Abstract
Ovarian cancer represents the fifth leading cause of death from all cancers for women. During the last decades overall survival has improved due to the use of new chemotherapy schedules. Still, the majority of patients die of this disease. Research reveals that ovarian cancer patients exhibit significant immune responses against their tumor. In this review the knowledge obtained thus far on the interaction of ovarian cancer tumor cells and the immune system is discussed. Furthermore the role of p53 as tumor antigen and its potential role as target antigen in ovarian cancer is summarized. Based on the increased knowledge on the role of the immune system in ovarian cancer major improvements are to be expected of immunotherapy based treatment of this disease.
Similar content being viewed by others
Introduction
Ovarian cancer is the most common cause of death from gynecological malignancies. Its nonspecific clinical presentation and the absence of effective screening methods are responsible for the 70% of patients who present with an advanced stage of disease at the time of diagnosis. Primary treatment for advanced stage ovarian cancer is cytoreductive surgery followed by platinum/paclitaxel based chemotherapy. An aggressive surgical approach has been advocated with the intent to remove all macroscopic disease which should yield better survival than leaving residual disease [1–3]. Response rates to primary chemotherapy are 65–80%. When residual or recurrent disease manifests itself, resistance to chemotherapy will prohibit further curative therapy, resulting in an overall survival for patients with advanced stage ovarian disease of only 10–20%[4, 5].
Research during the last decades has revealed that ovarian cancer patients exhibit significant immune responses against their tumor (reviewed in this paper). In designing alternative treatments to successfully eradicate ovarian cancer it is important to consider both the positive effects of immune responses to ovarian cancer and the confounding negative effects on the immune system caused by the tumor cells. As the main target for a potential vaccine is the (overexpressed / mutated) p53 protein we will focus on studies aimed at the induction of humoral and cellular responses against this antigen. However, before reviewing these studies we will briefly introduce some general aspects of the cellular immune system including antigen encounter, antigen processing and presentation and factors influencing the outcome of the immune response in ovarian cancer.
General introduction on the cellular immune system
Antigen presenting cells, most likely dendritic cells, can capture tumor antigens that are secreted or shed by tumor cells or by taking up dying tumor cells. The tumor antigens are processed and presented as peptides by major histocompatability complex (MHC) I and II molecules on the cell surface, and recognized by the T-cell receptor on T-cells. This phenomenon is often referred to as the first signal of activation. After cleavage of proteins into peptides by the proteasome complex and loading of peptides into the class I molecules in the endoplasmatic reticulum, these MHC class I – peptide complexes, recognized by cytotoxic T lymphocytes, are transported to the cell surface. MHC class II molecules mainly present exogenous endocytosed proteins. Antigen (peptide) loading of MHC class II molecules occurs within the endocytic pathway (MHC class II compartments). MHC class II – peptide complexes expressed on the cell surface are recognized by the CD4+ T helper cells. Next to this first antigen specific signal there is a need for a second signal. This signal involves the ligation of CD28 or CTLA-4 on lymphocytes by co-stimulatory molecules CD80 (B7.1) or CD86 (B7.2) respectively on antigen presenting cells or target cells. Binding of the CD28 receptor results in proliferation and activation of T cells, in contrast to binding of CTLA-4 which results in T cell anergy. Another important co-activation signal is mediated by interaction of CD40 ligand on T cells and CD40 on the antigen presenting cell. Fully activated CD8+ T cells differentiate into cytotoxic T lymphocytes and can lyse tumor cells. Memory CD4+ and CD8+ T cells play a critical role in maintaining protective immunity. Apart from their role in expanding CD8+ T-cells, CD4+ T-cells are also involved in the activation of CD8+ independent tumoricidal mechanisms which may play a role in the eradication of tumor cells that have lost MHC class I expression [6]. The CD4+ T cells can be divided into at least two subsets of T helper cells (Th), designated Th1 and Th2. Whereas a Th1 type immune response generally stimulates the generation of cellular immunity, a Th2 type response stimulates humoral immunity next to growth and differentiation of mast cells and eosinophils. Th1 cells secrete cytokines like IFN-γ, Il-2 and TNF-α, Th2 type cells mainly produce IL-4 and IL-10. Regulatory or suppressor T cells, represent potentially a major barrier to successful anti-tumor immune responses. These include Natural Killer T cells[7], CD25+CD4+ T cells [8, 9] and Th3 cells[10]. The balance of signals processed by regulatory T cells can determine vastly different scenarios in tumor surveillance [11]. In the mouse system, CD25+CD4+ regulatory T cells suppress the activation and proliferation of other CD4+ and CD8+ T cells specific for auto antigens which of course is important to prevent autoimmunity but on the other hand prevents the effective generation of immunity to tumor antigens.
The rules that govern the balance between immunity and tolerance is controlled by the conditions of antigen encounter and activation status of the antigen presenting cell [10, 12]. In general, systemic and persistent exposure of T cells to antigen in the absence of costimulation tends to result in T cell tolerization. The type and level of costimulation received during the first encounter with antigen are key determinants in the outcome of an immune response. This depends largely on the activation status of the professional antigen presenting cell that presents the antigenic peptide to naive T cells, in most cases the dendritic cell. The costimulatory state of professional antigen presenting cell is promoted by activated CD4+ T cells, in particular by interaction between CD40L on Th cells and CD40 on the APC [13–16]. This type of T cell help is essential for CTL induction under noninflammatory conditions, whereas lack of CD4+ T cell help can lead to CTL tolerization[17]. Direct demonstration that the activation status of antigen presenting cells influences the outcome of antigen recognition by CD8+ T cells was obtained in studies in which vaccination with mature dendritic cell induced cytotoxic T lymphocyte immunity, whereas infusion of immature dendritic cells failed to do so [15, 18]. The conditions involved in setting the balance between tolerance and immunity seem to be different for activated T cells, because circumstances that tolerize naive T cells may not be tolerogenic for memory T cells. More details on the cellular immune system are to be found in recent reviews [19–22])
Ovarian cancer and the immune system
While the interaction between the host immune system and ovarian cancer tumor cells is still not completely understood, several observations suggest that cell-mediated immune responses could be important in controlling ovarian cancer.
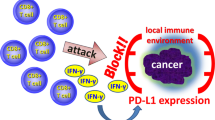
As already stated, the presence of antigen presenting cells, most favorable dendritic cells, is crucial in activating the immune system. In cancer patients the number of dendritic cells is decreased and functionally suppressed by the tumor microenvironment, inhibiting immune responses and thereby causing an impaired tumor immunity [23–27]. For several tumor types it was shown that the number of infiltrating dendritic cells correlated with good prognosis. In a retrospective study using immunohistochemistry the same phenomenon was observed in ovarian cancer [28]. The potential role of dendritic cells in ovarian cancer was demonstrated by Schlienger et al[29]. In 50% of ovarian cancer patients dendritic cells derived from peripheral blood mononuclear cells could, in vitro, induce tumor specific T cells upon loading the dendritic cells with tumor antigen derived from autologous tumor. The antigen(s) recognized by these T cells were not defined. Dendritic cells derived from peripheral blood mononuclear cells and tumor associated macrophages obtained from ascites from the same ovarian cancer patients, cultured with IL-4, GM-CSF and TNF-α, comparably stimulated T cell lines[30]. In contrast to the beneficial effects of macrophages and dendritic cells on the tumor specific immune responses, tumor associated macrophages have been shown to secrete the immunosuppressive cytokine IL-10[27, 31]. One of the effects of IL-10 is that it induces B7-H1 expression on myeloid derived dendritic cells [32]. B7-H1, belonging to the B7 family of costimulatory molecules, is thought to be involved in the regulation of cellular immune responses through its receptors on activated T and B cells [33, 34]. B7-H1 was first described to be expressed by ovarian cancer cells. Later it has been shown to be also present in other human carcinomas [33]. Tumor associated B7-H1 induces apoptosis of activated antigen specific T cells, contributing to the immune evasion of tumor cells [35]. Not only the ovarian cancer tumor cells but also myeloid derived dendritic cells obtained from ovarian tumor tissue and their draining lymph nodes express B7-H1, and are capable to downregulate T cell responses[32]. INF-γ upregulates B7-H1 on the surface of tumor cell lines [35], which might have implications for IFN-γ based cancer immunotherapy. To deal with this issue one could consider blockade of the B7-H1 pathway by e.g. neutralizing mAb. The efficacy of this approach has been shown very nicely in a mouse model for squamous cell carcinoma [36].
