Abstract
Overexpression of the human epidermal growth factor receptor (HER)-2 oncogenic receptor tyrosine kinase, which occurs in 25% of breast cancers, portends poor clinical outcome and consequently represents an attractive target for therapeutic intervention. Small molecule tyrosine kinase inhibitors that compete with ATP binding at the cytoplasmic catalytic kinase domain of HER-2 block autophosphorylation and activation of HER-2, resulting in inhibition of downstream proliferation and survival signals. These agents have exhibited clinical activity in patients with HER-2 overexpressing breast cancers. Here we review the development of HER-2 tyrosine kinase inhibitors, their mechanisms of action, their biological and clinical activities, their safety profile, and combination strategies including conventional cytotoxics and other targeted agents.
Similar content being viewed by others
Introduction
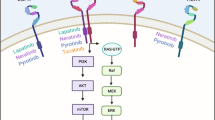
Members of the human epidermal growth factor receptor (HER) family of transmembrane receptor tyrosine kinases (HER-1/epidermal growth factor receptor [EGFR], HER-2, HER-3, and HER-4), particularly EGFR, HER-2 and HER-3, promote tumor cell proliferation and survival in a variety of epithelial malignancies. HER-2 protein overexpression or gene amplification, which occurs in approximately 25% to 30% of breast cancers, portends poor clinical outcome [1–3]. Members of the epidermal growth factor family of soluble ligands bind to their cognate HER receptors and induce formation of HER receptor homodimers or heterodimers, resulting in autophosphorylation of specific tyrosine residues within the cytoplasmic catalytic kinase domain of the activated receptor (Figure 1) [4]. These tyrosine autophosphorylation residues serve as docking sites for SH2 (Srchomology 2) and phosphotyrosine-binding domain containing protein, which links the activated, phosphorylated HER receptor with downstream cell proliferation (mitogen-activated protein kinase [MAPK]) and survival (phosphatidyl-inositol-3 kinase [PI3K]) signaling pathways (Figure 1) [5, 6]. HER receptor heterodimers are potent signaling complexes, with HER-2 being the preferred heterodimeric partner. Consequently, HER-2 represents an attractive target for cancer drug development.
HER-2 containing heterodimers and their downstream signaling effects. Shown are distinct HER-2 containing heterodimers, ligands that activate respective receptor complexes, downstream linked signaling pathways, and their putative functional effects in HER-2 overexpressing breast cancers. AR, amphiregulin; BTC, betacellulin; EGFR, epidermal growth factor receptor; EPR, epiregulin; HB-EGF, heparin-binding epidermal-like growth factor; HER, human epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; NRG, neuregulin; PI3K, phosphatidylinositol-3 kinase; TGF, transforming growth factor.
Trastuzumab (Herceptin®; Genentech, South San Francisco, CA, USA) is a humanized anti-HER-2 monoclonal antibody that has been approved for treatment of patients with breast cancers that overexpress HER-2 protein or that exhibit ErbB2 gene amplification. It has revolutionized the treatment of HER-2 overexpressing breast cancers by improving survival in metastatic breast cancer when combined with cytotoxic agents; recently, it also exhibited significant clinical efficacy in the adjuvant breast cancer setting [7–12]. The precise mechanism(s) by which trastuzumab exerts its anti-tumor effects is unknown, although it is probably multi-factorial, including antibody-dependent cell mediated cytotoxicity [13], downregulation of HER-2 signaling following antibody mediated receptor internalization [14], inhibition of cell proliferation and survival signals [15, 16], and interference with DNA repair [17]. However, the majority of HER-2 overexpressing breast cancers do not respond to trastuzumab therapy alone. Several mechanisms of resistance have been proposed, including the following [18–24]: expression of redundant survival signaling pathways (for example, the insulin-like growth factor [IGF] receptor); deficient expression of the PTEN tumor suppressor gene; expression of p95HER-2, a highly active truncated form of HER-2 that lacks the extracellular domain, which is the recognition site for trastuzumab; and downregulation of the cyclin-dependent kinase inhibitor p27kip1. However, these mechanisms of trastuzumab resistance do not appear to preclude the antitumor activity of small molecule inhibitors of HER-2 kinase, as is discussed below.
Small molecule tyrosine kinase inhibitors targeting HER-2
In addition to targeting HER-2 using antibody therapies, small molecule tyrosine kinase inhibitors (TKIs) that compete with ATP for binding at the HER-2 catalytic kinase domain block HER-2 signaling (Table 1). These compounds may be reversible (for instance GW572016 [lapatinib]; GlaxoSmithKline, King of Prussia, PA, USA) [25] or irreversible inhibitors (for example CI-1033 [canertinib]; Pfizer, Groton, CT, USA) [26]. Most of these compounds target more than one HER receptor, which has the potential advantage of simultaneously blocking two or more heterodimer components.
