Abstract
Background
Alcadeinα (Alcα) is a neuronal membrane protein that colocalizes with the Alzheimer's amyloid-β precursor protein (APP). Successive cleavage of APP by β- and γ-secretases generates the aggregatable amyloid-β peptide (Aβ), while cleavage of APP or Alcα by α- and γ-secretases generates non-aggregatable p3 or p3-Alcα peptides. Aβ and p3-Alcα can be recovered from human cerebrospinal fluid (CSF). We have previously reported alternative processing of APP and Alcα in the CSF of some patients with sporadic mild cognitive impairment (MCI) and AD (SAD).
Results
Using the sandwich enzyme-linked immunosorbent assay (ELISA) system that detects total p3-Alcα, we determined levels of total p3-Alcα in CSF from subjects in one of four diagnostic categories (elderly controls, MCI, SAD, or other neurological disease) derived from three independent cohorts. Levels of Aβ40 correlated with levels of total p3-Alcα in all cohorts.
Conclusions
We confirm that Aβ40 is the most abundant Aβ species, and we propose a model in which CSF p3-Alcα can serve as a either (1) a nonaggregatable surrogate marker for γ-secretase activity; (2) as a marker for clearance of transmembrane domain peptides derived from integral protein catabolism; or (3) both. We propose the specification of an MCI/SAD endophenotype characterized by co-elevation of levels of both CSF p3-Alcα and Aβ40, and we propose that subjects in this category might be especially responsive to therapeutics aimed at modulation of γ-secretase function and/or transmembrane domain peptide clearance. These peptides may also be used to monitor the efficacy of therapeutics that target these steps in Aβ metabolis
Similar content being viewed by others
Background
Alcadeins (Alcs) represent a family of neuronal type I membrane proteins (designated as Alcα, Alcβ, and Alcγ) that are encoded by independent genes [1]. In neurons, Alc forms a tripartite complex with Alzheimer's amyloid β-protein precursor (APP) via the crosslinking action of the neural adaptor protein X11-like (X11L) [2, 3]. In the absence of X11L, both the free Alc proteins and the free APP are subjected to coordinated proteolytic cleavage through similar mechanisms: APP and Alc are both cleaved by the identical α-secretase at the juxtamembrane region. This cleavage of Alc causes release of N-terminal soluble Alc ectodomain (sAlc) and leaves behind a C-terminal cell-membrane-associated AlcCTF. APP can undergo either an identical α-secretase cleavage (thereby generating a cell-associated APPCTFα) or instead (and unlike Alc) APP can undergo β-secretase cleavage leading to generation of sAPPβ and a cell-associated APPCTFβ [4]. All three CTFs (APPCTFα, APPCTFβ, AlcCTF) are subjected to regulated intramembranous cleavage by the γ-secretase complex, in which presenilin 1 or 2 (PS1, PS2) functions as the catalytic subunit [2]. Mutations in PS1 and PS2 are known to cause early onset familial Alzheimer's disease (FAD). The γ-secretase reaction involving APPCTFα generates the p3 fragment, while the reaction involving APPCTFβ generates the amyloid-β peptide (Aβ) [4]. Cleavage of AlcCTF by γ-secretase liberates a small peptide named p3-Alc (a named selected to be symmetrical with the name of the APP p3 peptide). The p3-Alc peptide is detectable in CSF, while the APP p3 peptide is very labile and difficult to detect in CSF [2, 5].
Most patients with FAD carry one of over 200 pathogenic mutations identified in the coding sequence of PS1 or PS2. These mutations alter intramembranous cleavage of APP so as to increase production of Aβ42, the most aggregation-prone, oligomerogenic, and fibrillogenic species of Aβ [6–8]. Other patients with FAD may carry pathogenic mutations in the coding sequence of the APP gene, all of which promote the accumulation of Aβ [4]. Furthermore, Down syndrome (DS) patients carry three copies of chromosome 21 which includes the APP gene locus, and therefore, DS patients have a “genetic overdose” of APP, leading them to develop AD by middle age [9]. Therefore, alterations in the generation of Aβ, in both quality and quantity, are considered to be causes of AD pathogenesis in genetic forms of the disease.
In the more common sporadic forms of AD (SAD), the molecular pathogenesis remains unknown. Aβ42 levels are reduced in the CSF of SAD patients [10–12], but the use of CSF Aβ42 as an in vivo marker for APP metabolism is complicated by its deposition in brain and cerebral vasculature as the disease progresses. Recent evidence suggests that a disturbance in an apolipoprotein E (APOE)-isoform-dependent step in Aβ clearance plays a role in the pathogenesis of SAD [13], although these data could not exclude the possibility that Aβ oligomerization or fibrillization (and not a defect in some clearance pathway alone) may also play a role, since apoE also plays a role in Aβ aggregation (Caesar et al., unpublished observations).