In ascites and tumors from patients with ovarian cancer myeloid dendritic cells are outnumbered by plasmacytoid dendritic cells [27, 37, 38]. The exact role of the plasmacytoid dendritic cells in priming naive T cells needs to be further elucidated. It seems that plasmacytoid dendritic cells produce high levels of the angiogenic cytokines TNFα and IL-8 in contrast to the myeloid dendritic cells which produce cytokine IL-12, an inhibitor of angiogenesis. Thus, the accumulation of plasmacytoid dendritic cells in ascites and ovarian cancer tumors is of benefit for the vascularization of the tumor and thereby promotes tumor growth[39].
In ovarian cancer tumor infiltrating CD4+ and CD8+ T cells have been studied extensively. MHC restricted tumor infiltrating lymphocytes cell lines and clones have been developed from lymphocytes derived from ascites and solid tumors of patients with ovarian cancer [40–44]. A clear association between tumor infiltrating lymphocytes and clinical outcome in ovarian cancer patients has been reported in a landmark paper by Zhang et al[45]. In a large cohort of 186 ovarian cancer patients, the five year survival rate was 38% among patients whose tumors contained T cells and only 4,5% among patients whose tumors contained no T cells. The presence of intratumoral T cells was an independent prognostic factor in a multivariate analysis. One of the other remarkable observations from this study was the correlation between high vascular endothelial growth factor expression and low number of T cells, suggesting that vascular endothelial growth factor reduces the number of T cells. T cells from patients with late-stage ovarian cancer contained increased proportions of regulatory CD25+CD4+ T cells, that secreted the immunosuppressive cytokine TGF-β[9]. In a very elegant study by Curiel et al it was shown that ovarian cancer tumor cells and associated macrophages produce the chemokine CCL22, which mediates trafficking of regulatory T cells in tumors and ascites but not to draining lymph nodes[46]. It was shown that these regulatory T cells suppressed tumor specific T cells and were associated with worse prognosis[46]. The regulatory T cells expressed high levels of CCR4, a receptor for CCL22. By blocking regulatory T cell attracting factors, like CCL22, patients might benefit to a higher extent of immunotherapeutic approaches. Also in the same paper by Curiel it was shown that HER-2/neu specific T cells were blocked by the regulatory T cells in their proliferative function, cytokine production and cytolytic activity. The papers of Zhang et al [45] and Curiel et al [46] seem to have conflicting results with Zhang et al showing a positive correlation between the presence of intratumoral T cells and survival and Curiel et al showing an inverse correlation. However in the first study the total number of T cells was taken into account and in the latter paper only the number of regulatory T cells. One can imagine that ovarian cancer patients with intratumoral T cells have a favorable prognosis as long as regulatory T cells are absent. Nevertheless, it will be important that the data from Zhang et al will be confirmed by others to elucidate the role of intratumoral T cells in ovarian cancer. It has been proposed by Conejo-Garcia et al that the ligand "Letal" (lymphocyte effector cell toxicity-activating ligand), expressed by ovarian cancer tumor cells has a role in survival and expansion of tumor infiltrating lymphocytes [47]. Higher levels of tumor derived "Letal" correlated with stronger lymphocyte infiltration. The same group recently published on a new mechanism of tumor vasculogenesis involving vascular endothelial growth factor in cooperation with antimicrobial inflammatory peptides called β-defensins mediated by a new population of CD11c positive leucocytes (DC precursors) named by these group "vascular leucocytes"' [48, 49]. These observations provide a role for the immune system in tumor angiogenesis and need further research to assess what the implications for the clinic could be.
Cytokines and their role in the normal ovary and in ovarian cancer is nicely reviewed by Nash et al[50] and will not be discussed extensively in this review. Ovarian cancer cells probably only partially retain the ability to produce cytokines with important immunostimulatory functions, that are expressed by normal ovarian epithelial cells but lost during neoplastic transformation e.g. the pro-inflammatory cytokine IL-18 [51]. Stat3, a mediator in inflammatory responses and overexpressed in ovarian cancer [52, 53], might play an important role in this change in cytokine production by tumor cells suppressing proinflammatory cytokine production[54].
MHC class I down regulation, an often observed immune escape mechanism in different types of cancer, has not been described frequently for ovarian cancer [55–57]. However recently, Vitale et al showed that MHC class I down regulation was associated with higher stage of disease, yet in a multivariate analysis not with survival [58].
The influence of cytoreductive surgery and platinum/paclitaxel based chemotherapy on the immune system in ovarian cancer has not been elucidated up to now. Whether the anti-tumor reactivity in ovarian cancer patients is influenced by surgery and / or chemotherapy remains to be determined. The immunogenicity of dying tumor cells upon chemotherapeutical treatment, does depend on the nature of the cell death (apoptosis or necrosis), but probably as important are local environment and the activation state of the dendritic cells. Platinum based chemotherapy induces apoptosis of ovarian cancer tumor cells. It is therefore encouraging that dendritic cells loaded with autologous apoptotic tumor cells are capable to induce strong tumor specific T cell responses[29]. T cells themselves are susceptible to chemotherapy [59], but high expression of "Letal" by tumor cells protects lymphocytes from cisplatinum induced cell death [47]. For tumor associated antigens like Mov18, OV-TL3 and OC125 only limited differences in expression on the cell surface of ovarian cancer cells were observed before and after chemotherapy[57].
p53 as tumor antigen
General introduction on p53
Specific T cell-mediated immunotherapy requires the identification of tumor-specific antigens carrying T cell epitopes presented in the context of MHC class I and/or MHC class II molecules (reviewed by[19, 20, 60, 61]) An attractive tumor specific antigen in ovarian cancer is the frequently overexpressed and mutated p53 protein. Other possible target antigens like HER-2/neu and MUC-1 are less frequently expressed by ovarian tumor cells. P53 is a tumor suppressor protein. The role of p53 and other cancer genes has been reviewed by Vogelstein and Vousden [62–64]. P53 acts as a transcription factor, playing a key role in coordinating cell cycle arrest, DNA repair and apoptosis following DNA damage to promote genomic stability. P53, as a transcription factor, mediates apoptosis by pathways involving the upregulation of pro-apoptotic genes as well as downregulation of anti-apoptotic genes [65]. P53 also has the capacity to induce apoptosis directly from the cytoplasm via direct activation of Bax to permeabilize mitochondria which will release cytochrome c leading to the induction of apoptosis [66]. In cancer cells loss of wild-type p53 function may lead to more aggressive tumor growth and failure to respond to standard therapy. The most common way of loss of function is through mutation. P53 is one of the most commonly mutated tumor suppressor proteins in human tumors [67], and already more than 4000 different mutations have been described. The majority are point mutations, resulting in single amino-acid substitutions, generally occurring in the central region of the protein (amino acid 100–300). Other tumor suppressor genes often lose their expression after mutation, but the point mutated p53 protein is often more stable and therefore overexpressed in tumor cells. The loss of function of p53 might be due to binding of the mutated protein to the wild type protein (non-functional tetramers) or to loss of the wild type allele (loss of heterozygosity) [67, 68]. P53 mutations are associated with poor prognosis. Other ways of inactivation include binding to overexpressed MDM2 or E6 protein of human papillomavirus, both causing rapid p53 protein degradation via the ubiquitin pathway[62, 63]. Increased resistance to chemotherapy by mutant p53 has been linked to loss of the presumed triggering role of wild-type p53 in the process of apoptosis.