However, many so-called HER specific inhibitors are promiscuous kinase inhibitors. A recent study [27] investigated the specificity of 20 TKIs that are approved by the US Food and Drug Administration or are currently in clinical trials. Their binding specificity against 113 kinases, mostly tyrosine kinases with an additional small number of serine/threonine kinases, was then determined using clinically relevant drug concentrations (namely plasma concentrations achieved in patients administered the clinically recommended dose). The following drugs with HER-2 kinase inhibitory activity were evaluated: canertinib (pan-HER irreversible inhibitor), EKB-569 (EGFR, HER-2 irreversible inhibitor), lapatinib (EGFR, HER-2 reversible inhibitor), and gefitinib and erlotinib (mono-EGFR reversible inhibitors). Of the 20 drugs evaluated, lapatinib was the most specific inhibitor, binding its intended targets (EGFR and HER-2) with high affinity and an additional two kinases (STK10 and SLK) with markedly lower affinities. In contrast, EKB-569 was found to be a rather promiscuous kinase inhibitor, binding 56 of the 113 kinases tested, as well as binding several non-HER kinases at similar affinities to its target EGFR. CI-1033 (canertinib), which is purportedly a specific inhibitor of EGFR, HER-2 and HER-4, is also promiscuous, binding 36 of the 113 kinases tested.
The promiscuous nature of these drugs has the potential to contribute to increased toxicity. In addition, not all HER receptors are necessarily desirable targets in breast cancer. For example, HER-4 is associated with a more differentiated, less aggressive breast cancer and is a favorable prognostic factor in breast cancer, and therefore it may not be a desirable therapeutic target in breast cancer [28].
Most of the small molecule HER kinase inhibitors share similar pharmacokinetic profiles. They tend to exhibit prolonged plasma half-lives (> 24 hours), to exhibit dose-proportional kinetics, to be highly protein bound, and to be metabolized rather than undergo renal excretion [29, 30].
Biologic activity of HER-2 kinase inhibitors in preclinical and clinical studies
Small molecule HER-2 kinase inhibitors are typically potent, with a 50% inhibitory concentration against HER-2 in the low nanomolar range, based on in vitro kinase assays [26, 31, 32]. Inhibition of HER-2 autophosphorylation triggers a cascade of events that block signaling via the MAPK-Erk1/2 and PI3K-Akt signaling networks in HER-2 overexpressing tumor cell lines and breast cancer xenografts [25, 26, 31, 33]. In contrast to antibody-based therapies, small molecule HER-2 kinase inhibitors reduce phosphorylated but not total HER-2 expression [25, 26, 31, 33].
Inhibition of HER-2 autophosphorylation and downstream signaling pathways in preclinical models is important, but ideally one would wish to demonstrate these effects in the clinic. Skin, an easily accessible EGFR expressing tissue, served as a surrogate to determine the effects of erlotinib and gefitinib on EGFR phosphorylation, and on the MAPK-Erk1/2 and PI3K-Akt pathways [34, 35]. Unfortunately, biologic effects in skin do not necessarily correlate with clinical response [34].
Studies have attempted to evaluate the biologic activity of HER-2 kinase inhibitors in tumor biopsies obtained from patients on clinical trials. For example, a phase Ib study of lapatinib monotherapy in 67 patients [36], 50% of whom had breast cancer, showed that lapatinib inhibited HER-2 and EGFR phosphorylation at day 28 of therapy, with consequent reduction in the expression of phospho-Erk1/2, phospho-Akt, and cyclin D1; importantly, it also increased tumor cell apoptosis (by terminal dUTP nick-end labeling [TUNEL]). Biologic responses were often associated with partial responses and prolonged stable disease. A panel of candidate tumor biomarkers was identified that predicted response to lapatinib monotherapy in women with breast cancer, which included overexpression of HER-2, expression of phosphorylated HER-2, and baseline TUNEL score greater than 0 (evidence of spontaneous tumor cell apoptosis). Although inhibition of HER-2 phosphorylation, phospho-Erk1/2, and phospho-Akt may be necessary for clinical response to lapatinib, they are not sufficient. Downregulation of survivin, a member of the IAP (inhibitor of apoptosis protein) family and a predictor of adverse clinical outcome in breast cancer, appears to represent a more robust correlate of clinical response associated with inhibition of HER-2 autokinase activity by lapatinib in HER-2 overexpressing breast cancers [37].
In addition to lapatinib, sequential tumor biopsies were obtained during a phase I study (n = 53) conducted in patients with solid tumors treated with canertinib [38]. The biologic effects of canertinib on its intended targets (namely phospho-EGFR and phospho-HER-2), cell proliferation (Ki67), and expression of the cyclin-dependent kinase inhibitor p27 were assessed. Immunoprecipitation and Western blot analysis conducted in nine tumor biopsies showed a median reduction in phospho-EGFR protein levels of 44%, a 26% reduction in Ki67, and a 56% increase in p27 steady-state protein expression at day 15 of therapy compared with baseline (pretreatment) biopsies.