We recently reported that the CSF of subjects with sporadic MCI and early AD showed a relative overrepresentation of a minor p3-Alcα species, p3-Alcα38, raising the possibility that γ-secretase dysfunction can exist even in the absence of an FAD-linked genetic mutation [14]. The previous study [14] was performed using immunoprecipitation-mass spectrometry which, as performed, is considered to be a semi-quantitative method. Therefore, we have begun moving toward the development of sensitive ELISAs that will permit convenient, reliable, and sensitive quantitation of total p3-Alcα and selected minor species (with p3-Alcα38 being the top priority in that respect). Here we report the application of a recently developed ELISA system (antibody and assay development described elsewhere) [15] that can quantify total p3-Alcα in the range of 40 to 600 pg/mL. Using this system, we have quantified total p3-Alcα levels in the CSF of three independent cohorts that consist of subjects with MCI/CDR 0.5 or AD (CDR 1–3), as well as subjects that are either cognitively intact, age-matched controls or suffer from frontotemporal lobar degeneration (FTLD). This latter population served as other neurological disease (OND) controls.
Results
CSF p3-Alcα levels in elderly nondemented subjects and in subjects with MCI, or mild or moderate SAD in Cohort 1 (Japan) and Cohort 2 (US).
We first examined p3-Alcα levels in the CSF of subjects with MCI (CDR 0.5), mild or moderate AD (CDR 1 and CDR 2) and age-matched elderly nondemented controls (CDR 0) in Cohort 1 (Japan) (Table 1). The p3-Alcα levels in subjects were compared according to CDR (Figure 1A, left panel). Subjects with MCI (CDR 0.5, n = 20) showed a trend toward higher levels than controls (CDR 0, n = 18), but the trend did not reach statistical significance. p3-Alcα levels in subjects with mild AD (CDR 1, n = 13) were significantly higher than those in controls (p < 0.05; Tukey-Kramer’s multiple comparison test). Interestingly, p3-Alcα levels in AD subjects with moderate dementia (CDR 2, n = 13) were indistinguishable from those observed in non-demented controls. When the p3-Alcα levels of the CDR 1 and CDR 2 subjects were compared, these groups were also significantly different (p < 0.05).
Levels of p3-Alc α , Aβ40, and Aβ42, and Aβ42/40 ratios in CSF of three cohorts. (A) Cohort 1 (Japan). Non-demented healthy controls (CDR 0, n = 18), very mild AD subjects (CDR 0.5, n = 20), AD subjects with mild dementia (CDR 1, n = 13) and AD subjects with moderate dementia (CDR 2, n = 13) were analyzed for levels of p3-Alcα (left) and Aβ40 (middle left), Aβ42 (middle right) and Aβ42/40 ratio (right). (B) Cohort 2 (USA). Non-demented healthy controls (CDR 0, n = 20), MCI subjects (CDR 0.5, n = 20) and AD subjects with mild dementia (CDR 1, n = 13) were analyzed for levels of p3-Alcα (left) and Aβ40 (middle left), Aβ42 (middle right) and Aβ42/40 ratio (right). Dashed line on the middle right panel indicates cut-off value of Aβ42 (500 pg/mL). (C) Cohort 3 (Japan). Non-demented healthy controls (CDR 0, n = 23), MCI subjects (CDR 0.5, n = 9), AD subjects with mild dementia (CDR 1, n = 13) and AD subjects with moderate and severe dementia (CDR 2–3, n = 12), and FTLD subjects (n = 37) of Japanese cohort (Cohort 3) were analyzed for levels of p3-Alcα (left) and Aβ40 (middle left), Aβ42 (middle right) and Aβ42/40 ratio (right). Statistical analysis was performed using the Dunn's multiple comparisons test following the Kruskal-Wallis test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
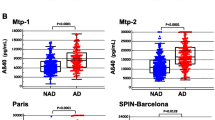
In Cohort 1 (Japan), we observed a significant increase in Aβ40 levels in subjects with MCI/CDR 0.5 (p < 0.05) and mild AD (CDR 1, p < 0.01), while Aβ42 levels did not significantly change as a function of disease progression (Figure 1A, middle panels). Furthermore, the total p3-Alcα levels significantly correlated with the levels of Aβ40 (R2 = 0.536, p < 0.0001) in subjects with CDR 0.5, 1 and 2 (Figure 2A). The Aβ42/40 ratio was reduced in MCI and AD subjects as compared to the Aβ42/40 of non-demented controls (CDR 0) (Figure 1A, right panel). This is a standard effect and is believed to be a consequence of reduced Aβ42 levels due to its deposition in cerebral vessels and parenchyma [16, 17]. No differences between male and female subjects were detected for p3-Alcα and Aβ40 levels (Additional file 1, Figure S1).(Additional file 2, Figure S2).
Correlation of p3-Alc α levels with Aβ40 level in subjects with CDR 0.5, 1 and 2–3. Correlation of CSF p3-Alcα and Aβ40 levels was explored in subjects with CDR 0.5, 1 and 2 in cohort 1 (A, n = 46), in subjects with CDR 0.5 and 1 in cohort 2 (B, n = 33), and CDR 0.5, 1 and 2–3 in cohort 3 (C, n = 34). The relation between p3-Alcα and levels was investigated by Pearson's correlation coefficient test (GraphPad Prism 5). Statistical significance is indicated in figure with asterisks.