P53 as tumor antigen (preclinical studies)
P53 protein is overexpressed in 50–60% of ovarian cancers [69–73]. Restoration of the function of p53 in tumor cells is one therapeutic approach. Important progress has been made recently in this field, using viral and non-viral vectors [74], or p53 activating peptides [75]. On the other hand, p53 seems an attractive target for cancer immunotherapy. Due to mutation, nuclear and cytoplasmatic levels of p53 are strongly increased in tumor cells compared to normal cells, thereby providing an immunological window for p53 wild-type specific immune effector cells [76, 77]. Still, tolerance against an autoantigen as wild type p53 needs to be overcome, without development of autoreactive T cells. Mutant and wild-type p53 specific CTL have been described in mice [78–85] In mice, eradication of tumors was achieved with vaccines composed of p53 wild type and mutant peptides [81–83], as well as with adoptive transfer of wild type p53 specific T cells [78, 85–87]. To immunize with whole p53 protein expressed by e.g. viral vectors or long peptides overlapping a whole protein has the advantage of multiple MHC class I and II restricted epitope expression (dominant as well as cryptic). Mouse dendritic cells transduced with an adenoviral wild type p53 encoding construct generated wild type p53 specific CTL (after i.v. or s.c. immunization) capable of preventing the outgrowth of sarcoma tumors[88, 89]. Moreover, the same construct used intratumorally, induced a systemic antitumor response against p53 overexpressing tumors, despite the fact that anti p53 T cell responses could not be measured[90]. Intratumoral injections with recombinant canarypox virus expressing wild type murine p53 (ALVAC-p53) showed antitumor effects in 66% of the mice, however without detectable anti p53 CTL responses [91]. Using different routes of ALVAC-p53 immunizations only intravenous administration was capable of inducing anti-p53 CTL response [92]. More successful than the ALVAC-p53 immunizations in mice was the approach using a recombinant modified vaccinia virus Ankara, expressing wild-type murine p53 (MVAp53). This cell free immunization strategy protected mice for the outgrowth of a syngeneic murine sarcoma by intraperitoneal injection of MVAp53[93]. Mice immunized s.c. with a recombinant vaccinia virus construct expressing wild type p53 were protected against challenge with a p53 overexpressing glioblastome cell line (GL261). Achieving successful p53 based immunization in the presence of well established tumors probably requires active adjuvants. CTLA-4 plays an important role in (negative) regulation of T cell responses [94]. The p53 specific CTL and Th responses can be enhanced by using anti-CTLA-4 at the time of antigenic stimulation, thereby even more effectively breaking tolerance [93, 95]. Anti-CTLA-4 blockade in combination with a vaccine adjuvant, CpG ODN (synthetic oligodeoxynucleotide containing unmethylated cytosine-phosphate-guanine motifs) had a synergistic effect on the improvement of MVAp53 induced antitumor immunity[96]. Using MVAp53 based immunization Dafterian et al showed eradication of large, well established tumors in three different tumor models in two different strains of mice[96]. The immune response against p53 can also be enhanced by the activation of CD40 [89, 97]. Triggering of the CD40 receptor on dendritic cells is vital for their adequate activation and maturation. Both compounds, anti-CTLA4 and activators of CD40, will become available to test on a wide-based scale in clinical studies within the near future. Another route of enhancement of p53 specific immune response after immunization was obtained by administration of Flt3 Ligand, a strong DC stimulating adjuvant[98]. High steady state levels of p53 are not a pre-requisite for tumor eradication by p53 specific CTL as mentioned in one study[99]. Instead, p53 turnover is an important factor in determining the sensitivity of tumor cells to these CTL [87, 100]. CD4+ T helper cells are crucial in the recruitment and regulation of the innate and adaptive immune effector cells[101]. We have demonstrated that CD4+ p53 specific T-helper cells are able to help tumor-specific CTL in controlling p53 overexpressing tumors [102]. Using MHC-transgenic mice has shown to be very efficient in obtaining MHC class I restricted CTL against p53 with high avidity capable of lysing p53 overexpressing tumor cells without lysis of normal cells expressing normal levels of p53 [77]. Very elegantly Kuball et al showed that a CD8-independent p53 specific T cell receptor, generated in HLA A2.1 transgenic mice, could be expressed in human CD8+ and CD4+ T cells with p53 specific tumor recognition[103]. This is at least a very efficient way to obtain p53 specific class I restricted T cells with very high affinity. These model systems might help to answer questions on self tolerance for tumor antigens like p53 and intriguing aspects like cross presentation, cross priming and different aspects of immunotherapy in cancer. So far neither clinical nor immunopathological damage to normal tissue has been observed in different mouse models, despite the fact that wild type p53 is expressed in normal tissue. This indicates that p53 specific T cells are truly tumor-specific. Data available so far support the view that p53 specific immunotherapy may offer a wide therapeutic margin in cancer patients. Proof of the pudding is still in the eating, knowing that their might be important differences in the immune system between preclinical models and men as nicely reviewed by Mestas et al [104].
Cicinnati et al studied the potential of prophylactic vaccination with p53 epitopes using DNA and /or peptide pulsed dendritic cell vaccination in the tumor model giving rise to sarcomas[105]. Compared to control mice a higher incidence of epitope loss tumors were detected in the prophylactic vaccinated group resulting in an increase in tumor growth. Vaccine induced tumor escape therefore could be an important risk in p53 based prophylactic vaccines.