Safety and tolerability
Cardiotoxicity is a significant concern among patients treated with trastuzumab who were previously treated with anthracyclines [39]. In first-line treatment of advanced stage breast cancer, trastuzumab in combination with anthracycline and cyclophosphamide (AC; n = 143) resulted in 27% and 16% incidences of any cardiac dysfunction and New York Heart Association class III-IV heart failure, respectively, as compared with 7% and 5% with trastuzumab alone, and 7% and 3% with anthracycline and cyclophosphamide alone [8]. Although the precise mechanism of trastuzumab-induced cardiotoxicity is unknown, HER-2 appears to serve as a survival factor for cardiac myocytes [40]. Recently, an increased incidence of cardiotoxicity was demonstrated in patients receiving imatinib [41], which targets members of the abl kinase family, raising questions as to whether small molecule TKIs, particularly those targeting HER-2, might also have cardiotoxic effects. Lapatinib appears to carry lower risk for cardiotoxicity compared with trastuzumab [42]. For example, in a randomized phase III clinical trial comparing the combination of lapatinib plus capecitabine with capecitabine alone in women with relapsed HER-2 positive breast cancer previously treated with an anthracycline and trastuzumab [43], there were four asymptomatic cardiac events in the lapatinib/capecitabine arm (n = 163). All lapatinib trials excluded patients with left ventricular ejection fraction of 50% or less, or below the lower limit of institutional normal levels, potentially biasing the data by selecting those individuals who are at lower risk for developing cardiotoxicity.
Because dual EGFR/HER-2 and pan-HER inhibitors are potent inhibitors of EGFR signaling, it is not surprising that their major toxicity is EGFR related, including skin rash and diarrhea, the latter representing the dose-limiting toxicity for most of these compounds [30, 44]. In addition, caneritinib use was associated with thrombocytopenia [30].
Clinical data in breast cancer
Lapatinib
Lapatinib, a dual EGFR/HER-2 TKI, is the most clinically advanced of the HER-2 kinase inhibitors in breast cancer. The initial suggestion of clinical activity in breast cancer was demonstrated in a phase Ib dose-ranging study in which 30 heavily pretreated breast cancer patients received lapatinib monotherapy [44]; of these patients, four experienced confirmed partial responses and 10 others had prolonged stable disease. The four partial responses were all in patients with HER-2 overexpressing breast cancers [36, 44]. Interestingly, four out of five patients with inflammatory breast cancer (IBC) treated in phase I lapatinib trials (monotherapy and combination studies) achieved a partial response; of these two received lapatinib monotherapy and one each on lapatinib and paclitaxel, and lapatinib and capecitabine combination studies [44–46]. All of these IBC responders overexpressed HER-2.
This encouraging activity led to a phase II trial of lapatinib monotherapy in patients with recurrent/anthracycline-refractory IBC. Patients were assigned to one of two cohorts depending on whether their tumor overexpressed HER-2 or did not overexpress HER-2 but expressed EGFR. The preliminary data were recently reported at the 31st Annual Meeting of the European Society of Medical Oncology [47]. Patients received oral lapatinib (1500 mg/day) monotherapy on a continuous basis. Approximately 50% (16 out of 30) of the patients in the HER-2 overexpressing cohort achieved a complete or partial response in skin/chest wall lesions and/or RECIST target lesions, as compared with only about 7% (1 out of 15) of patients in the EGFR expressing, HER-2 non-overexpressing cohort. These results are encouraging in light of the heavily pretreated nature of these patients with aggressive IBC, and they further highlight the significance of HER-2 overexpression as a predictor of response to lapatinib monotherapy in breast cancer. Further studies investigating the use of lapatinib in IBC, both as a monotherapy and in combination with other agents, are currently underway.
Two large phase II clinical trials in which heavily pretreated patients with HER-2 overexpressing breast cancer received lapatinib monotherapy demonstrated marginal clinical activity, with seven of the initial 81 evaluable patients achieving an objective response [48]. Targeted therapies such as lapatinib will probably be more effective in the setting of earlier disease, especially when they are used as monotherapy. In this context, a phase II clinical trial of lapatinib monotherapy was conducted in chemotherapy-naïve patients with metastatic HER-2 overexpressing (positive by fluorescent in situ hybridization) breast cancer. An interim analysis of the first 40 patients [49] identified a response rate of approximately 30%, with a similar percentage of patients experiencing stable disease.