We next analyzed p3-Alcα levels in the CSF of subjects in Cohort 2 (USA) (Figure 1B), in subjects with very mild dementia (CDR 0.5) (N.B., in this cohort, the term MCI is not utilized) and mild dementia due to AD (CDR 1) (Table 1) [18]. CSF from Cohort 2 (USA) showed the expected relatively stable levels of Aβ40 in very mild dementia (CDR 0.5) and mild AD (CDR 1) subjects when compared to those of non-demented controls. In this cohort, both p3-Alcα levels and Aβ40 levels remained relatively stable in the subjects with CDR 0.5 and 1, and the levels of the two peptides did not show the direct correlation observed in Cohort 1 (R2 = 0.07922, p = 0.1126) (Figure 2B). It is worth noting that, in this cohort, semiquantitative data on amyloid deposition were available on all subjects, based on 11 C] Pittsburgh compound B (11 C]PiB) amyloid imaging. No differences between male and female subjects were detected for p3-Alcα and Aβ40 levels (Additional file 1, Figure S1).
In Cohort 1 (Japan), we noticed that the Aβ42 levels in MCI and AD subjects appeared to be dimorphic, and we suspect that this may reflect differential levels of Aβ42 deposition (i.e., subjects with low Aβ42 may have more amyloid deposition than those with normal levels of Aβ42), but no 11 C]PiB data were available to enable us to assess this possibility. Using an Aβ42 cut-off value [19] of 500 pg/ml, we divided the subjects in Cohort 1 (Japan) into two populations and analyzed the level of p3-Alcα and Aβ in the “low Aβ42” (Figure 3A-D) and “high Aβ42” (Figure 3E-H) subpopulations. In the subpopulation of subjects with low CSF Aβ42 levels (<500 pg/mL) in MCI/CDR 0.5 and AD (CDR 1 and CDR 2), neither CSF Aβ40 nor CSF p3-Alcα levels were increased in MCI and AD subjects when compared to the corresponding levels in the CSF of non-demented controls (CDR 0) (Figure 3A-C). Therefore, the overall profiles of Aβ40 and Aβ42 levels in this low Aβ42 subpopulation of Cohort 1 (Japan) closely resembled those of Cohort 2 (USA) (compare Figure 3A-D with Figure 1B). In this low Aβ42 subpopulation of Cohort 1 (Japan), the correlation between CSF Aβ40 levels and CSF p3-Alcα levels observed for the entire cohort was no longer evident.
Levels of p3-Alc α , Aβ40, and Aβ42, and Aβ42/40 ratios in CSF of Cohort 1 (Japan) following subgrouping into “low Aβ42” and “high” Aβ42” subpopulations. Subjects of Japanese cohort (Cohort 1) were divided into two subpopulations with a cut-off value of Aβ42 (500 pg/mL). (A-D, low Aβ42 subgroup). Control (Aβ42 >500 pg/mL) and MCI and AD subjects (Aβ42 < 500 pg/mL) were analyzed for p3-Alcα (A), Aβ40 (B), Aβ42 (C) and Aβ42/40 ratio (D). (E-H, high Aβ42 subgroup). Control (Aβ42 <500 pg/mL) and MCI and AD subjects (Aβ42 > 500 pg/mL) were analyzed for p3-Alcα (E), Aβ40 (F), Aβ42 (G) and Aβ42/40 ratio (H). Dashed line on panels C and G indicates the cut-off value of Aβ42 (500 pg/mL). Statistical analysis was performed using the Dunn's multiple comparisons test following the Kruskal-Wallis test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The second subpopulation of Cohort 1 was composed of MCI and AD subjects who showed relatively higher Aβ42 levels (>500 pg/mL). In this high Aβ42 subpopulation, we observed an increase in levels of both Aβ40 and p3-Alcα in MCI and AD subjects (Figure 3E-H). These data suggest that there might be subpopulations of MCI and SAD subjects that exhibit relatively higher CSF levels of both Aβ and p3-Alcα. These subpopulations might represent differential depletion of CSF Aβ42 by progressive cerebral and cerebrovascular amyloid deposition. We would tentatively propose that the subjects distinguished by their high (vs low) Aβ42 levels might define separate endophenotypes that might be useful for understanding the heterogeneity in causes and/or progression of SAD. For example, the low Aβ42 subpopulation might have a relatively greater proportion of their Aβ42 in fibrillar form, while those normal or high Aβ42 might have a relatively greater proportion of their Aβ42 in nonfibrillar, soluble oligomer form.
CSF p3-Alcα levels in elderly non-demented subjects and in subjects with MCI, AD and FTLD in cohort 3 (Japan)
In an effort to confirm the observations described above, we next analyzed another cohort (Cohort 3; Japan), which is independent of Cohort 1 (Japan). This Japanese cohort (Cohort 3) includes non-demented controls (CDR 0; n = 23), MCI/CDR 0.5 (n = 9), mild AD (CDR 1; n = 13), moderate and severe AD (CDR 2 + CDR 3; n = 12), and FTLD (n = 37) subjects (Table 1). There were no significant differences across the various subject groups with respect to average ages or age range (Table 1). Cohort 3 (Japan) showed typical profiles for CSF Aβ levels (Figure 1C); CSF Aβ42 levels were decreased and CSF Aβ40 levels did not change in MCI/CDR 0.5 and AD subjects as compared with the corresponding values from non-demented controls (Figure 1C, middle panels). Aβ42/40 ratios were decreased in MCI and AD, consistent with the typical pattern for those diagnostic groups (Figure 1C, right panel). FTLD subjects (OND controls) also showed the typical trends in Aβ levels. In Cohort 3 (Japan), no significant increase of CSF p3-Alcα levels in AD was observed as compared to non-demented controls, although the CSF of FTLD subjects showed a significant reduction in the p3-Alcα levels as compared to non-demented controls and MCI/AD subjects (Figure 1C, left panels). In this cohort, a strong correlation of p3-Alcα with Aβ40 (R2 = 0.8483, p < 0.0001) was observed in subjects with CDR 0.5, 1 and 2–3 as was observed in Cohort 1 (Figure 2C). No differences between male and female subjects were observed for the p3-Alcα levels or for the Aβ40 levels (Additional file 1, Figure S1).