P53 as tumor antigen (clinical studies)
In humans MHC class I restricted p53 specific CTL [106–121], MHC class II restricted p53 specific proliferating Th cells [122–125], and p53 antibody responses (summarized in Table 1) have been observed [123, 126–133]. The first phase I/II immunization trials using p53 as an antigen have just finished and new trials are being initiated. In a phase I study, six advanced stage cancer patients were immunized with an adenoviral vector encoding wild type p53[134]. Neither tumor responses nor anti p53 responses were observed, however all patients showed an adenoviral immune response. This strong anti adenoviral specific response may limit a p53 specific response. Based on the results in the mouse system[91, 92, 135] and rhesus macaques [136], a phase I/II clinical study involving vaccination of end-stage colorectal cancer patients with a recombinant canarypox virus (ALVAC) encoding wild type p53 was performed[137]. Patients were immunized intravenously with an increasing dosage of ALVAC-p53. From this study it appeared that this modality is safe and capable of stimulating p53-specific Th1 (IFNγ) responses in several of these patients. One out of 16 patients showed stable disease for a short period of time after immunization with the highest dose. Fever was the only vaccine related adverse effect. The authors conclude from this trial that repeated immunizations are probably necessary to obtain good clinical responses. Again, anti-vector responses were observed in all patients after vaccination which might have impaired the anti-p53 immune responses. Preclinical data have shown the superiority of prime and boost vaccine strategies using different viral vectors [138, 139]. Whether or not the route of administration plays a role is under debate[140]. Clinical studies have shown the safety and effectiveness of prime and boost vaccination protocols using different viral vectors to deliver the antigen of interest[141, 142]. An analysis of the p53 specific Th response before and after surgery for colorectal cancer showed that the majority of the Th responses detected were not associated with the immunostimulatory cytokine IFNγ, whereas a number of Th responses even involved secretion of the immunomodulatory cytokine IL-10, pointing at the activity of T-regulatory cells that are known to suppress T cell immunity[143]. These results more or less resemble the cytokine profiles of tumor associated T cells derived from ovarian tumors, which were also associated with a lower zeta chain expression[144]. It is important to further investigate the character of the p53 specific T cell responses, because p53-based vaccination of patients should be aimed at boosting only the desired Th1-type immunity, while stimulation of T-regulatory cells should be avoided. This finding would argue in favor of application of a p53-specific vaccination using a delivery mode specifically stimulating the anti p53 (cytotoxic T cell and) Th1 responses. Autologous dendritic cells expressing the antigen of interest is one of these ways. Svane et al reported on their phase I immunization study in breast cancer patients with p53 peptide pulsed DC[145]. Dendritic cells were pulsed with three wild-type and three modified HLA-A2 restricted p53 peptides combined with a MHC class II binding peptide (PADRE). Patients received ten subcutaneous immunizations with at least 5 × 106 peptide pulsed dendritic cells combined with 6 mIU/m2 IL2. Two out of six patients had a clinical response and three out of six had p53 specific T cell responses (including the two patients with a clinical response), without inducing significant toxicity. Another vaccination strategy would be the use of long peptides encoding the whole protein of interest. The advantage of using long peptides is that, if delivered in the appropriate adjuvant (with dendritic cell stimulatory capacity), all potential MHC class I and class II epitopes within the delivered peptides will be processed and presented to host T cells. Table 2 and 3 summarize the naturally processed wild-type p53 epitopes in MHC class I and II known so far. These vaccines will thus become independent of MHC binding motif prediction or processing algorithms and can be administered to subjects independent of their MHC type. A phase I – II trial using wild- type p53 derived long peptides in ovarian cancer patients will be initiated at the University Medical Center Groningen in 2005.
Conclusion
Progress in the fight against ovarian cancer has been hampered by the lack of highly effective therapy to permanently eradicate disseminated intraperitoneal metastases, which are present in most patients at the time of diagnosis. In order to improve the poor outcome for ovarian cancer patients standard and new treatment modalities, such as targeted or biologic agents and immunotherapy should be combined. In this review we pointed out that ovarian cancer tumor cells may (over)express immunoregulatory molecules such as ligand "Letal", CD40 and Stat-3 which stimulate immune response. On the other hand molecules are expressed which downregulate MHC class I molecules and / or simultaneously produce ligands such as CCL22 attracking regulatory T cells as immune-escape mechanism. Recent data showing the importance of the immune response in the course of ovarian cancer and the availability of new potent immunization strategies urge further exploration of immunotherapy as adjuvant treatment modality in ovarian cancer patients. The immune response against p53 can be enhanced by the activation of CD40, anti CTLA-4 blockade, coadministration of Flt3 Ligand and CpG ODN. Compounds capable of activating or blocking these molecules will become available within the near future to be tested on a wide-based scale in clinical studies. The role of p53 as tumor antigen in ovarian cancer in immunotherapy based trials will be unravled within the near future as well. Next to important issues as safety and immunogenicity of vaccination strategies, clinical effectiveness should be one of the major aims of future trials.
HW Nijman is supported by the Dutch Cancer Society (Grant nr. 2002-2768)
References
Eisenkop SM, Spirtos NM: Procedures required to accomplish complete cytoreduction of ovarian cancer: is there a correlation with "biological aggressiveness" and survival?. Gynecol Oncol. 2001, 82: 435-441. 10.1006/gyno.2001.6313.
Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, Ball H, Berek JS: The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994, 170: 974-979.
Eisenkop SM, Friedman RL, Wang HJ: Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998, 69: 103-108. 10.1006/gyno.1998.4955.
McGuire WP, Ozols RF: Chemotherapy of advanced ovarian cancer. Semin Oncol. 1998, 25: 340-348.
Thigpen JT: Chemotherapy for advanced ovarian cancer: overview of randomized trials. Semin Oncol. 2000, 27: 11-16.
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H: The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998, 188: 2357-2368. 10.1084/jem.188.12.2357.
Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG: NKT cells: facts, functions and fallacies. Immunol Today. 2000, 21: 573-583. 10.1016/S0167-5699(00)01735-7.
Sakaguchi S: Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000, 101: 455-458. 10.1016/S0092-8674(00)80856-9.
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH: Regulatory CD4(+)CD25(+) T cells in tumors from patients with early- stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001, 61: 4766-4772.
Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Schoenberger SP, Ohashi PS: Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000, 191: 2021-2027. 10.1084/jem.191.11.2021.
Smyth MJ, Godfrey DI, Trapani JA: A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001, 2: 293-299. 10.1038/86297.
Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H: Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997, 156: 199-209.
Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR: Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998, 393: 478-480. 10.1038/30996.
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G: Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996, 184: 747-752. 10.1084/jem.184.2.747.
Ridge JP, Di Rosa F, Matzinger P: A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998, 393: 474-478. 10.1038/30989.
Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ: T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998, 393: 480-483. 10.1038/31002.
Guerder S, Matzinger P: A fail-safe mechanism for maintaining self-tolerance. J Exp Med. 1992, 176: 553-564. 10.1084/jem.176.2.553.
Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ, van der Voort EI, Rea D, Offringa R, Geuze HJ, Melief CJ, Ossendorp F: Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000, 192: 145-150. 10.1084/jem.192.1.145.
Melief CJ, Toes RE, Medema JP, Van der Burg SH, Ossendorp F, Offringa R: Strategies for immunotherapy of cancer. Adv Immunol. 2000, 75: 235-282.
Rosenberg SA: A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999, 10: 281-287. 10.1016/S1074-7613(00)80028-X.
Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S: Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003, 24: 335-342. 10.1016/S1471-4906(03)00116-9.
Turtle CJ, Hart DN: Dendritic cells in tumor immunology and immunotherapy. Curr Drug Targets. 2004, 5: 17-39. 10.2174/1389450043490640.
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI: Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000, 6: 1755-1766.
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI: Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001, 166: 678-689.
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP: Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996, 2: 1096-1103. 10.1038/nm1096-1096.
Kusmartsev S, Gabrilovich DI: Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002, 51: 293-298. 10.1007/s00262-002-0280-8.
Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ: Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001, 7: 1339-1346. 10.1038/nm1201-1339.
Eisenthal A, Polyvkin N, Bramante-Schreiber L, Misonznik F, Hassner A, Lifschitz-Mercer B: Expression of dendritic cells in ovarian tumors correlates with clinical outcome in patients with ovarian cancer. Hum Pathol. 2001, 32: 803-807. 10.1053/hupa.2001.26455.
Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, Maus MV, Riley JL, Choi Y, Coukos G, Kaiser LR, Rubin SC, Levine BL, Carroll RG, June CH: TRANCE- and CD40 Ligand-matured Dendritic Cells reveal MHC class I restricted T cells specific for autologous tumor in late stage ovarian cancer patients. Clin Cancer Res. 2003, 9: 1517-1527.
Chu CS, Woo EY, Toll AJ, Rubin SC, June CH, Carroll RG, Schlienger K: Tumor-associated macrophages as a source of functional dendritic cells in ovarian cancer patients. Clin Immunol. 2002, 102: 291-301. 10.1006/clim.2001.5179.
Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS: Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J Immunol. 1999, 163: 6251-6260.
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W: Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003, 9: 562-567. 10.1038/nm863.
Dong H, Zhu G, Tamada K, Chen L: B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999, 5: 1365-1369. 10.1038/70932.
Dong H, Chen L: B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003, 81: 281-287.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L: Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002, 8: 793-800.
Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L: B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003, 63: 6501-6505.
Colonna M, Krug A, Cella M: Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002, 14: 373-379. 10.1016/S0952-7915(02)00349-7.
Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, Shepherd D, Colonna M, Cerundolo V: Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003, 33: 1052-1062. 10.1002/eji.200323676.
Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W: Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004, 64: 5535-5538.
Freedman RS, Tomasovic B, Templin S, Atkinson EN, Kudelka A, Edwards CL, Platsoucas CD: Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods. 1994, 167: 145-160. 10.1016/0022-1759(94)90084-1.
Freedman RS, Platsoucas CD: Immunotherapy for peritoneal ovarian carcinoma metastasis using ex vivo expanded tumor infiltrating lymphocytes. Cancer Treat Res. 1996, 82: 115-146.
Ioannides CG, Platsoucas CD, Rashed S, Wharton JT, Edwards CL, Freedman RS: Tumor cytolysis by lymphocytes infiltrating ovarian malignant ascites. Cancer Res. 1991, 51: 4257-4265.
Ioannides CG, Fisk B, Fan D, Biddison WE, Wharton JT, O'Brian CA: Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER-2/neu proto-oncogene. Cell Immunol. 1993, 151: 225-234. 10.1006/cimm.1993.1233.
Ioannides CG, Fisk B, Pollack MS, Frazier ML, Taylor WJ, Freedman RS: Cytotoxic T-cell clones isolated from ovarian tumour infiltrating lymphocytes recognize common determinants on non-ovarian tumour clones. Scand J Immunol. 1993, 37: 413-424.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G: Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003, 348: 203-213. 10.1056/NEJMoa020177.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W: Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004, 10: 942-949. 10.1038/nm1093.
Conejo-Garcia JR, Benencia F, Courreges MC, Gimotty PA, Khang E, Buckanovich RJ, Frauwirth KA, Zhang L, Katsaros D, Thompson CB, Levine B, Coukos G: Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of. Cancer Res. 2004, 64: 2175-2182.
Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G: Vascular Leukocytes Contribute to Tumor Vascularization. Blood. 2004
Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G: Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004, 10: 950-958. 10.1038/nm1097.
Nash MA, Ferrandina G, Gordinier M, Loercher A, Freedman RS: The role of cytokines in both the normal and malignant ovary. Endocr Relat Cancer. 1999, 6: 93-107. 10.1677/erc.0.0060093.
Wang ZY, Gaggero A, Rubartelli A, Rosso O, Miotti S, Mezzanzanica D, Canevari S, Ferrini S: Expression of interleukin-18 in human ovarian carcinoma and normal ovarian epithelium: evidence for defective processing in tumor cells. Int J Cancer. 2002, 98: 873-878. 10.1002/ijc.10268.
Chen H, Ye D, Xie X, Chen B, Lu W: VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004, 94: 630-635. 10.1016/j.ygyno.2004.05.056.
Huang M, Page C, Reynolds RK, Lin J: Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000, 79: 67-73. 10.1006/gyno.2000.5931.
Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H: Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004, 10: 48-54. 10.1038/nm976.
Kooi S, Zhang HZ, Patenia R, Edwards CL, Platsoucas CD, Freedman RS: HLA class I expression on human ovarian carcinoma cells correlates with T-cell infiltration in vivo and T-cell expansion in vitro in low concentrations of recombinant interleukin-2. Cell Immunol. 1996, 174: 116-128. 10.1006/cimm.1996.0301.
Nijman HW, van Diest PJ, Poort-Keesom RJ, Mensdorff-Pouilly S, Verstraeten RA, Kummer A, Meijer CJ, Melief CJ, Hilgers J, Kenemans P: T cell infiltration and MHC I and II expression in the presence of tumor antigens: An immunohistochemical study in patients with serous epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2001, 94: 114-120. 10.1016/S0301-2115(00)00294-3.
Ravenswaay Claasen HH, Fleuren GJ: The influence of combination chemotherapy on antigen expression in ovarian cancer. Gynecol Oncol. 1995, 58: 16-23. 10.1006/gyno.1995.1177.
Vitale M, Pelusi G, Taroni B, Gobbi G, Micheloni C, Rezzani R, Donato F, Wang X, Ferrone S: HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res. 2005, 11: 67-72.
Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP: Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999, 162: 3273-3279.
Offringa R, Van der Burg SH, Ossendorp F, Toes RE, Melief CJ: Design and evaluation of antigen-specific vaccination strategies against cancer. Curr Opin Immunol. 2000, 12: 576-582. 10.1016/S0952-7915(00)00145-X.
Van Den Eynde BJ, van der BP: T cell defined tumor antigens. Curr Opin Immunol. 1997, 9: 684-693. 10.1016/S0952-7915(97)80050-7.
Vogelstein B, Lane D, Levine AJ: Surfing the p53 network. Nature. 2000, 408: 307-310. 10.1038/35042675.
Vousden KH, Lu X: Live or let die: the cell's response to p53. Nat Rev Cancer. 2002, 2: 594-604. 10.1038/nrc864.
Vogelstein B, Kinzler KW: Cancer genes and the pathways they control. Nat Med. 2004, 10: 789-799. 10.1038/nm1087.
Coukos G, Rubin SC: Chemotherapy resistance in ovarian cancer: new molecular perspectives. Obstet Gynecol. 1998, 91: 783-792. 10.1016/S0029-7844(98)00054-4.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR: Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004, 303: 1010-1014. 10.1126/science.1092734.
Greenblatt MS, Bennett WP, Hollstein M, Harris CC: Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994, 54: 4855-4878.
de Vries A, Flores ER, Miranda B, Hsieh HM, van Oostrom CT, Sage J, Jacks T: Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc Natl Acad Sci U S A. 2002, 99: 2948-2953. 10.1073/pnas.052713099.
Nijman HW, Kenemans P, Poort-Keesom RJ, Verstraeten RA, Mensdorff-Pouilly S, Verheijen RH, Melief CJ, Hilgers J, Meijer CJ: Influence of chemotherapy on the expression of p53, HER-2/neu and proliferation markers in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1999, 83: 201-206. 10.1016/S0301-2115(98)00317-0.
Herod JJ, Eliopoulos AG, Warwick J, Niedobitek G, Young LS, Kerr DJ: The prognostic significance of Bcl-2 and p53 expression in ovarian carcinoma. Cancer Res. 1996, 56: 2178-2184.
Klemi PJ, Pylkkanen L, Kiilholma P, Kurvinen K, Joensuu H: p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer. 1995, 76: 1201-1208.
