Abstract
Zinc sulfide (ZnS) semiconductor nanocrystals with mercaptoethanol (ME) as a stabilizer (capping agent) were synthesized by coprecipitation method in room temperature using the solution of zinc chloride (ZnCl2) and sodium sulfide (Na2S) as starting material. The effect of ME and Na2S dropwise addition rate on the preparation of these samples was measured using UV–vis absorption and X-ray diffraction (XRD). The ultraviolet–visible (UV–vis) absorption and XRD of the prepared ZnS nanoparticles show increase of band gap and decrease of particle size with decrease in ME and Na2S dropwise addition rate to the reaction medium. This behavior is related to the size quantization effect due to the small size of the particles. The photoluminescence emission peak positions exhibit obvious blue shift from 510 to 455 nm. The particle sizes were obtained from transmission electron microscopy images.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Nanocrystalline semiconductor particles have attracted considerable interest in recent years because of their novel properties, such as large surface-to-volume ratio and the three-dimensional confinement of the electrons [1–7]. Interest in semiconductor nanoparticles is justified by the fact that their fundamental physical and chemical properties can be very different from those of the bulk materials. Their reduced dimensions enable one to reduce the size of electronic circuitry. They are expected to have higher quantum efficiencies due to increased oscillator strengths as a result of quantum confinement. A threshold for the occurrence of quantum effects is given by the value for the Bohr radius of exciton in the bulk material [8]. As a representative of wide band gap II-VI semiconductor nanocrystals, zinc sulfide (ZnS) has been synthesized by different methods [9–14]. ZnS has a band gap of 3.66 eV at the temperature of 300 K. This corresponds to ultraviolet (UV) radiation for optical interband transitions. It is an important inorganic material for a variety of applications including photoconductors, solar cells, field effect transistors, sensors, transducers, optical coatings, and light-emitting materials [15–18]. It has been investigated extensively because of its potential optical applications [19–26]. The systematic exploration of growing inorganic nanocolloids is an increasingly popular research subject. Physical methods such as X-ray or electron beam lithography [27], which are commonly used for the production of low-dimensional solids, have inherent resolution limits that restrict their use at the nanometer scale. On the other hand, the colloid chemistry route offers a simple means to synthesize such particles with good control of the size and, more importantly, the size distribution. There have been extensive reports in the past few years [28, 29] demonstrating that by changing the reaction conditions (i.e., the concentration of the starting materials, the nature of the solvents, and the suitable capping/stabilizing agents), it is possible to synthesize a variety of nanoparticles with different sizes. In this paper, we report the synthesis of ZnS quantum dots by the chemical coprecipitation method at room temperature and in aqueous solutions with zinc chloride (ZnCl2), sodium sulfide (Na2S) as starting materials, and mercaptoethanol (ME) as a capping agent for controlling particles size and double-distilled water as dispersing solvent. The effect of ME and Na2S dropwise addition rate to the reaction medium on the preparation of these samples has been studied.
Results and discussion
Figure 1 shows the X-ray diffraction (XRD) pattern of the ZnS nanoparticles with different dropwise addition rates at room temperature. Three different peaks are obtained at 2θ values of 29.0°, 48.1°, and 57.0° for the ZnS nanoparticles, which indicate that the samples have face centered cubic structure and the peaks correspond to diffraction at (111), (220), and (311) planes, respectively. Peaks related to high angles have submerged in the background due to the large line broadening, which is attributed to the nanoscale size of the particles.
The peak broadening at the lower angle is more meaningful for the calculation of particle size. Using the (111) reflection from the XRD pattern, the mean crystallite size (D) of nanoparticles was also estimated using the Scherrer formula [30] as follows:
where λ, B, and θ are the X-ray wavelength of the radiation used (Kα(Cu) = 0.154056 nm), the full width at half maximum of the diffraction peak, and the Bragg diffraction angle (with 2θ ranging from 10° to 100°), respectively. The lattice parameter has been computed to be 6.29 Å, which is close to the standard value (5.42 Å) [31].