The treatment of most cancers relies on the use of combinations of non-cross-resistant drugs. In this context, a multicenter, open-label, randomized phase III clinical trial comparing lapatinib and capecitabine versus capecitabine alone [43] was conducted in patients with HER-2 over-expressing (3+ by immunohistochemistry or positive by fluorescent in situ hybridization) metastatic or locally advanced breast cancer. Eligibility required documented progression on prior anthracycline, taxane, and trastuzumab therapy. The primary clinical end-point was time to progression in the intention-to-treat patient population. Overall survival, response rate, and progression-free survival were secondary end-points. Based on an interim analysis conducted by an independent safety review board in 321 patients (160 in the lapatinib plus capecitabine arm, and 161 in the capecitabine monotherapy arm), there was a statistically significant improvement in median time to progression in the lapatinib plus capecitabine arm (36.9 weeks) as compared with the capecitabine monotherapy arm (19.7 weeks; P = 0.00016). Similarly, there was a statistically significant increase in progression-free survival, with median progression-free survival in the combination arm being 36.9 weeks as compared with 17.9 weeks in the capecitabine monotherapy arm (P = 0.000045). There did not appear to be statistically significant differences in response rate between groups, although there was a trend in favor of the combination arm. The study was terminated early because of superiority, based on the recommendation of the independent safety review board, making it difficult to determine whether there was a difference in overall survival between the two arms.
Additional lapatinib combination studies in various settings of breast cancer are currently ongoing, including combinations with taxanes, trastuzumab, aromatase inhibitors, and antiestrogens.
Canertinib (CI-1033)
Early phase clinical trials conducted in patients with breast cancer suggested that this pan-HER irreversible TKI has clinical activity in this setting. Results from a phase II trial of canertinib monotherapy in advanced stage breast cancer (n = 32), which has completed accrual, are pending. In addition to the typical EGFR-related toxicity, there is a 28% incidence of thrombocytopenia associated with canertinib, which might complicate its combination with myelosuppressive cytotoxic agents [30].
HKI-272
HKI-272 is a dual EGFR, HER-2 irreversible TKI that is currently in early phase clinical development. Recently presented preliminary phase I data from 51 patients with solid tumors, 23 of whom had advanced stage breast cancer, indicate that there were two confirmed and two unconfirmed partial responses in breast cancer [50]. The encouraging response rate in this phase I, heavily pretreated patient population led to the initiation of a phase II clinical trial of HKI-272 monotherapy in patients with advanced stage breast cancer.
Other HER-2 tyrosine kinase inhibitors
BIBW 2992 is an irreversible inhibitor of HER-2 and EGFR tyrosine kinases. Phase I studies investigating different dosing schedules (14-day treatment/28-day cycle and 21-day treatment/28-day cycle) have been undertaken in 22 patients with solid tumors. Prolonged stable disease rather than complete or partial response have been observed in these phase I studies [51]. BMS-599626 is an oral pan-HER receptor kinase inhibitor that is currently in phase I clinical trials. TAK 165 is a selective irreversible inhibitor of HER-2 kinase, which has demonstrated activity against HER-2 overexpressing breast cancer cell lines. It is also worth noting that EGFR mono-inhibitors, such as gefitinib and erlotinib, have exhibited very limited clinical activity when used as monotherapy in the setting of advanced stage breast cancer. Their use in combination with antiestrogens to prevent the development of tamoxifen resistance continues to be an intriguing application pursued in the clinic [52, 53].
Potential advantages of small molecule HER-2 kinase inhibitors over antibody therapies
In addition to the convenience of an oral drug compared with an antibody requiring weekly intravenous infusions, there appears to be reduced risk for cardiotoxicity with lapatinib compared with trastuzumab; the reasons for this difference are probably inherent to the disparate biologic effects of lapatinib and trastuzumab. Drugs with reduced risk for cardiotoxicity may be particularly desirable in the adjuvant setting, in which the long-term effects of cardiotoxicity are less acceptable.
As patients with HER-2 overexpressing breast cancers live longer on trastuzumab-based therapies, the incidence of central nervous system (CNS) metastasis increases. Large molecular weight molecules (for instance, trastuzumab) do not effectively cross the blood-brain barrier. Small molecule HER-2 kinase inhibitors have the advantage that they are able to cross into the CNS. A pilot study of lapatinib monotherapy in breast cancer patients with brain metastases demonstrated that lapatinib crosses the blood-brain barrier, has a biologic effect in brain tumors (as determined by changes in fluorodeoxyglucose positron emission tomography), and exhibits clinical activity [54]. In addition, in a randomized phase III clinical trial comparing lapatinib plus capecitabine (n = 163) with capecitabine monotherapy (n = 161) in relapsed breas cancer [43], there were fewer CNS relapses in the combination arm (four CNS relapses) than in capecitabine monotherapy arm (12 CNS relapses). Additional studies are in progress to expand upon these initial observations.