Marks JR, Davidoff AM, Kerns BJ, Humphrey PA, Pence JC, Dodge RK, Clarke-Pearson DL, Iglehart JD, Bast RCJ, Berchuck A: Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991, 51: 2979-2984.
van der Zee AG, Hollema H, Suurmeijer AJ, Krans M, Sluiter WJ, Willemse PH, Aalders JG, de Vries EG: Value of P-glycoprotein, glutathione S-transferase pi, c-erbB-2, and p53 as prognostic factors in ovarian carcinomas. J Clin Oncol. 1995, 13: 70-78.
McCormick F: Cancer gene therapy: fringe or cutting edge?. Nat Rev Cancer. 2001, 1: 130-141. 10.1038/35101008.
Snyder EL, Meade BR, Saenz CC, Dowdy SF: Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide. PLoS Biol. 2004, 2: E36-10.1371/journal.pbio.0020036.
Offringa R, Vierboom MP, Van der Burg SH, Erdile L, Melief CJ: p53: a potential target antigen for immunotherapy of cancer. Ann N Y Acad Sci. 2000, 910: 223-233.
Voss RH, Lotz C, Cellary A, Theobald M: Targeting p53, hdm2, and CD19: vaccination and immunologic strategies. Bone Marrow Transplant. 2000, 25 Suppl 2: S43-S45. 10.1038/sj.bmt.1702353.
Hilburger RM, Abrams SI: Characterization of CD8+ cytotoxic T lymphocyte/tumor cell interactions reflecting recognition of an endogenously expressed murine wild-type p53 determinant. Cancer Immunol Immunother. 2001, 49: 603-612. 10.1007/s002620000156.
Yanuck M, Carbone DP, Pendleton CD, Tsukui T, Winter SF, Minna JD, Berzofsky JA: A mutant p53 tumor suppressor protein is a target for peptide-induced CD8+ cytotoxic T-cells. Cancer Res. 1993, 53: 3257-3261.
Mathiassen S, Lauemoller SL, Ruhwald M, Claesson MH, Buus S: Tumor-associated antigens identified by mRNA expression profiling induce protective anti-tumor immunity. Eur J Immunol. 2001, 31: 1239-1246. 10.1002/1521-4141(200104)31:4<1239::AID-IMMU1239>3.0.CO;2-C.
Mayordomo JI, Loftus DJ, Sakamoto H, De Cesare CM, Appasamy PM, Lotze MT, Storkus WJ, Appella E, DeLeo AB: Therapy of murine tumors with p53 wild-type and mutant sequence peptide- based vaccines. J Exp Med. 1996, 183: 1357-1365. 10.1084/jem.183.4.1357.
Noguchi Y, Chen YT, Old LJ: A mouse mutant p53 product recognized by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994, 91: 3171-3175.
Noguchi Y, Richards EC, Chen YT, Old LJ: Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci U S A. 1995, 92: 2219-2223.
Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA: Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995, 92: 11993-11997.
Vierboom MP, Nijman HW, Offringa R, van der Voort EI, van Hall T, van den BL, Fleuren GJ, Kenemans P, Kast WM, Melief CJ: Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997, 186: 695-704. 10.1084/jem.186.5.695.
Peralta EA, Liu X, McCarthy TM, Wilson TG, Diamond DJ, Ellenhorn JD: Immunotherapy of bladder cancer targeting P53. J Urol. 1999, 162: 1806-1811. 10.1097/00005392-199911000-00074.
Vierboom MP, Zwaveling S, Bos GMJ, Ooms M, Krietemeijer GM, Melief CJ, Offringa R: High steady-state levels of p53 are not a prerequisite for tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. Cancer Res. 2000, 60: 5508-5513.
Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, Carbone DP: Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin Exp Immunol. 1999, 117: 244-251. 10.1046/j.1365-2249.1999.00913.x.
Nikitina EY, Chada S, Muro-Cacho C, Fang B, Zhang R, Roth JA, Gabrilovich DI: An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther. 2002, 9: 345-352. 10.1038/sj.gt.3301670.
Murakami T, Tokunaga N, Waku T, Gomi S, Kagawa S, Tanaka N, Fujiwara T: Antitumor effect of intratumoral administration of bone marrow-derived dendritic cells transduced with wild-type p53 gene. Clin Cancer Res. 2004, 10: 3871-3880.
Odin L, Favrot M, Poujol D, Michot JP, Moingeon P, Tartaglia J, Puisieux I: Canarypox virus expressing wild type p53 for gene therapy in murine tumors mutated in p53. Cancer Gene Ther. 2001, 8: 87-98. 10.1038/sj.cgt.7700279.
Hurpin C, Rotarioa C, Bisceglia H, Chevalier M, Tartaglia J, Erdile L: The mode of presentation and route of administration are critical for the induction of immune responses to p53 and antitumor immunity. Vaccine. 1998, 16: 208-215. 10.1016/S0264-410X(97)00190-4.
Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, Ellenhorn JD: CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol. 2003, 170: 3401-3407.
Krummel MF, Allison JP: CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995, 182: 459-465. 10.1084/jem.182.2.459.
Hernandez J, Ko A, Sherman LA: CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001, 166: 3908-3914.
Daftarian P, Song GY, Ali S, Faynsod M, Longmate J, Diamond DJ, Ellenhorn JD: Two distinct pathways of immuno-modulation improve potency of p53 immunization in rejecting established tumors. Cancer Res. 2004, 64: 5407-5414.
Erdile LF, Smith D: CD40 activation enhances the magnitude of cellular immune responses against p53 but not the avidity of the effectors. Cancer Immunol Immunother. 2000, 49: 410-416. 10.1007/s002620000135.
Parajuli P, Pisarev V, Sublet J, Steffel A, Varney M, Singh R, LaFace D, Talmadge JE: Immunization with wild-type p53 gene sequences coadministered with Flt3 ligand induces an antigen-specific type 1 T-cell response. Cancer Res. 2001, 61: 8227-8234.
Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet JG, Choppin J: Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J Immunol. 1998, 160: 328-333.
Sirianni N, Ha PK, Oelke M, Califano J, Gooding W, Westra W, Whiteside TL, Koch WM, Schneck JP, DeLeo A, Ferris RL: Effect of human papillomavirus-16 infection on CD8+ T-cell recognition of a wild-type sequence p53264-272 peptide in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004, 10: 6929-6937.
Mitra R, Singh S, Khar A: Antitumour immune responses. Expert Rev Mol Med. 2003, 2003: 1-22. 10.1017/S1462399403005623.
Zwaveling S, Vierboom MP, Ferreira Mota SC, Hendriks JA, Ooms ME, Sutmuller RP, Franken KL, Nijman HW, Ossendorp F, Van der Burg SH, Offringa R, Melief CJ: Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res. 2002, 62: 6187-6193.
Kuball J, Schmitz FW, Voss RH, Ferreira EA, Engel R, Guillaume P, Strand S, Romero P, Huber C, Sherman LA, Theobald M: Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity. 2005, 22: 117-129. 10.1016/j.immuni.2004.12.005.
Mestas J, Hughes CC: Of mice and not men: differences between mouse and human immunology. J Immunol. 2004, 172: 2731-2738.
Cicinnati VR, Dworacki G, Albers A, Beckebaum S, Tuting T, Kaczmarek E, DeLeo AB: Impact of p53-based immunization on primary chemically-induced tumors. Int J Cancer. 2005, 113: 961-970. 10.1002/ijc.20686.