The optical absorption spectra of the nanoparticles were measured using a USB-2000 UV–VIS spectrophotometer (Ocean Optics, Dunedin, FL, USA). The absorption edge is observed in the range of 290 to 320 nm. The band gap of nanoparticles was also calculated using the formula as follows:
where h, c, λ, and Eg are the Planck’s constant, the speed of light in vacuum, the optical absorption wavelength (range of 290 to 320 nm), and the band gap, respectively. This blue shift of the absorption edges for different nanocrystals is related to the size decrease of the particles and is attributed to the quantum confinement effect of nanoparticles. A threshold for the occurrence of quantum effects is given by the value for the Bohr radius of exciton in the bulk material. The absorption spectrum of B sample nanoparticles synthesized at 4 to 5 rates is shown in Figure 2. It shows an excitonic absorption peak at 300 nm (4.13 eV). There is a clear blue shift of band gap with respect to bulk ZnS, since band gap of bulk ZnS with cubic structure is 3.6 eV (345 nm). This is due to the modification of valence and conduction bands by quantum confinement effects. Even though the nanocrystallites exhibit bulk-like crystal structure, they are too small to have bulk-like electronic wave functions. Applying the confinement effects, the optical band gap energy of nanocrystallites is given by Brus equation [32] as follows:
where Eg(bulk) in eV is the band gap energy of bulk, me and mh are electron and hole effective masses, and R in nanometers is the particle size. For cubic ZnS, Eg = 3.6 eV, me = 0.34 mo, and mh = 0.23 mo; mo is the free electron rest mass and ϵ = 8.76 is the permittivity of the sample [33]. Taking the band gap of synthesized nanoparticles as 4.13 eV with substitution in Equation 4 gives the particle optical size as 2.69 nm. Table 1 shows the calculated size of ZnS nanoparticles from Scherrer formula and Brus equation. The band gap of a particle can be tuned by changing the size of nanocrystals below Bohr radius of exciton in the bulk material. Table 1 shows the variation of the optical band gaps, particle sizes, and absorption edges at different dropwise addition rates.
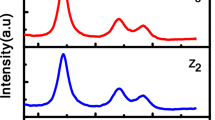
These semiconductor nanocrystals were then characterized using transmission electron microscopy (TEM, JEOL JEM3010; JEOL Ltd., Tokyo, Japan). Figures 3 and 4 show the TEM image of the ZnS semiconductor nanocrystals. According to the TEM images, the agglomeration occurred in the ZnS semiconductor nanocrystals. The agglomeration is due to van der Waals force between particles [34]. The TEM image shows clearly that the particles are not spherical.
Conclusions
The nanoparticles of zinc sulfide have been successfully synthesized using a simple chemical method. It is also observed that the decrease in size of the particle and the increase of band gap occur with the decrease in ME and Na2S dropwise addition rates to the reaction medium. The XRD analysis shows that the samples prepared are in a cubical phase. The broad peak of the XRD pattern indicates the nanocrystalline behavior of the particles. The absorption peak appears at around 290 to 320 nm, which is fairly blue-shifted from the absorption edge of the bulk (345 nm).
The solid-state theory based on the delocalized electron and hole within the confined volume can explain the blue-shifted optical absorption spectra [35, 36].