Finally, three proposed mechanisms that mediate resistance to trastuzumab do not appear to be relevant to HER kinase inhibitors such as lapatinib. First, expression of IGF receptor 1 in HER-2 overexpressing breast cancers, which confers resistance to trastuzumab, does not preclude response to lapatinib and may predict a more favorable clinical outcome [36, 47, 49, 55]. Second, PTEN deficiency, which purportedly mediates trastuzumab resistance [15], does not appear to affect response to lapatinib [47, 56]. Finally, the presence of p95HER-2 (the truncated HER-2 receptor that lacks the extracellular domain), which exhibits increased expression with disease progression and confers resistance to trastuzumab, remains sensitive to lapatinib in preclinical models [33].
Although infrequent, activating mutations in the HER-2 kinase domain are present in certain epithelial tumors [57]. Recently, Arteaga and coworkers [58] showed that lapatinib and caneritinib, but not mono-EGFR inhibitors, were active against cells expressing these mutations. Thus, in the future, the specific HER-2 mutation identified could be used to direct choices regarding the optimal HER targeted therapy.
Combination strategies with HER-2 kinase inhibitors in breast cancer
HER-2 targeted therapies are more effective when they are combined with other agents, as indicated by the enhanced clinical efficacy of trastuzumab when it is used in combination with cytotoxics as compared with trastuzumab monotherapy. Is there a rationale for selecting drugs that are more likely to enhance the efficacy of HER-2 kinase inhibitors? The answer is 'yes'. There may be a biologic explanation for why the combination of lapatinib and capecitabine is effective, which includes lapatinib-mediated downregulation of thymidine synthase, an enzyme associated with resistance to 5-fluorouracil [59]. Whether HER-2 kinase inhibitors will exhibit enhanced efficacy in combination with other classes of cytotoxic agents remains to be determined.
We have an opportunity to combine HER-2 kinase inhibitors with other targeted therapies. Preclinical studies have demonstrated enhanced antitumor activity and inhibition of survivin in HER-2 overexpressing breast cancer cell lines in response to combined trastuzumab and lapatinib therapy as compared with either agent as monotherapy [60]. Furthermore, a recent phase I trial of trastuzumab and lapatinib [61] identified a 23% response rate in advanced stage, heavily pretreated breast cancers. These results triggered an ongoing phase III randomized clinical trial of trastuzumab and lapatinib.
Crosstalk between estrogen and HER receptors provides a rationale for combining antiestrogens with HER targeted therapies. We established a model of autoresistance to lapatinib in which resistance was mediated in part through the upregulation of estrogen receptor signaling [62]. Combining specific antiestrogens with lapatinib prevented the onset of lapatinib autoresistance. These preclinical studies provided a rationale for subsequent phase II/III clinical trials combining lapatinib with various antiestrogen therapies.
In light of the crosstalk between IGF receptor 1 and HER receptors, combining therapies targeting both pathways makes sense scientifically. Recently, Esteva and colleagues [55] reported enhanced antitumor effects of combined lapatinib with IGF receptor 1 specific antibodies in breast cancer cells. Similarly, the crosstalk between the vascular endothelial growth factor (VEGF) receptors and HER receptors provides a rationale for combining HER-2 kinase inhibitors with anti-VEGF antibodies (for example, bevacizumab) or small molecule VEGF receptor TKIs [63, 64]. Clinical trials investigating these combinations in breast cancer are currently underway, with interesting preliminary responses recently reported. Additional combinations include the combination of HER-2 kinase inhibitors with hsp90 antagonists, the latter inducing proteolysis of HER-2, and combination with inhibitors of the PI3K-Akt-mTOR (mammalian target of rapamycin) pathway, especially in tumors where there is evidence of pathway deregulation (for example, PI3KCA mutation).
Conclusion
In light of its role in promoting tumor cell proliferation and survival in breast cancer, HER-2 is an attractive target. Trastuzumab validated HER-2 as a therapeutic target by changing the natural history of HER-2 overexpressing breast cancers by extending survival. One alternative approach to targeting HER-2 is via small molecule HER-2 kinase inhibitors, currently developed in the clinic. Lapatinib, the most advanced of these compounds, recently exhibited clinical efficacy when combined with capecitabine in a randomized phase III trial of patients with HER-2 over-expressing breast cancer. Lapatinib has also shown clinical activity as a monotherapy in women with heavily pretreated HER-2 overexpressing IBC. A number of other small molecule inhibitors with differing activity profiles are under development. Small molecule HER-2 kinase inhibitors have several potential advantages over trastuzumab, not the least of which is the possibility of reduced risk for cardiotoxicity and efficacy in settings of trastuzumab resistance. The use of oral small molecule HER-2 kinase inhibitors in combination with conventional cytotoxics and other targeted therapies based on scientific rationale represents the future for this important class of therapeutics.
