Abstract
Specific heats of two types of lead tellurides, namely p-type Pb1-xSn x Te (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0) and n-type PbTe doped with y mass% PbI2 (y = 0.0, 0.2, 0.5, 0.8, 1.0), were observed in the temperature range of 50°C to 500°C using a differential scanning calorimeter (DSC). The specific heat of pure Te is also observed in the same temperature range to confirm the dominance of Te. At lower temperatures below 200°C, all the observed data were fairly consistent with those predicted on the basis of Dulong-Petit’s law, while above 200°C, the observed data showed curious temperature dependences. At high temperatures above 200°C, DSC analysis, with the aid of the experiment on pure Te, revealed an anomalously large specific heat that can be predicted from the Schottky defects caused by decomposition and sublimation of Te. In the n-type case, the anomaly in specific heats is rather smaller than that in the p-type case, which fact suggested that the dopant PbI2 may suppress the decomposition and sublimation of Te from lead tellurides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead tellurides, namely n-type PbTe doped with 0.5 mass% PbI2 and p-type Pb0.5Sn0.5Te, are widely used for practical thermoelectric generation applications[1, 2]. However, it has recently been reported that heating over 400°C causes the decomposition and sublimation of Te from lead tellurides[3]. This report clarified that heating over 400°C will debase the thermoelectric performance of lead tellurides.
The output characteristics of thermoelectric generators depend on the thermal characteristics as well as the electrical characteristics. For this reason, we examined the temperature dependence of specific heat of lead telluride to investigate the behavior of Te in lead telluride from the viewpoint of the evaluation of thermal characteristics.
Experimental procedure
Sample preparation
Pure materials, namely 6N Pb, 6N Te, and 5N PbI2, were weighed out at desirable composition ratios of Pb1-xSn x Te (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0) and PbTe + y mass% PbI2 (y = 0.0, 0.2, 0.5, 0.8, 1.0) in a globe box filled with argon. They were put in quartz ampoules and encapsulated in a vacuum of 2×10−3 Pa. The ampoules were put in a locking furnace. The materials in ampoules were melted and stirred for 1 h at 1,100°C and then cooled down at a rate of 150°C/h with a temperature gradient of 0.5°C/mm and a locking frequency of 0.3 Hz. The ingots obtained were identified by X-ray diffraction analysis as Pb1-xSn x Te and PbTe + y mass% PbI2.
Measurement of the specific heats
The specific heats of the samples taken out of the ingots were measured with a differential scanning calorimeter (DSC; SHIMADZU DSC-60, Kyoto, Japan). The temperature of the soaking block in DSC is controlled to supply the heat Qs to the sample holder and the heat Qr to the reference holder, while the sample temperature Ts and the reference temperature Tr are measured to evaluate the sample-reference temperature difference Δ T, which determines the feedback heat flows. The difference between the heat flows is output as an electrical signal.
Three types of DSC signals were observed in three different conditions, namely (a) the sample holder was empty, (b) it contained the standard material, and (c) it contained the sample whose specific heat was unknown, while the reference holder was kept to be empty. In this experiment, 99% pure α-alumina was used as the standard sample. The specific heats were determined with the analyzing software attached to the DSC machine under the conditions shown in Table1.
Results and discussion
P-type Pb1-xSn x Te
Figure1 shows the result of the p-type Pb1-xSn x Te (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0). At low temperatures below 200°C, the observed specific heats were approximately constant for all the samples, while above 200°C, they tended to increase with increasing temperature. Specific heats of the contents Pb, Sn, and Te were observed to verify the experiment. The results are shown in Figures2,3, and4.
As shown in Figure4, the specific heat of Te rapidly increased with increasing temperature at temperatures ranging from 200°C to 400°C, which suggested that Te strongly affects the thermal characteristics of Pb1-xSn x Te.
Assuming that individual atoms in Pb1-xSn x Te are moving independently, the specific heat was estimated on the basis of Dulong-Petit’s law, and it was compared with that observed at low temperatures below 200°C, where the gas constant is assumed to have a theoretical value of 8.3145 J/(mol K). The results are shown in Tables2 and3.
The calculated values were roughly equal to the observed values, but all the observed values were larger than the calculated values, which suggests that other energies than the lattice vibration energy are also included in the total kinetic energy. The difference between the observed values at high temperature above 200°C and those at low temperature below 200°C may be due to the contribution from the free motion of atoms and/or ions in the experiment performed for Te, which might cause the abnormally large values at high temperatures above 200°C in the specific heat of Te as shown in Figure5.
Anomaly in the specific heat that shows the apparent value of the specific heat rapidly increases with increasing temperature suggests that the number of atoms and/or ions moving freely rapidly increases with increasing temperature. The facts and considerations mentioned above lead us to the following discussion:
-
1.
At low temperatures below 200°C, atoms and/or ions move through the defects that exist from the outset in the sample, so that the observed values will be rather larger than the calculated values.
-
2.