Experimental procedure
First, 0.01 M of ZnCl2, Na2S, and ME as capping agent was dissolved in double-distilled water for 30 min at room temperature, and the obtained solution was magnetically stirred for 30 min at room temperature. Afterwards, the capping agent solution was added drop by drop at different rates of 2 to 3, 4 to 5, and 6 to 7 per second to the ZnCl2 solution under vigorous stirring in N2 atmosphere. After 10 min, the solution of Na2S was poured drop by drop at different rates of 2 to 3, 4 to 5, and 6 to 7 per second to the solution containing ZnCl2 and ME under vigorous stirring in N2 atmosphere. Nanoparticles of ZnS were fabricated by chemical reaction by Ying Wang [37] as follows:
The nanoparticles were collected by centrifugation at 2,000 rpm for 15 min. In the final step, the obtained precipitate was filtered and dried at room temperature to remove water, organic cappings, and other by products formed during the reaction process. After sufficient drying, the precipitate was crushed to fine powder using mortar and pestle. The three samples, called A (2 to 3 s), B (4 to 5 s), and C (6 to 7 s) with different dropwise addition rates, were obtained.
References
Henglein A: Small-particle research: physico-chemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89: 1861–1873.
Steigerwald ML, Brus LE: Semiconductor crystallites: a class of large molecules. Acc. Chem. Res. 1990, 23: 183–188.
Bawendi MG, Steigerwald ML, Brus LE: The quantum-mechanics of larger semiconductor clusters (quantum dots). Annu. Rev. Phys. Chem. 1990, 41: 477–496.
Wang Y, Herron N: Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photo physical properties. J. Phys. Chem. 1991, 95: 525–532.
Weller H: Quantized semiconductor particles: a novel state of matter for materials science. Adv. Mater. 1993, 5: 88–95.
Alivisatos AP: Semiconductor clusters, nano crystals, and quantum dots. Science 1996, 271: 933–937.
Eychmüller A: Structure and photo physics of semi conductor nano crystals. J. Phys. Chem. B 2000, 104: 6514–6528.
van Dijken A, Janssen AH, Smitsmans MHP, Vanmaekelbergh D, Meijerink A: Size-selective photo etching of nano crystalline semiconductor particles. Chem. Mater. 1998, 10: 3513–3522.
Xu JF, Ji W, Lin JY, Tang SH, Du YW: Preparation of ZnS nanoparticles by ultrasonic radiation method. Appl Phys A 1998, 66: 639–641.
Dinsmore AD, Hsu DS, Qadri SB, Cross JO, Kennedy TA, Gray HF, Ratna BR: Structure and luminescence of annealed nanoparticles of ZnS:Mn. J. Appl. Phys. 2000, 88: 4985.
Que W, Zhou Y, Lam YL, Chan YC, Kam CH, Liu B, Gan LM, Chew CH, Xu GQ, Chua SJ, Xu SJ, Mendis FVC: Structural and luminescence properties of nanostructured ZnS:Mn. Appl. Phys. Lett. 1998, 73: 2727–2729.
Huang J, Yang Y, Xue S, Yang B, Liu S, Shen J: All-inorganic light emitting device based on ZnO nanoparticles. Appl. Phys. Lett. 1997, 70: 2335–2337.
Shao LX, Chang Q, Hwang HL: Zinc sulfide thin films deposited by RF reactive sputtering for photovoltaic applications. Appl. Surf. Sci. 2003, 212: 305–310.
Habib Ullah M, Il K, Ha CS: pH selective synthesis of ZnS nanocrystals and their growth and photoluminescence. Mater. Lett. 2007, 61: 4267–4271.
Chestnoy N, Hull R, Brus LE: Higher excited electronic states in clusters of ZnSe, CdSe, and ZnS: spin‒orbit, vibronic, and relaxation phenomena. J. Chem. Phys. 1996, 85: 2237–2242.
Lee SM, Hwang CS: Synthesis of a white-light-emitting ZnSe:Mn nanocrystal via thermal decomposition reaction of organometallic precursors. Bull Korean Chem Soc 2013, 34: 321–324.
Brus LE: Electron–electron and electron‒hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80: 4403.
Wang Y, Herron N: Nanometer-sized semiconductor clusters: materials synthesis, quantum size effects, and photo physical properties. J. Phys. Chem. 1987, 91: 257.
Awschalom DD, Kikkawa JM: Electron spin and optical coherence in semiconductors. Phys. Today 1999, 52: 33.