Note
This article is part of a review series on HER2 therapy, edited by Mark Pegram.
Other articles in the series can be found online at http://breast-cancer-research.com/articles/review-series.asp?series=BCR_HER2
Abbreviations
- CNS:
-
central nervous system
- EGFR:
-
epidermal growth factor receptor
- HER:
-
human epidermal growth factor receptor
- IBC:
-
inflammatory breast cancer
- IGF:
-
insulin-like growth factor
- MAPK:
-
mitogen-activated protein kinase
- PI3K:
-
phosphatidylinositol-3 kinase
- TKI:
-
tyrosine kinase inhibitor
- TUNEL:
-
terminal dUTP nick-end labeling
- VEGF:
-
vascular endothelial growth factor.
References
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stewart SG, Udove J, Ullrich A, et al: Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989, 244: 707-712. 10.1126/science.2470152.
Bacus SS, Zelnich CR, Plowman G, Yarden Y: Expression of the erbB-2 family of growth factor receptors and their ligands in breast cancer: Implication for tumor biology and clinical behavior. Am J Clin Pathol. 1994, 102 (4 Suppl 1): S13-S24.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL: Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987, 235: 177-182. 10.1126/science.3798106.
Yarden Y, Sliwkowski MX: Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001, 2: 127-137. 10.1038/35052073.
Hynes N, Lane H: ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005, 5: 341-354. 10.1038/nrc1609.
Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE: ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998, 18: 5042-5051.
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fahrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, et al: Multinational study of the efficacy and safety of humanized anti-Her2 monoclonal antibody in women who have Her2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999, 17: 2639-2648.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001, 344: 783-792. 10.1056/NEJM200103153441101.
Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, et al: Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998, 16: 2659-2671.
Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, et al: Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996, 14: 737-744.
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al: Trastuzumab plus chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005, 353: 1673-1684. 10.1056/NEJMoa052122.
Piccart-Gebhart MJ, Proctor M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005, 353: 1659-1672. 10.1056/NEJMoa052306.
Clynes RA, Towers TL, Presta LG, Ravetch JV: Inhibitory Fc receptors modulates in vivo cytotoxicity against tumor targets. Nat Med. 2000, 6: 443-446. 10.1038/74704.
Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A: p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989, 9: 1165-1172.
Nahta R, Esteva FJ: Herceptin: mechanisms of action and resistance. Cancer Lett. 2006, 232: 123-138. 10.1016/j.canlet.2005.01.041.
Sliwkowski MX, Lofgren J, Lewis GD, Hotaling TE, Fendly BM, Fox JA: Nonclinical studies addressing the mechanism of action of trastuzumab (herceptin). Semin Oncol. 1999, 26 (4 Suppl 12): 60-70.
Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ: Antibody to Her-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994, 9: 1829-1838.
Smith BL, Chin D, Maltzman W, Crosby K, Hortobagyi GN, Bacus SS: The efficacy of Herceptin therapies is influenced by the expression of other erbB receptors, their ligands and the activation of downstream signalling proteins. Br J Cancer. 2004, 91: 1190-1194.
Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M: Insulin-like growth factor-1 receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst. 2001, 93: 1852-1857. 10.1093/jnci/93.24.1852.
Nahta R, Yu D, Hong MC, Hortobagyi GN, Esteva FJ: Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006, 3: 269-280. 10.1038/ncponc0509.
Nagata Y, Lan K-H, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al: PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004, 6: 117-127. 10.1016/j.ccr.2004.06.022.
Saez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, Lluch A, Garcia-Conde J, Baselga J, Clinton GM: p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006, 12: 424-431. 10.1158/1078-0432.CCR-05-1807.
Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ: P27 (kip1) down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004, 64: 3981-3986. 10.1158/0008-5472.CAN-03-3900.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe J-P, Tong F, et al: A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006, 10: 515-527. 10.1016/j.ccr.2006.10.008.
Xia W, Mullin RJ, Keith BR, Liu L, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL: Anti-tumor activity of GW572016: A dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002, 21: 6255-6263. 10.1038/sj.onc.1205794.
Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, et al: Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA. 1998, 95: 12022-12027. 10.1073/pnas.95.20.12022.
Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Aziomioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen T, Floyd M, et al: A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005, 23: 329-336. 10.1038/nbt1068.
Sartor CI, Zhou H, Kozlowska E, Guttridge K, Kawata E, Caskey L, Harrelson J, Hynes N, Ethier S, Calvo B, et al: HER4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001, 21: 4265-4275. 10.1128/MCB.21.13.4265-4275.2001.
Bence AK, Anderson EB, Doukas MA, DeSimone PA, Davis GA, Smith DA, Koch KM, Stead AG, Mangum S, Spector NL, et al: Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW57 a dual EGFR-ErbB2 inhibitor, in healthy subjects. Invest New Drugs. 2005, 23: 39-49. 10.1023/B:DRUG.0000047104.45929.ea.