CSF samples in Cohort 3 (Japan) (like Cohort 1) appeared to be dimorphic with regard to levels of CSF Aβ42. Therefore, we again divided subjects into two populations with a cut-off value of CSF Aβ42 (500 pg/mL), and we again analyzed CSF p3-Alcα levels and Aβ40 levels as shown in Figure 3 (compare Figure 3 vs Figure 4). In samples from the low Aβ42 subpopulation of MCI/CDR 0.5 and AD (CDR >1) subjects, CSF levels of Aβ40 and p3-Alcα were indistinguishable from the corresponding values from non-demented controls and were not correlated (Figure 4A-D). On the other hand, CSF from a subpopulation of MCI (CDR 0.5) and AD (CDR >1) subjects who showed high Aβ42 levels (>500 pg/mL) showed high p3-Alcα and high Aβ40 levels when compared with non-demented controls and FTLD subjects (Figure 4E-H).
Levels of p3-Alc α , and Aβ40, Aβ42 and Aβ42/40 ratios in CSF of Cohort 3 (Japan) subpopulations. Subjects of cohort 3 were divided into two subpopulations with cut-off value of Aβ42 (500 pg/mL). (A-D, low Aβ42 subgroup). Control and FTLD subjects (Aβ42 >500 pg/mL) and MCI and AD subjects (Aβ42 < 500 pg/mL) were analyzed for p3-Alcα (A), Aβ40 (B), Aβ42 (C) and Aβ42/40 ratio (D). (E-H, high Aβ42 subgroup). Control and FTLD subjects (Aβ42 <500 pg/mL) and MCI and AD subjects (Aβ42 > 500 pg/mL) were analyzed for p3-Alcα (E), Aβ40 (F), Aβ42 (G) and Aβ42/40 ratio (H). Dashed line on panels C and G indicates cut-off value of Aβ42 (500 pg/mL). Statistical analysis was performed using the Dunn's multiple comparisons test following the Kruskal-Wallis test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Correlation of p3-Alcα levels between CSF and plasma in same individuals
We previously reported that plasma p3-Alcα levels are increased in AD patients [15]. Therefore, we wanted to study the relationship between CSF and plasma in p3-Alcα levels in the same individuals. In the cohorts we have described here so far, we do not have access to matched CSF and plasma samples from same subjects and drawn at same time. Thus, we used samples from a fourth cohort which includes pre-clinical stage subjects without significant impairment for memory and recognition (MMSE average score 28.5, Table 1), but who show a slight lower score of California Verbal Learning Test. Such subjects are currently the targets for various early intervention trials [20]. For this population, we had access to plasma and CSF from the same individuals taken at the same time. We examined p3-Alcα levels in plasma and CSF of aged population (n = 57), and sought potentially relevant correlations. We identified a significant positive correlation between plasma p3-Alcα levels and CSF levels (p = 0.032 and R2 = 0.0809 by Pearson's correlation coefficient test). The results suggest that CSF p3-Alcα levels can correlate with plasma levels (Figure 5).
Positive correlation of p3-Alc α levels in CSF with those in plasma of same subjects. Correlation of CSF p3-Alcα levels with plasma p3-Alcα levels were examined in same subjects of pre-clinical stages for dementia, whose MMSE score did not decrease remarkably (n = 57, Table 1). The relation between CSF p3-Alcα levels and plasma levels was investigated by Pearson's correlation coefficient test (Graph Pad Prism 5) (R2 = 0.0809; *p = 0.032).
Discussion
In our previous studies, we demonstrated that the products of alternative cleavage of non-APP substrates (known as Alcs) by γ-secretase gave rise to a modified p3-Alcα peptide profile in media conditioned by transfected cells expressing an FAD-linked mutant PS1 and a similar modified profile was also identified in the CSF of subjects with sporadic MCI (known as CDR 0.5 in Cohort 2 and recently renamed “prodromal AD” in the revised lexicon for dementia syndromes [21]), and mild AD [5, 14]. In order to quantify these peptides reliably and conveniently, we have recently developed an ELISA for p3-Alcα[15]. This ELISA system quantifies total p3-Alcα levels, but does not specifically measure the individual species of p3-Alcα. In the current paper, we have employed this ELISA to quantify total p3-Alcα levels in the CSF of three independent cohorts of subjects who were categorized as either nondemented controls, sporadic MCI, sporadic AD, or FTLD.