Wang X, Ding Y, Summers CJ, Wang ZL: Large-scale synthesis of six-nanometer-wide ZnO nanobelts. J. Phys. Chem. B 2004, 108: 8773–8777.
Narayanaswamy A, Xu HF, Pradhan N, Kim M, Peng X: Synthesis of urea capped ZnS nanoparticles. J. Am. Chem. Soc. 2006, 128: 10310.
Khosravi AA, Kundu M, Jatwa L, Deshpande SK, Bhagwat UA, Sastry M, Kulkarni SK: Green luminescence from copper doped zinc sulphide quantum particles. Appl. Phys. Lett. 1995, 67: 2702–2704.
Li Y, Meng GW, Zhang LD, Phillipp F: Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl. Phys. Lett. 2000, 76: 2011–2013.
Zhang YS, Wang LS, Liu XH, Yan YJ, Chen CQ, Zhu J: Effect of surface stress on the stiffness of micro/nanocantilevers: nanowire elastic modulus measured by nano-scale tensile and vibrational techniques. J. Phys. Chem. B 2005, 109: 13091.
Ibanga EJ, Le Luyer C, Mugnier J: Zinc oxide waveguide produced by thermal oxidation of chemical bath deposited zinc sulphide thin films. Matter Chem Phys 2003, 80: 490.
Archana J, Navaneethan M, Ponnusamy S, Hayakawa Y, Muthamizhchelvan C: Optical, structural and surface morphological studies of bean-like triethylamine capped zinc selenide nanostructures. Mat Lett 2009, 63: 1931.
Kumar A, Biebuyck HA, Whitesides GM: Patterning self-assembled monolayers: applications in materials science. Langmuir 1994, 10: 1498.
Vossmeyer T, Katsikas L, Giersig M, Popovic IG, Diesner K, Chemseddine A, Eychmuller A, Weller H: CdS nano clusters: synthesis, characterization, size dependent oscillator strength, temperature shift of the excitonic transition energy, and reversible absorbance shift. J. Phys. Chem. 1994, 98: 7665.
Murray CB, Norris DB, Bawendi MG: Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115: 8706.
Patterson A: The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56: 978–982. http://link.aps.org/doi/10.1103/PhysRev.56.978 10.1103/PhysRev.56.978
Sharma R, Chandra BP, Bisen DP: Optical properties of ZnS:Mn nanoparticles prepared by chemical routs. Chalcgenide Letters 2009, 6: 339–342.
Bawendi MG, Steigerwald ML, Brus LE: The quantum mechanics of larger semiconductor cluster. Rev Phys Chem 1990, 41: 477–496.
Landolt-Bornstein: Numerical data and functional relationships in science and technology. Berlin: Springer Verlag; 1987:168.
Hartley PA, Parfitt GD, Pollack LB: The role of the van der Waals force in the agglomeration of powders containing submicron particles. Power Tech. 1985, 42: 35–46.
Rema Devi BS, Raveendran R, Vaidyan AV: Synthesis and characterization of Mn2+-doped ZnS nanoparticles. Pramana-J. Phys. 2007, 68: 679–687.
Rahdar A: Effect of 2-mercaptoethanol as capping agent on ZnS nanoparticles: structural and optical characterization. J. Nano. Chem. 2013, 3: 1–5.
Wang Y, Herron N: Quantum size effects on the exciton energy of CdS clusters. Physical Review B 1990, 42: 7253–7255.
Acknowledgments
The author would like to thank Dr. M. Ali-Ahmad, Mrs. M. Asudeh, Mr. H. Rahdar, and Mrs. Heidari Mokarrar for their support and assistance with this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declare that he has no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rahdar, A. Effect of mercaptoethanol and Na2S dropwise addition rate on zinc sulfide semiconductor nanocrystals: synthesis and characterization. J Nanostruct Chem 3, 61 (2013). https://doi.org/10.1186/2193-8865-3-61
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-61