Nemunaitis J, Eiseman I, Cunningham C, Senzer N, Williams A, Lenehan P, Olson SC, Bycott P, Schlicht M, Zentgraff R, et al: Phase I clinical and pharmacokinetics evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2005, 11: 3846-3853. 10.1158/1078-0432.CCR-04-1950.
Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Brawner Floyd M, Golas J, Hallett WA, Johnson BD, Nilakantan R, et al: Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004, 64: 3958-3965. 10.1158/0008-5472.CAN-03-2868.
Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, Ellis B, Pennisi C, Horne E, Lackey K, et al: A unique structure for epidermal growth factor receptor bound to GW572016 (lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004, 64: 6652-6659. 10.1158/0008-5472.CAN-04-1168.
Xia W, Liu L-H, Ho P, Spector NL: Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3, yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004, 23: 646-653. 10.1038/sj.onc.1207166.
Baselga J: Skin as a surrogate tissue for pharmacodynamic end points: is it deep enough?. Clin Cancer Res. 2003, 9: 2389-2390.
Malik SN, Siu LL, Rowinsky EK, deGraffenried L, Hammond LA, Rizzo J, Bacus S, Brattain MG, Kreisberg JI, Hidalgo M: Pharma-codynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patients. Clin Cancer Res. 2003, 9: 2478-2486.
Spector NL, Xia W, Burris HA, Hurwitz H, Claire Dees E, Dowlati A, O'Neil B, Overmoyer B, Liu L, Marcom K, et al: A Study of the biological effects of GW572016, a reversible inhibitor of EGFR (ErbB1) and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005, 23: 1-11.
Xia W, Bisi J, Strum J, Liu L, Carrick K, Graham KL, Hardwicke MA, Treece AL, Bacus S, Spector NL: Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overex-pressing breast cancers. Cancer Res. 2006, 66: 1640-1647. 10.1158/0008-5472.CAN-05-2000.
Zinner RG, Donato NJ, Nemunaitis JJ, Cunningham CC, Shin HJ, Zentgraf RE, Ayers GD, Glisson BS, Khuri FR, Kies MS, et al: Biomarker modulation in tumor and skin biopsy samples from patients with solid tumors following treatment with the panerbB tyrosine kinase inhibitor CI-1033. Proc Am Soc Clin Oncol. 2002, 21: 58a-
Guarneri V, Lenihan DJ, Valero V, Durand JB, Durand JB, Broglio K, Hess KR, Michaud LB, Gonzalez-Angulo AM, Hortobagyi GN, et al: Long-term cardiac tolerability of trastuzumab in meta-static breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol. 2006, 24: 4107-4115. 10.1200/JCO.2005.04.9551.
Chien KR: Myocyte survival pathways and cardiomyopathy: implications for trastuzumab cardiotoxicity. Semin Oncol. 2000, 27: 9-14.
Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, et al: Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006, 12: 908-916. 10.1038/nm1446.
Perez EA, Byrne JA, Hammond IW, Rafi R, Martin AM, Berger MS, Zaks TZ, Oliva CR, Roychowdhury DF, Stein SH: Results of an analysis of cardiac function in 2812 patients treated with lapatinib [abstract]: ASCO Annual Meeting Proceedings. J Clin Oncol. 2006, 24 (18S): 583a-
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006, 355: 2733-2743. 10.1056/NEJMoa064320.
Burris HA, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O'Neil B, Marcom PK, Ellis MJ, Overmoyer B, Jones SF, et al: Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005, 23: 5305-5313. 10.1200/JCO.2005.16.584.