Interestingly, applying our new, quantitative p3-Alc ELISA to CSF for the first time, we were surprised to observe in two cohorts of Japanese subjects the apparent existence of subpopulations of sporadic MCI and AD subjects in whose CSF there was differential elevation of the levels of the reaction products generated by γ-secretase cleavage of multiple substrates; i.e., APP and Alcadein. Since Aβ40 and total p3-Alcα were highly correlated in these cohorts, the current data support the use of p3-Alcα as a surrogate for total APP-derived γ-cleaved products. Elevated levels of p3-Alcα and Aβ were also observed in plasma samples of some female AD patients [15]. However, in CSF, we did not detect any differences in levels between male and female subjects. In another independent cohort study with plasma samples, we confirmed the significant increase of p3-Alcα levels in MCI and AD patients, but we observed no systematic differences between male and female subjects [22]. Therefore, it is worth noting that the observation of a sex specific increase in p3-Alcα levels in plasma of female AD patients has not been consistently observed in all cohorts studied.
The increase in p3-Alcα level could arguably be caused by (1) increased primary α-cleavage by α-secretase; (2) increased intramembranous γ-cleavage by γ-secretase and/or (3) diminished clearance of transmembrane-derived fragments such as p3-Alcα. Because Aβ, a product of primary β-cleavage of APP by β-secretase, is also increased in this subpopulation, and because we have previously linked PS1 mutations to variant p3-Alc speciation [5], we have argued on the basis of parsimony, that the molecular pathology was more likely attributable to dysfunction of γ-secretase. However, in light of the new data herein, it is possible that both p3-Alcα speciation and also p3-Alcα levels may be affected. When these observations are taken together with the model of altered CSF peptide clearance [23], and the evidence that clearance of Aβ from CSF is modulated in an APOE-isoform-specific manner [13], we now must consider it equally likely that altered p3-Alcα levels and speciation could be attributable to a defect in clearance from CSF of transmembrane domain metabolite peptides.
A stratification of the current (this paper) and prior data [14] according to APOE genotype, followed by re-analysis, is underway. We have attempted a preliminary APOE genotype-dependent analysis using cohort 3 samples. ApoE4 carriers tended to show higher values of both p3-Alcα and Aβ40 in MCI (CDR 0.5) and AD (CDR 1) patients but not in more advance AD (CDR 2–3) or in FTLD patients (Additional file 3, Figure S2). However, the increase of p3-Alcα and Aβ40 in APOE4 carriers did not reach statistical significance when compared to the corresponding levels in non-APOE 4 carriers. Because this was a small scale pilot analysis, we consider unresolved the issue of whether APOE4 genotype influences the level of p3-Alcα in AD. In order to address this issue directly, analysis of the p3-Alcα levels in the identical samples studied by Castellano et al.[13] is under consideration.
It is interesting to note that both the quality and quantity of p3-Alcα accumulation in CSF may be transient, occurring in MCI and mild AD but not evident in later stages (see ref 14 and this paper). Serial examinations of CSF from the same subjects at different stages of AD will be required in order to establish whether or not such a phenomenon truly exists within the same individual. The Biomarker Core of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [24] should be a useful resource in pursuing this hypothesis.
Since p3-Alcα is not incorporated into cerebral or cerebrovascular amyloid, the decrease in p3-Alcα levels in later stage AD subjects (CDR 2 or more) may be due to progressive neuronal degeneration, thereby eliminating the main cellular source of p3-Alc peptides. This is also consistent with other data suggesting that AD may be divisible into an early Aβ-driven phase (beginning presymptomatically and extending into mild stages of dementia) and a later phase that may be driven by inflammation and/or tauopathy [25]. Consistent with this formulation are the recent reports that fibrillar amyloid burden, as indicated by 11 C]PiB signal, begins accumulating perhaps 10–15 years before symptoms are evident [26] and then plateaus [27]. This reformulation of AD pathogenesis also fits with recent data from Rinne and colleagues, showing that a reduction in the fibrillar amyloid burden caused by ~1.5 yrs of bapineuzumab infusion had no obvious impact on cognition [28].
If the apparent transient elevation of levels of p3-Alcα and/or Aβ is due, at least in part, to transient γ-secretase dysfunction, the identification of this “spike” of dysfunction could be important for the timing and nature of interventions aimed at this enzyme. For example, elevated CSF p3-Alcα levels (or the coordinate elevation of CSF Aβ40 and p3-Alcα levels) could be used as an endophenotype that marks a subpopulation of sporadic MCI/ CDR 0.5/prodomal AD and mild AD subjects that might be especially amenable to γ-secretase modulators [29]. Again, serial CSF examinations of normal elderly and presymptomatic and prodromal AD (such as those performed by the ADNI [24]) will be required in order to determine precisely if and when any CSF p3-Alcα spike exists and whether the beginning of the p3-Alcα spike heralds the onset of the Aβ accumulation phase. If so, then periodic determination of a panel of CSF biomarkers (including Aβ42, Aβ40, and p3-Alcα) in populations at risk might be useful in determining when to initiate clinical trials of Aβ−lowering agents [25]. This concept dovetails well with recent evidence showing that dramatic changes in CSF Aβ42/Aβ40 are observed in some subjects, and these dramatic outlier values can be used to reveal subjects with spontaneous PS1 mutations [30]. Plasma levels of p3-Alcα were parallel to CSF levels in preclinical stages of disease of subjects (Figure 5). Therefore peripheral sampling may be informative, thereby avoiding the inconvenience of serial CSF sampling, although we have not examined the correlation in MCI/CDR 0.5/prodromal AD and AD subjects. Finally, if the addition of CSF p3-Alcα determination turns out to contribute useful information about clinical state or pathogenesis, one might consider adding additional γ-secretase reaction products to the panel (e.g., ephrin B [31], ephrin B receptor [32]) in order to establish whether many or all γ-secretase substrates are implicated in this putative stage in the molecular pathogenesis of AD that is characterized by γ-secretase dysfunction, impaired transmembrane domain peptide clearance, or both.