Hoffmann TK, Nakano K, Elder EM, Dworacki G, Finkelstein SD, Appella E, Whiteside TL, DeLeo AB: Generation of T cells specific for the wild-type sequence p53(264-272) peptide in cancer patients: implications for immunoselection of epitope loss variants. J Immunol. 2000, 165: 5938-5944.
Houbiers JG, Nijman HW, Van der Burg SH, Drijfhout JW, Kenemans P, van de Velde CJ, Brand A, Momburg F, Kast WM, Melief CJ: In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993, 23: 2072-2077.
Nijman HW, Houbiers JG, Van der Burg SH, Vierboom MP, Kenemans P, Kast WM, Melief CJ: Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immunother. 1993, 14: 121-126.
Nijman HW, Van der Burg SH, Vierboom MP, Houbiers JG, Kast WM, Melief CJ: p53, a potential target for tumor-directed T cells. Immunol Lett. 1994, 40: 171-178. 10.1016/0165-2478(94)90189-9.
Nikitina EY, Clark JI, van Beynen J, Chada S, Virmani AK, Carbone DP, Gabrilovich DI: Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001, 7: 127-135.
Ropke M, Regner M, Claesson MH: T cell-mediated cytotoxicity against p53-protein derived peptides in bulk and limiting dilution cultures of healthy donors. Scand J Immunol. 1995, 42: 98-103.
Ropke M, Hald J, Guldberg P, Zeuthen J, Norgaard L, Fugger L, Svejgaard A, Van der BS, Nijman HW, Melief CJ, Claesson MH: Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. Proc Natl Acad Sci U S A. 1996, 93: 14704-14707. 10.1073/pnas.93.25.14704.
Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA: Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997, 185: 833-841. 10.1084/jem.185.5.833.
Umano Y, Tsunoda T, Tanaka H, Matsuda K, Yamaue H, Tanimura H: Generation of cytotoxic T cell responses to an HLA-A24 restricted epitope peptide derived from wild-type p53. Br J Cancer. 2001, 84: 1052-1057. 10.1054/bjoc.2000.1715.
Wurtzen PA, Pedersen LO, Poulsen HS, Claesson MH: Specific killing of P53 mutated tumor cell lines by a cross-reactive human HLA-A2-restricted P53-specific CTL line. Int J Cancer. 2001, 93: 855-861. 10.1002/ijc.1417.
Asai T, Storkus WJ, Mueller-Berghaus J, Knapp W, DeLeo AB, Chikamatsu K, Whiteside TL: In vitro generated cytolytic T lymphocytes reactive against head and neck cancer recognize multiple epitopes presented by HLA-A2, including peptides derived from the p53 and MDM-2 proteins. Cancer Immun. 2002, 2: 3-
Azuma K, Shichijo S, Maeda Y, Nakatsura T, Nonaka Y, Fujii T, Koike K, Itoh K: Mutated p53 gene encodes a nonmutated epitope recognized by HLA-B*4601-restricted and tumor cell-reactive CTLs at tumor site. Cancer Res. 2003, 63: 854-858.
McArdle SE, Rees RC, Mulcahy KA, Saba J, McIntyre CA, Murray AK: Induction of human cytotoxic T lymphocytes that preferentially recognise tumour cells bearing a conformational p53 mutant. Cancer Immunol Immunother. 2000, 49: 417-425. 10.1007/s002620000137.
Papadopoulos KP, Hesdorffer CS, Suciu-Foca N, Hibshoosh H, Harris PE: Wild-type p53 epitope naturally processed and presented by an HLA-B haplotype on human breast carcinoma cells. Clin Cancer Res. 1999, 5: 2089-2093.
Petersen TR, Buus S, Brunak S, Nissen MH, Sherman LA, Claesson MH: Identification and design of p53-derived HLA-A2-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001, 53: 357-364. 10.1046/j.1365-3083.2001.00887.x.
Tokunaga N, Murakami T, Endo Y, Nishizaki M, Kagawa S, Tanaka N, Fujiwara T: Human monocyte-derived dendritic cells pulsed with wild-type p53 protein efficiently induce CTLs against p53 overexpressing human cancer cells. Clin Cancer Res. 2005, 11: 1312-1318.
Tilkin AF, Lubin R, Soussi T, Lazar V, Janin N, Mathieu MC, Lefrere I, Carlu C, Roy M, Kayibanda M, .: Primary proliferative T cell response to wild-type p53 protein in patients with breast cancer. Eur J Immunol. 1995, 25: 1765-1769.
Van der Burg SH, de Cock K, Menon AG, Franken KL, Palmen M, Redeker A, Drijfhout J, Kuppen PJ, van V, Erdile L, Tollenaar RA, Melief CJ, Offringa R: Long lasting p53-specific T cell memory responses in the absence of anti-p53 antibodies in patients with resected primary colorectal cancer. Eur J Immunol. 2001, 31: 146-155. 10.1002/1521-4141(200101)31:1<146::AID-IMMU146>3.0.CO;2-T.
Chikamatsu K, Albers A, Stanson J, Kwok WW, Appella E, Whiteside TL, DeLeo AB: P53(110-124)-specific human CD4+ T-helper cells enhance in vitro generation and antitumor function of tumor-reactive CD8+ T cells. Cancer Res. 2003, 63: 3675-3681.
Fujita H, Senju S, Yokomizo H, Saya H, Ogawa M, Matsushita S, Nishimura Y: Evidence that HLA class II-restricted human CD4+ T cells specific to p53 self peptides respond to p53 proteins of both wild and mutant forms. Eur J Immunol. 1998, 28: 305-316. 10.1002/(SICI)1521-4141(199801)28:01<305::AID-IMMU305>3.0.CO;2-3.
Angelopoulou K, Rosen B, Stratis M, Yu H, Solomou M, Diamandis EP: Circulating antibodies against p53 protein in patients with ovarian carcinoma. Correlation with clinicopathologic features and survival. Cancer. 1996, 78: 2146-2152. 10.1002/(SICI)1097-0142(19961115)78:10<2146::AID-CNCR15>3.0.CO;2-Z.
Angelopoulou K, Diamandis EP: Detection of the TP53 tumour suppressor gene product and p53 auto- antibodies in the ascites of women with ovarian cancer. Eur J Cancer. 1997, 33: 115-121. 10.1016/S0959-8049(96)00295-X.
Crawford LV, Pim DC, Bulbrook RD: Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982, 30: 403-408.
Gadducci A, Ferdeghini M, Buttitta F, Fanucchi A, Annicchiarico C, Prontera C, Bevilacqua G, Genazzani AR: Preoperative serum antibodies against the p53 protein in patients with ovarian and endometrial cancer. Anticancer Res. 1996, 16: 3519-3523.
Gadducci A, Ferdeghini M, Buttitta F, Cosio S, Fanucchi A, Annicchiarico C, Genazzani AR: Serum anti-p53 antibodies in the follow-up of patients with advanced ovarian carcinoma. Anticancer Res. 1998, 18: 3763-3765.
Gadducci A, Ferdeghini M, Buttitta F, Cosio S, Fanucchi A, Annicchiarico C, Gagetti O, Bevilacqua G, Genazzani AR: Assessment of the prognostic relevance of serum anti-p53 antibodies in epithelial ovarian cancer. Gynecol Oncol. 1999, 72: 76-81. 10.1006/gyno.1998.5101.
Labrecque S, Naor N, Thomson D, Matlashewski G: Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993, 53: 3468-3471.