Jones SF, Burris HA, Yardley DA, Greco FA, Spigel DR, Raefsky EL, Hainsworth JD, Willcutt NT, Calvert SA, Versola MJ, et al: Lapatinib (an oral dual kinase inhibitor) plus weekly or every 3 week paclitaxel [abstract]. San Antonio Breast Cancer Symposium. Breast Cancer Res Treat. 2004, 88 (Suppl 1): 1069a-
Schwartz G, Chu QSC, Hammond LA, Mita M, Curtright J, Versola MJ, Kock K, Pandite LN, Ho PT, Rowinsky EK: Phase I clinical, biology and pharmacokinetic study of the combination of GW572016 and capecitabine in patients with advanced solid tumors [abstract]: ASCO Annual Meeting Proceedings. J Clin Oncol. 2004, 22 (14S): 3070a-
Trudeau M, Johnston S, Kaufman B, Blackwell K, Ahmed SB, Boussen H, Frikha M, Ayed FB, El-Hariry I, Spector N: Lapatinib (Tycerb) monotherapy in patients with recurrent inflammatory breast cancer: clinical activity and biological predictors of response. Annals Oncol. 2006, 1400a-Suppl 16
Blackwell KL, Burstein H, Pegram M, Storniolo A, Salazar VM, Maleski JE, Lin X, Spector N, Stein SH, Berger MS: Determining relevant biomarkers from tissue and serum that may predict response to single agent lapatinib in trastuzumab refractory metastatic breast cancer [abstract]: ASCO Annual Meeting Proceedings. J Clin Oncol. 2005, 23 (16S): 3004a-
Gomez HL, Chavez MA, Doval DC, Chow LW, Wood BA, Berger MS, Sledge GW: A Phase II, randomized trial using the small molecule tyrosine kinase inhibitor lapatinib as a first-line treatment in patients with FISH positive advanced or metastatic breast cancer: ASCO Annual Meeting Proceedings. J Clin Oncol. 2004, 23 (14S): 3046a-
Wong KK, Fracasso PM, Bukowski RM, Munster PN, Lynch T, Abbas R, Quinn SE, Zacharchuk C, Burris H: HKI-272, an irreversible pan erbB receptor tyrosine kinase inhibitor: preliminary phase 1 results in patients with solid tumors: ASCO Annual Meeting Proceedings. J Clin Oncol. 2006, 24 (18S): 3018a-
Agus DB, Terlizzi E, Stopfer P, Amelsberg A, Gordon MS: Phase 1 dose escalation study of BIBW-2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumors: ASCO Annual Meeting Proceedings. J Clin Oncol. 2006, 24 (18S): 2074a-
Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, McClelland RA, Jordan N, Wakeling AE, Nicholson RI: The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. 2003, 144: 5105-5117. 10.1210/en.2003-0705.
Arpino G, Weiss H, Lee AV, Schiff R, DePlacido S, Osborne CK, Elledge RM: Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005, 97: 1254-1261.
Lin NU, Carey LA, Liu MC, Younger J, Come SE, Bullitt E, Van Den Abbeele AD, Li X, Hockberg FH, Winer EP: Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancer. J Clin Oncol. 2006, 24: 503a-10.1200/JCO.2005.05.1458.
Nahta R, Yuan LXH, Zhang B, Kobayashi R, Esteva FJ: Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor-I receptor 1 signaling. Mol Cancer Ther. 2007,
Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J, Pry K, Westlund R, Stein S, Spector NL: Lapatinib anti-tumor activity is not dependent upon PTEN in ErbB2-overexpressing breast cancers. Cancer Res. 2007,
Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, Stevens C, O'Meara S, Smith R, Parker A, et al: Intragenic ERBB2 kinase mutations in tumors. Nature. 2004, 431: 525-526. 10.1038/431525b.
Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK, Arteaga CL: HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006, 10: 25-38. 10.1016/j.ccr.2006.05.023.
Budman DR, Soong R, Calabro A, Tai J, Diasio R: Identification of potentially useful combinations of epidermal growth factor receptor tyrosine kinase antagonists with conventional cytotoxic agents using median effect analysis. Anti-Cancer Drugs. 2006, 17: 921-928. 10.1097/01.cad.0000224457.36522.60.
Xia W, Gerard C, Lui L, Baudson N, Ory T, Spector NL: Lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases synergizes with anti-ErbB2 antibodies to inhibit mediators of tumor cell survival and induce apoptosis in ErbB2 overexpressing breast cancer cells. Oncogene. 2005, 24: 6213-6221. 10.1038/sj.onc.1208774.
Storniolo A, Burris H, Pegram M, Overmoyer B, Mille K, Jones S, Silverman P, Paul E, Loftiss J, Pandite L: A phase I, open-label study of lapatinib (GW572016) plus trastuzumab; a clinically active regimen [abstract]: ASCO Annual Meeting Proceedings. J Clin Oncol. 2005, 16S: 559a-
Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, Paulozzo G, Trusk P, Lyass L, Spector NL: A model of acquired autoresistance to ErbB2 tyrosine kinase inhibitors and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006, 103: 7795-7800. 10.1073/pnas.0602468103.
Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, Stieber P, Vernes JM, Gutierrez J, Hong K, et al: Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004, 10: 1706-1716. 10.1158/1078-0432.CCR-0951-3.
Miller KD, Trigo JM, Wheeler C, Barge A, Rowbottom J, Sledge G, Baselga J: A multicenter phase II trial of ZD a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibition, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005, 11: 3369-3376. 10.1158/1078-0432.CCR-04-1923.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Neil Spector and Wenle Xia worked at GlaxoSmithKline within the past 5 years, and Iman El-Hariry is currently a GlaxoSmithKline employee.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Spector, N., Xia, W., El-Hariry, I. et al. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Res 9, 205 (2007). https://doi.org/10.1186/bcr1652
Published:
DOI: https://doi.org/10.1186/bcr1652