In the case of pure Te, PbTe, SnTe, and Pb1-xSn x Te, Schottky defects increase with increasing temperature and Te atoms and/or ions freed from the crystal lattice intensely move around in the sample, which causes a large increase of the specific heat.
On the basis of the discussion above, we calculated the activation energy and the number of Schottky defects equal to the number of free atoms and ions. With the excess specific heat denoted by Q, the frequency factor by A, the activation energy by E V , the absolute temperature by T, and Boltzmann constant by kB, the following equation is given:
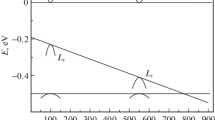
The value of E V was estimated at 0.20 eV from the inclination of the Arrhenius plot for Te activation based on Equation 1 as shown in Figure6. With the number of Schottky defects equal to the number of free atoms and ions denoted by n and the number of lattice points by N, which is assumed to be about 1023 cm-3, the following equation is given:
The calculation results of n are shown in Table4 and Figure7. It is obvious from the results that the number of Schottky defects or the number of free atoms and ions parabolically increases with increasing temperature.
N-type PbTe + y mass% PbI2
Figure8 shows the observed specific heats of PbTe + y mass% PbI2 (y = 0.0, 0.2, 0.5, 0.8, 1.0). The specific heats slightly increased with increasing temperature for all the samples, except for the non-doped (y = 0) sample. These results differ from the results in the case of pure Te, PbTe, SnTe, and Pb1-xSn x Te, i.e., the excess specific heats decrease at high temperatures above 200°C. Such a difference may be due to the effect of the dopant PbI2. PbI2 is usually added to PbTe as an n-type dopant. It may be reasoned that PbI2 filled up some vacancies and suppressed the decomposition of Te at high temperature above 200°C, which reduced the number of Schottky defects and consequently decreased the excess specific heat.
From the results obtained in this work and those published in the previous work[3], the atomic behavior may be described for the three temperature ranges as follows:
-
1.
100°C to 200°C
Atoms and ions move around via vacancies in the crystalline sample. The observed specific heat is rather larger than that calculated on the basis of Dulong-Petit’s law.
-
2.
200°C to 400°C
Decomposition of Te is caused and consequently Schottky defects are increased by heating. Te atoms move onto the sample surface to form a liquid or amorphous state.
-
3.
400°C and up
Te on the sample is volatilized.
The experiments performed in this work revealed the curious properties of Te, which is the main component of tellurides. It is concluded that PbI2 added as a dopant suppresses the sublimation of Te and consequently Schottky defects.
Conclusions
In order to investigate the behavior of Te in lead tellurides, DSC analysis was made on Pb1-xSn x Te (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0) and PbTe + y mass% PbI2 (y = 0.0, 0.2, 0.5, 0.8, 1.0). The results are concluded as follows:
-
1.
In the temperature range of 100°C to 200°C, the observed specific heats were rather greater than those predicted from Dulong-Petit’s law. It is concluded that the deviation from Dulong-Petit’s law is due to that the atoms more intensively move via the vacancies increased by heating.
-
2.
At high temperatures above 200°C, an excess specific heat is observed for PbTe system compounds. Discussion with the aid of the Arrhenius plot revealed the increase in the number of Schottky defects caused by the decomposition of Te from the crystal lattice sites.
-
3.
The excess specific heats of n-type PbTe + y mass% PbI2 at high temperatures above 200°C decreased with increasing temperature, which differed from those of Pb1-xSn x Te. It is concluded that PbI2 added as a dopant suppresses the decomposition of Te from the crystal lattice sites, which reduces the number of Schottky defects.
-
4.
The results of these experiments do not directly indicate that Te in PbTe devices is readily sublimated and dissociated by heating. Many types of PbTe devices, in fact, have operated for extended periods of time at higher temperatures than 200°C. It is, however, suggested that Te in PbTe devices might be sublimated and dissociated at lower temperatures when bare surfaces of the devices include any defects such as scratches, cracks, and voids, which would induce the effects discussed in this report.
References
Rowe DM: CRC Handbook of Thermoelectrics. Boca Raton: CRC; 1995.
Yoneda S, Ohta E, Kaibe HT, Ohsugi IJ, Shinohara Y, Nishida IA: Possibility of improvement of thermoelectric properties of Pb1-xSnxTe by the functionally graded material. J. Adv. Sci 2001, 12: 379–384.
Yoneda S, Kato M, Ohsugi IJ: Anomalous thermal expansion of Pb–Te system semiconductors. J. Appl. Phys 2010, 107: 074901. 10.1063/1.3361282
Acknowledgements
The authors gratefully acknowledge the financial and other support of this research provided by the Faculty of Engineering, Kanagawa University, Yokohama, Kanagawa, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SY carried out all the analysis and calculation, conceived and designed the study, and drafted the manuscript. IJO and MK carried out the X-ray analysis and participated in the discussion and writing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yoneda, S., Kato, M. & Ohsugi, I.J. Anomaly in the specific heat of lead tellurides. J Theor Appl Phys 7, 11 (2013). https://doi.org/10.1186/2251-7235-7-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7235-7-11