G, Mason R, McColl I: Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA 1993, 270: 1203-1210. 10.1001/jama.270.10.1203
Mythen MG, Webb AR: Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg 1995, 130: 423-429.
Marik PE: Gastric intramucosal pH. A better predictor of multi-organ dysfunction syndrome and death than oxygen-derived variables in patients with sepsis. Chest 1993, 104: 225-229.
Bouachour G, Guiraud MP, Gouello JP, Roy PM, Alquier P: Gastric intramucosal pH: an indicator of weaning outcome from mechanical ventilation in COPD patients. Eur Respir J 1996, 9: 1868-1873. 10.1183/09031936.96.09091868
Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM: A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg 1996, 183: 145-154.
Kirton OC, Windsor J, Wedderburn R, Hudson-Civetta J, Shatz DV, Mataragas NR, Civetta JM: Failure of splanchnic resuscitation in the acutely injured trauma patient correlates with multiple organ system failure and length of stay in the ICU. Chest 1998, 113: 1064-1069.
Knichwitz G, Mertes N, Kuhmann M: Improved PCO2 measurement in six standard blood gas analysers using a phosphate-buffered solution for gastric tonometry. Anaesthesia 1995, 50: 532-534.
Heinonen PO, Jousela IT, Blomqvist KA, Olkkola KT, Takkunen OS: Validation of air tonometric measurement of gastric regional concentrations of CO2 in critically ill septic patients. Intensive Care Med 1997, 23: 524-529. 10.1007/s001340050368
Venkatesh B, Morgan J, Jones RD, Clague A: Validation of air as an equilibration medium in gastric tonometry: an in vitro evaluation of two techniques for measuring air PCO2. Anaesth Intensive Care 1998, 26: 46-50.
Creteur J, De Backer D, Vincent JL: Monitoring gastric mucosal carbon dioxide pressure using gas tonometry: in vitro and in vivo validation studies. Anesthesiology 1997, 87: 504-510. 10.1097/00000542-199709000-00008
Bams Jl, Mariani MA, Groeneveld AB: Predicting outcome after cardiac surgery: comparison of global haemodynamic and tonometric variables. Br J Anaesth 1999, 82: 33-37.
Groeneveld AB, Vervloet M, Kolkman JJ: Gastric tonometry in the fed or fasting state? Crit Care Med 1998, 26: 1937-1939. 10.1097/00003246-199812000-00006
Levy B, Perrigault PF, Gawalkiewicz P, Sebire F, Escriva M, Colson P, Wahl D, Frederic M, Bollaert PE, Larcan A: Gastric versus duodenal feeding and gastric tonometric measurements. Crit Care Med 1998, 26: 1991-1994. 10.1097/00003246-199812000-00026
Kolkman JJ, Groeneveld AB, Meuwissen SG: Effect of gastric feeding on intragastric P(CO2) tonometry in healthy volunteers. J Crit Care 1999, 14: 34-38.
Fiddian-Green RG, Pittenger G, Whitehouse WMJ: Back-diffusion of CO2 and its influence on the intramural pH in gastric mucosa. J Sur Res 1982, 33: 39-48.
Knichwitz G, Rotker J, Mollhoff T, Richter KD, Brussel T: Continuous intramucosal PCO2 measurement allows the early detection of intestinal malperfusion. Crit Care Med 1998, 26: 1550-1557. 10.1097/00003246-199809000-00023
Guzman JA, Lacoma FJ, Kruse JA: Gastric and esophageal intra-mucosal PCO2 (PiCO2) during endotoxemia: assessment of raw PiCO2 and PCO2 gradients as indicators of hypoperfusion in a canine model of septic shock. Chest 1998, 113: 1078-1083.
Weil MH, Nakagawa Y, Tang W, Sato Y, Ercoli F, Finegan R, Grayman G, Bisera J: Sublingual capnometry: a new noninvasive measurement for diagnosis and quantitation of severity of circulatory shock. Crit Care Med 1999, 27: 1225-1229. 10.1097/00003246-199907000-00001
Weil MH, Rackow EC, Trevino R, Grundler W, Falk JL, Griffel MI: Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med 1986, 315: 153-156.
Adrogue HJ, Rashad MN, Gorin AB, Yacoub J, Madias NE: Assessing acid-base status in circulatory failure. Differences between arterial and central venous blood. N Engl J Med 1989, 320: 1312-1316.
Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ: Veno-arterial carbon dioxide gradient in human septic shock. Chest 1992, 101: 509-515.
Zhang H, Vincent JL: Arteriovenous differences in PCO2 and pH are good indicators of critical hypoperfusion. Am Rev Respir Dis 1993, 148: 867-871.
Van der Linden P, Rausin I, Deltell A, Bekrar Y, Gilbart E, Bakker J, Vincent JL: Detection of tissue hypoxia by arteriovenous gradient for PCO2 and pH in anesthetized dogs during progressive hemorrhage. Anaesth Analg 1995, 80: 269-275.
Jin X, Weil MH, Sun S, Tang W, Bisera J, Mason EJ: Decreases in organ blood flows associated with increases in sublingual PCO2 during hemorrhagic. J Appl Physiol 1998, 85: 2360-2364.
Schlichtig R, Mehta N, Gayowski TJ: Tissue-arterial PCO2 difference is a better marker of ischemia than intramural pH (pHi) or arterial pH-pHi difference. J Crit Care 1996, 11: 51-56.
Knichwitz G, Rotker J, Brussel T, Kuhmann M, Mertes N, Mollhoff T: A new method for continuous intramucosal PCO2 measurement in the gastrointestinal tract. Anaesth Analg 1996, 83: 6-11.
Morgan TJ, Venkatesh B, Endre ZH: Continuous measurement of gut luminal PCO2 in the rat: responses to transient episodes of graded aortic hypotension. Crit Care 1997, 25: 1575-1578.
Bernardin G, Lucas P, Hyvernat H, Deloffre P, Mattei M: Influence of alveolar ventilation changes on calculated gastric intramu-cosal pH and gastric-arterial PCO2 difference. Intensive Care Med 1999, 25: 269-273. 10.1007/s001340050834
Tao W, Zwischenberger JB, Kramer GC: Rapid monitoring of gastrointestinal intraluminal PCO2 as an end-organ perfusion index. Crit Care Med 1997, 25: 1458-1459. 10.1097/00003246-199709000-00009
Kolkman JJ, Groeneveld AB, Meuwissen SG: Effect of ranitidine on basal and bicarbonate enhanced intragastric PCO2: a tonometric study. Gut 1994, 35: 737-741.
Nakagawa Y, Weil MH, Tang W, Sun S, Yamaguchi H, Jin X, Bisera J: Sublingual capnometry for diagnosis and quantitation of circulatory shock. Am J Respir Crit Care Med 1998, 157: 1838-1843.
Marik PE: Sublingual capnography. A clinical validation study. Chest 2001, 120: 923-927. 10.1378/chest.120.3.923
Povoas HP, Weil MH, Tang W, Moran B, Kamohar T, Bisera J: Comparisons between sublingual and gastric tonometry during hemorrhagic shock. Chest 2000, 118: 1127-1132. 10.1378/chest.118.4.1127
Weiss IK, Fink S, Edmunds S, Harrison R, Donnelly K: Continuous arterial gas monitoring: initial experience with the Para-trend 7 in children. Intensive Care Med 1996, 22: 1414-1417. 10.1007/s001340050275
Hatherill M, Tibby SM, Durward A, Rajah V, Murdoch IA: Continuous intra-arterial blood-gas monitoring in infants and children with cyanotic heart disease. Br J Anaesth 1997, 79: 665-667.
Venkatesh B, Hendry SP: Continuous intra-arterial blood gas monitoring. Intensive Care Med 1996, 22: 818-828. 10.1007/s001340050173
Porter JM, Ivatury RR: In search of the optimal end-point of resuscitation in trauma patients: a Review. J Trauma 1998, 44: 908-914.
Guzman JA, Kruse JA: Gastric intramucosal PCO 2 as a quantitative indicator of the degree of acute hemorrhage. J Crit Care 1998, 13: 49-54.
Guzman JA, Kruse JA: Continuous assessment of gastric intra-mucosal PCO2 and pH in hemorrhagic shock using capnometric recirculating gas tonometry. Crit Care Med 1997, 25: 533-537. 10.1097/00003246-199703000-00025
Acknowledgement
Supported by Miami Children's Hospital Foundation's grant to BRT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Totapally, B.R., Fakioglu, H., Torbati, D. et al. Esophageal capnometry during hemorrhagic shock and after resuscitation in rats. Crit Care 7, 79 (2002). https://doi.org/10.1186/cc1856
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc1856