Conclusions
The causes of sporadic AD may be various, and some clinical populations with sporadic mild cognitive impairment (also known as CDR0.5 and prodromal AD) and mild AD showed an increase in the CSF levels of transmembrane domain peptides derived from integral membrane proteins such as Alc and APP. The CSF p3-Alcα levels paralleled the plasma levels, indicating that peripheral information might reflect the pathological state in brain, at least where γ-secretase malfunction is concerned. This endophenotype may caused by (1) disturbed processing of APP and Alc by γ-secretase; (2) a reduction in clearance mechanism of these peptides; or (3) both. Patients in this category might be especially responsive to drug therapeutics aimed at modulation of γ-secretase function and/or transmembrane domain peptide clearance.
Methods
CSF collection
CSF collection was approved by the ethical board at each institution, and each subject underwent a standard lumbar puncture (LP) while in the lateral decubitus position. The subjects at Washington University in St Louis underwent lumbar puncture at a specific time of day (8 AM) and after an overnight fast. After the disposal of the first 1 mL of CSF, the remaining fluid was collected in polypropylene tubes. Tubes were subjected to centrifugation (1,000 x g for 10 min at 4°C) to remove any debris and then stored in small aliquots at −80°C. Alzheimer's disease was clinically diagnosed based on two major criteria: Diagnostic and Statistical Manual Disorders; 4th Edition (DSM-IV) and the National Institute of Neurological and Communicational Disorders and Stroke - Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria. In one US cohort (Table 1; Cohort 2), complete details of collection protocols were provided in a previous report [14]. CDR 0 subjects in this US cohort were verified as controls with CSF Aβ42 >500 pg/mL, which suggests absence of amyloid plaques [19]. In cohort 3 (Table 3), the clinical diagnoses of patients with FTLD were made on the basis of established clinical criteria [7].
Subject characteristics and data are summarized in Table 1. A detailed description of all subjects (including their clinical descriptions and raw values for p3-Alcα, Aβ42 and Aβ40) are provided in the (Additional file 3, Tables S1, Additional file 3, Tables S2, Additional file 3, Tables S3, Additional file 3, Tables S4).
Quantification of p3-Alcα and Aβ in CSF with ELISA
We used a quantitative ELISA system for total p3-Alcα as described [15]. In brief, a 25 μL aliquot of CSF was diluted 10-fold for the measurement of p3-Alcα and Aβ42, and 10 μL of CSF was diluted 25-fold for measurement of Aβ40. Diluent was PBS containing 1% (w/v) BSA and 0.05% (v/v) Tween-20. To remove debris, the samples (250 μL) were centrifuged at 15,000 x g for 10 min. The supernatant (100 μL) was assayed in duplicate using synthetic p3-Alcα35 peptide as a standard. Aβ40 and Aβ42 levels were measured according the instructions of the respective manufacturers (IBL, Fujioka Japan for cohort 1; INNOTEST, Innogenetics, Ghent Belgium for cohort 2; Wako Pure Chemical Industries, Osaka Japan for cohort 3). All analyses were performed with operators blinded to diagnosis until data tables were generated. Diagnoses and data tables were exchanged among the authors at the time of unblinding.
Authors' information
Sam Gandy, Katsuya Urakami, Toshiharu Suzuki are co-senior authors for this study.
Abbreviations
- AD:
-
Alzheimer's disease
- Aβ:
-
Amyloid β-protein
- Alc:
-
Alcadein
- APP:
-
Amyloid β-protein precursor
- p3-Alc:
-
APP p3-like peptide derived from Alc
- CDR:
-
Clinical dementia rating
- CSF:
-
Cerebrospinal fluid
- FAD:
-
Familial Alzheimer's disease
- HDS-R:
-
Revised Hasegawa Dementia Scale
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental Status Examination
- SAD:
-
Sporadic Alzheimer's disease
- PS:
-
Presenilin.
References
Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T: Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein mediated stabilization of amyloid β-protein precursor metabolism. J Biol Chem. 2003, 278: 49448-49458. 10.1074/jbc.M306024200.
Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, Yamamoto T, De Strooper B, Yamamoto K, Suzuki T: Coordinated metabolism of Alcadein and amyloid β-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem. 2004, 279: 24343-24354. 10.1074/jbc.M401925200.
Suzuki T, Nakaya T: Regulation of amyloid β-protein precursor by phosphorylation and protein interactions. J Biol Chem. 2008, 283: 29633-29637. 10.1074/jbc.R800003200.