Vennegoor CJ, Nijman HW, Drijfhout JW, Vernie L, Verstraeten RA, Mensdorff-Pouilly S, Hilgers J, Verheijen RH, Kast WM, Melief CJ, Kenemans P: Autoantibodies to p53 in ovarian cancer patients and healthy women: a comparison between whole p53 protein and 18-mer peptides for screening purposes. Cancer Lett. 1997, 116: 93-101. 10.1016/S0304-3835(97)00168-7.
Kuball J, Schuler M, Antunes FE, Herr W, Neumann M, Obenauer-Kutner L, Westreich L, Huber C, Wolfel T, Theobald M: Generating p53-specific cytotoxic T lymphocytes by recombinant adenoviral vector-based vaccination in mice, but not man. Gene Ther. 2002, 9: 833-843. 10.1038/sj.gt.3301709.
Roth J, Dittmer D, Rea D, Tartaglia J, Paoletti E, Levine AJ: p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc Natl Acad Sci U S A. 1996, 93: 4781-4786. 10.1073/pnas.93.10.4781.
Rosenwirth B, Kuhn EM, Heeney JL, Hurpin C, Tartaglia J, Bonnet MC, Moingeon P, Erdile L: Safety and immunogenicity of ALVAC wild-type human p53 (vCP207) by the intravenous route in rhesus macaques. Vaccine. 2001, 19: 1661-1670. 10.1016/S0264-410X(00)00416-3.
Menon AG, Kuppen PJ, Van der Burg SH, Offringa R, Bonnet MC, Harinck BI, Tollenaar RA, Redeker A, Putter H, Moingeon P, Morreau H, Melief CJ, van de Velde CJ: Safety of intravenous administration of a canarypox virus encoding the human wild-type p53 gene in colorectal cancer patients. Cancer Gene Ther. 2003, 10: 509-517. 10.1038/sj.cgt.7700600.
Aarts WM, Schlom J, Hodge JW: Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002, 62: 5770-5777.
Grosenbach DW, Barrientos JC, Schlom J, Hodge JW: Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001, 61: 4497-4505.
Kudo-Saito C, Schlom J, Hodge JW: Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005, 11: 2416-2426.
Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KY, Schlom J: Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000, 18: 3964-3973.
Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J: Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005, 23: 720-731. 10.1200/JCO.2005.10.206.
Van der Burg SH, Menon AG, Redeker A, Franken KL, Drijfhout JW, Tollenaar RA, Hartgrink HH, van de Velde CJ, Kuppen PJ, Melief CJ, Offringa R: Magnitude and polarization of P53-specific T-helper immunity in connection to leukocyte infiltration of colorectal tumors. Int J Cancer. 2003, 107: 425-433. 10.1002/ijc.11419.
Rabinowich H, Suminami Y, Reichert TE, Crowley-Nowick P, Bell M, Edwards R, Whiteside TL: Expression of cytokine genes or proteins and signaling molecules in lymphocytes associated with human ovarian carcinoma. Int J Cancer. 1996, 68: 276-284. 10.1002/(SICI)1097-0215(19961104)68:3<276::AID-IJC2>3.0.CO;2-Z.
Svane IM, Pedersen AE, Johnsen HE, Nielsen D, Kamby C, Gaarsdal E, Nikolajsen K, Buus S, Claesson MH: Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a phase I study. Cancer Immunol Immunother. 2004, 53: 633-641. 10.1007/s00262-003-0493-5.
Abendstein B, Marth C, Muller-Holzner E, Widschwendter M, Daxenbichler G, Zeimet AG: Clinical significance of serum and ascitic p53 autoantibodies in epithelial ovarian carcinoma. Cancer. 2000, 88: 1432-1437. 10.1002/(SICI)1097-0142(20000315)88:6<1432::AID-CNCR22>3.0.CO;2-8.
Vogl FD, Frey M, Kreienberg R, Runnebaum IB: Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br J Cancer. 2000, 83: 1338-1343. 10.1054/bjoc.2000.1446.
Vogl FD, Stickeler E, Weyermann M, Kohler T, Grill HJ, Negri G, Kreienberg R, Runnebaum IB: p53 autoantibodies in patients with primary ovarian cancer are associated with higher age, advanced stage and a higher proportion of p53-positive tumor cells. Oncology. 1999, 57: 324-329. 10.1159/000012069.
Hogdall EV, Hogdall CK, Blaakaer J, Heegaard NH, Glud E, Christensen L, Bock JE, Norgaard-Pedersen B, Wiik A, Kjaer SK: P53 autoantibodies in sera from Danish ovarian cancer patients and their correlation with clinical data and prognosis. APMIS. 2002, 110: 545-553. 10.1034/j.1600-0463.2002.11007805.x.
Mayerhofer K, Tempfer C, Kucera E, Hefler L, Zeisler H, Kainz C, Zeillinger R, Sliutz G: Humoral p53 antibody response is a prognostic parameter in ovarian cancer. Anticancer Res. 1999, 19: 875-878.
Marx D, Frey M, Zentgraf H, Adelssen G, Schauer A, Kuhn W, Meden H: Detection of serum autoantibodies to tumor suppressor gene p53 with a new enzyme-linked immunosorbent assay in patients with ovarian cancer. Cancer Detect Prev. 2001, 25: 117-122.
Numa F, Umayahara K, Suehiro Y, Hirakawa H, Nawata S, Suminami Y, Oga A, Ito T, Sasaki K, Kato H: Serum anti-p53 antibodies in uterine and ovarian cancer: association with dna sequence copy number abnormalities. Tumour Biol. 2001, 22: 162-168. 10.1159/000050611.
Montenarh M, Harlozinska A, Bar JK, Kartarius S, Gunther J, Sedlaczek P: p53 autoantibodies in the sera, cyst and ascitic fluids of patients with ovarian cancer. Int J Oncol. 1998, 13: 605-610.
Green JA, Robertson LJ, Campbell IR, Jenkins J: Expression of the p53 gene and presence of serum autoantibodies in ovarian cancer: correlation with differentiation. Cancer Detect Prev. 1995, 19: 151-155.
Barfoed AM, Petersen TR, Kirkin AF, Thor SP, Claesson MH, Zeuthen J: Cytotoxic T-lymphocyte clones, established by stimulation with the HLA- A2 binding p5365-73 wild type peptide loaded on dendritic cells In vitro, specifically recognize and lyse HLA-A2 tumour cells overexpressing the p53 protein. Scand J Immunol. 2000, 51: 128-133. 10.1046/j.1365-3083.2000.00668.x.
Eura M, Chikamatsu K, Katsura F, Obata A, Sobao Y, Takiguchi M, Song Y, Appella E, Whiteside TL, DeLeo AB: A wild-type sequence p53 peptide presented by HLA-A24 induces cytotoxic T lymphocytes that recognize squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000, 6: 979-986.
Wurtzen PA, Claesson MH: A HLA-A2 restricted human CTL line recognizes a novel tumor cell expressed p53 epitope. Int J Cancer. 2002, 99: 568-572. 10.1002/ijc.10375.
Rojas JM, McArdle SE, Horton RB, Bell M, Mian S, Li G, Ali SA, Rees RC: Peptide immunisation of HLA-DR-transgenic mice permits the identification of a novel HLA-DRbeta1*. Cancer Immunol Immunother. 2005, 54: 243-253. 10.1007/s00262-004-0596-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nijman, H., Lambeck, A., van der Burg, S. et al. Immunologic aspect of ovarian cancer and p53 as tumor antigen. J Transl Med 3, 34 (2005). https://doi.org/10.1186/1479-5876-3-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-3-34