St George-Hyslop PH: Molecular genetics of Alzheimer's disease. Biol Psychiatry. 2000, 47: 183-199. 10.1016/S0006-3223(99)00301-7.
Hata S, Fujishige S, Araki Y, Kato N, Araseki M, Nishimura M, Hartmann D, Saftig P, Fahrenholz F, Taniguchi M, Urakami K, Akatsu H, Martins RN, Yamamoto K, Maeda M, Yamamoto T, Nakaya T, Gandy S, Suzuki T: Alcadein cleavages by APP α- and γ-secretases generate small peptides p3-Alcs indicating Alzheimer disease-related γ-secretase dysfunction. J Biol Chem. 2009, 284: 36024-36033. 10.1074/jbc.M109.057497.
Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM: St George-Hyslop PH: Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995, 37: 754-760.
Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS: Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vitro. Neuron. 1996, 17: 1005-1013. 10.1016/S0896-6273(00)80230-5.
De Strooper B: Loss-of-function presenilin mutations in Alzheimer disease. Taking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007, 8: 141-146.
Schupf N: Genetic and host factors for dementia in Down's syndrome. Br J Psychiatry. 2002, 180: 405-410. 10.1192/bjp.180.5.405.
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM: Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006, 59: 512-519. 10.1002/ana.20730.
Grimmer T, Riemenschneider M, Förstl H, Henriksen G, Klunk WE, Mathis CA, Shiga T, Wester HJ, Kurz A, Drzezga A: Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009, 65: 927-934. 10.1016/j.biopsych.2009.01.027.
Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BN: Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009, 50: 1464-1470. 10.2967/jnumed.109.064360.
Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, Holtzman DM: Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011, 3: 89ra57-10.1126/scitranslmed.3002156.
Hata S, Fujishige S, Araki Y, Taniguchi M, Urakami K, Peskind E, Akatsu H, Araseki M, Yamamoto K, Martins RN, Maeda M, Nishimura M, Levey A, Chung KA, Montine T, Leverenz J, Fagan A, Goate A, Bateman R, Holtzman DM, Yamamoto T, Nakaya T, Gandy S, Suzuki T: Alternative processing of γ-secretase substrates in common forms of mild cognitive impairment and Alzheimer's disease: Evidence for γ-secretase dysfunction. Ann Neurol. 2011, 69: 1026-1031. 10.1002/ana.22343.
Konno T, Hata S, Hamada Y, Horikoshi-Sakuraba Y, Nakaya T, Saito Y, Yamamoto T, Yamamoto T, Maeda M, Ikeuchi T, Gandy S, Akatsu H, Suzuki T: Neuroimaging Initiative TJ: Coordinated increase of γ-secretase products in the plasma of some female Japanese sporadic Alzheimer's disease patients: Quantitative analysis of p3-Alcαwith a new ELISA system. Mol Neurodegener. 2011, 6: 76-10.1186/1750-1326-6-76.
Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM: Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000, 57: 100-105. 10.1001/archneur.57.1.100.
Buerger K, Frisoni G, Uspenskaya O, Ewers M, Zetterberg H, Geroldi C, Binetti G, Johannsen P, Rossini PM, Wahlund LO, Vellas B, Blennow K, Hampel H: Validation of Alzheimer's disease CSF and plasma biological markers: The multicentre reliability study of the pilot Europian Alzheimer's disease neuroimaging initiative (E-ADNI). Exp. Geron. 2009, 44: 579-585. 10.1016/j.exger.2009.06.003.
Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM: Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009, 65: 176-183. 10.1002/ana.21559.
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM: Inverse relation between in vivo amtloid imaging load and cerebrosoinal fluid Abeta42 in humans. Ann Neurol. 2006, 59: 512-519. 10.1002/ana.20730.
Sperling RA, Jack CR Jr, Aisen PS: Testing the right target and right drug at the right stage. Sci Transl Med. 2011, 3: 111cm33-10.1126/scitranslmed.3002609.
Dubois B, Feldman HH, Jacova C, DeKosky ST, Delacourte A, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P: Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010, 9: 1118-10.1016/S1474-4422(10)70223-4.
Kamogawa K, Kohara K, Tabara Y Y, Takita R, Miki T, Konno T, Hata S, Suzuki T: Utility of plasma levels of soluble p3-Alcadein () as a biomarker for sporadic Alzheimer's disease. J Alzheimers Dis. 2011
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ: Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010, 330: 1774-10.1126/science.1197623.
Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde AL, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H, Woodcock J, Blennow K: Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010, 9: 560-574. 10.1038/nrd3115.
Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, Feldman HH, Petersen RC, Siemers E, Doody RS, Hendrix SB, Grundman M, Schneider LS, Schindler RJ, Salmon E, Potter WZ, Thomas RG, Salmon D, Donohue M, Bednar MM, Touchon J, Vellas B: Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011, 76: 280-286. 10.1212/WNL.0b013e318207b1b9.
Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE: Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008, 65: 1509-1517. 10.1001/archneur.65.11.1509.
Hyman BT, Marzloff K, Arriagada PV: The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993, 52: 594-600. 10.1097/00005072-199311000-00006.
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, de Liano Rodriguez Martinez S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M: 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010, 9: 363-372. 10.1016/S1474-4422(10)70043-0.
Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL: Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010, 67: 769-780. 10.1016/j.neuron.2010.08.018.
Kauwe JS, Jacquart S, Chakraverty S, Wang J, Mayo K, Fagan AM, Holtzman DM, Morris JC, Goate AM: Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer's disease presenilin 1 mutation. Ann Neurol. 2007, 61: 446-53. 10.1002/ana.21099.
Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK: Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006, 25: 1242-1252. 10.1038/sj.emboj.7601031.
Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK: Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007, 282: 16155-16163. 10.1074/jbc.M611449200.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (20390018 to TS) and Grant-in-Aid for Research Activity Start-up (22890001 to SH) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), a grant from the Ministry of Health, Labor and Welfare (MHLW), and a grant from the New Energy and Industrial Technology Development Organization (NEDO) in Japan. S.H. was supported by Northern Advancement Center for Science & Technology (NOASTEC) and Terumo Life Science Foundation. S.G. was supported by the NIA Alzheimer's Disease Research Center grant P50 005138 to Mary Sano and by the Veterans Administration. The Washington University in St Louis Alzheimer’s Disease Center Biomarker Core is supported by P50 AG05681 [J.C.M.], P01-AG03991 [J.C.M.], P01-AG26276 [J.C.M.]. This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interest
D.H. declares potential conflicts of interest as follows; board membership on the Satori advisory board and En Vivo advisory board; consultancy with Pfizer, Bristol Myers Squibb, and Innogenetics; Grants/grants pending Eli Lilly, C2N Diagnostics, Astra Zeneca, and Pfizer; Patents (planned, pending or issued): “Predictive Diagnostic for Alzheimer's Disease” (US patent number 6,465,195), “Humanized Antibodies that Sequester Amyloid Beta Peptide” (US patent number 7,195,761), “Diagnostic for early stage Alzheimer's Disease” (US patent number 7,015,044), and “Assay method for Alzheimer's disease” (US patent 7,771,722). D.M.H. and R.J.B. are scientific advisors to C2N Diagnostics, which uses the SILK methodology in human studies and are co-inventors on U.S. patent 7,892,845 “Methods for measuring the metabolism of neurally derived biomolecules in vivo.” Washington University, with D.M.H. and R.J.B. as co-inventors, has also submitted the U.S. nonprovisional patent application “Methods for measuring the metabolism of CNS derived biomolecules in vivo,” serial #12/267,974. S.G. has consultancies with Diagenic and the Pfizer/Janssen Alzheimer Immunotherapy Initiative, and he holds grants from Amicus Therapeutics and Baxter Pharmaceuticals. S.G. holds the following issued patents: “Method of screening for modulators of amyloid formation” (US patent 5,348,963); “Treatment of amyloidosis associated with Alzheimer disease using modulators of protein phosphorylation (US patent 5,385,915); “Treatment of amyloidosis associated with Alzheimer disease” (US patent 5,242,932); and “Use of phosphoprotein patterns for diagnosis of neurological and psychiatric disorders” (US patent 4,874,694). T.S. is one of inventors of the issued patents "Marker peptide for Alzheimer's disease" (US patent 7,807,777). The remaining authors declare no conflict of interest.
Authors’ contribution
SH, MT, YP and TI carried out all of the experiments. TI, KU, AMF, DMH and RB collected samples. TI, KU, SG and TS participated in the design of the study, and TS conceived the study. All authors read and approved the final manuscript.
Electronic supplementary material
13024_2011_340_MOESM1_ESM.doc
Additional file 1 : Figure S1. Difference between male and female subjects for p3-Alc α and Aβ40 levels. Subjects in respective cohorts are analyzed for p3-Alcα and Aβ40 levels in different gender. F, female subjects; M, male subjects. Bars indicate average. No significance, using the Dunn's multiple comparisons test following the Kruskal-Wallis test, was detected for p3-Alcα and Aβ40 levels between male and female subjects in respective CDR and OND. (DOC 492 KB)
13024_2011_340_MOESM2_ESM.pdf
Additional file 2 : Figure S2. Difference between ApoE4 carriers and non-carriers for p3-Alc a and Aβ40 levels of cohort 3. ApoE4 carriers (+) and non-carriers (-) are compared for p3-Alca and Aβ40 levels. Nosignificance, using the Dunn's multiple comparisons test following the Kruskal-Wallis test, was detected for p3-Alca and Aβ40 levels between ApoE4 carriers and non-carriers. (PDF 174 KB)
13024_2011_340_MOESM3_ESM.pdf
Additional file 3 : Table S1. Details of individual subjects in Cohort 1 (Japanese cohort) Table S2. Details of individual subjects in Cohort 2 (US cohort) Table S3. Details of individual subjects in Cohort 3 (Japanese cohort) Table S4. Details of individual subjects in Cohort 4 (Australian cohort). (PDF 86 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hata, S., Taniguchi, M., Piao, Y. et al. Multiple γ-secretase product peptides are coordinately increased in concentration in the cerebrospinal fluid of a subpopulation of sporadic Alzheimer’s disease subjects. Mol Neurodegeneration 7, 16 (2012). https://doi.org/10.1186/1750-1326-7-16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1326-7-16