Abstract
A considerable number of central nervous system pathologies remain undiagnosed during the first two trimesters of pregnancy. This group of disorders includes anomalies of brain proliferation, migration and cortical organization. Due to the fact that a detailed ultrasound examination of the fetal brain is usually not performed during the third trimester the diagnosis of these disorders is usually only made in families with a previously affected child or in many cases be mere chance. In this article we review the feasibility of prenatal diagnosis of disorders of brain proliferation: microcephaly, macrocephaly, hemimegalencephaly and neoplastic and non-neoplastic abnormal cell types. We discuss the differential diagnosis and offer a stepwise approach to the diagnosis of the more common disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Review
Thirty years have passed since the first prenatal diagnosis of a fetus with a brain malformation was made by ultrasound [1]. Since then, rapid development of new US machines and transducers have established the basis for a new field in Obstetrics: the diagnosis of congenital anomalies[2].
Shortly after the report by Campbell et al [1] Kratochwil et al diagnosed fetal hydrocephaly [3], Michell and Bradley-Watson described a case of fetal meningocele [4] and Karp et al reported the use of ultrasound for the exclusion of primary microcephaly [5].
During the second half of the 70's and during the 80's studies on the normal anatomy and biometry of the fetal brain enabled prenatal screening for CNS malformations [6–9].
By 1988 the now classical textbook on the prenatal diagnosis of congenital anomalies by Romero et al [2] included a comprehensive chapter on the normal anatomy and pathology of the fetal CNS. In their book they describe the ultrasonographic features of holoprosencephaly, agenesis of the corpus callosum, intracranial arachnoid cysts, and choroid plexus cysts.
One year later, Filly et al suggested the use of 3 specific axial planes for the evaluation of the fetal CNS [10]. By routine visualization of the transventricular, transthalamic and transcerebellar planes, they were able to diagnose, retrospectively, most cases with CNS anomalies.
Further progress was made after the introduction of high-resolution transvaginal probes. Transvaginal ultrasound permits the study of the brain in fetuses in vertex presentation in planes that are usually difficult to obtain with the transabdominal approach [11, 12]. Recently, fetal magnetic resonance emerged as an additional potential useful method for the diagnosis of CNS anomalies [13].
The modern approach to the diagnosis of fetal CNS pathologies requires an in-depth knowledge of brain anatomy and embryology, expertise in the different imaging techniques and proficiency in the different genetic aspects of congenital brain disorders. Moreover, in order to establish the specific prognosis in a given case experience in pediatric neurology is essential. These requirements can only be met by a multidisciplinary team that includes experts in the fields of fetal medicine, neuroradiology, genetics, pediatric neurology, neonatology and pathology [14].
A cornerstone in the understanding of the development of the normal and pathologic brain is the understanding that the brain develops as a continuum during pregnancy and even after delivery and that different insults at a specific time may produce similar pathologies [15]. The current recommendation for screening of fetal anomalies is a second trimester ultrasound examination between 19 and 22 weeks of pregnancy. However a substantial amount of significant brain anomalies will remain undiagnosed at this gestational age [16].
We present a structured approach to the diagnosis of late manifesting fetal intracranial pathologies. In this first part we review the different aspects of cell proliferation disorders.
Brain Development
Volpe describes the sequential development of the human brain [15]. Early events include dorsal and ventral induction while later events, occurring from the second month of gestation through the postnatal period, include cell proliferation, migration, organization and myelination.
New classifications based on information obtained from MRI and genetic studies have recently been published [17, 18]. The classification system proposed by Barkovich et al [17] is based on Volpe's framework [15] but expands the sub-classifications. On the other hand, Sarnat [18] proposed a new etiologic classification based fundamentally on the different patterns of genetic expression of malformations without consideration of the temporal pattern of development.
The most useful classification for the fetal neurosonographer is the one proposed by Barkovich et al [17]. Description of the abnormal ultrasonographic findings in a particular fetus enables categorization into one of the following entities: 1. Cell proliferation anomalies (i.e. microcephaly, megalencephaly and hemimegalencephaly); 2. Neuronal migration anomalies (i.e. lissencephaly, cobblestone complex and heterotopia); or 3. Abnormal cortical organization (i.e. polymicrogyria, schizencephaly and cortical dysplasia).
Cell proliferation disorders
Microcephaly
Microcephaly is defined postnatally as low brain weight and a small head circumference (HC) more than two standard deviations (SD) below the mean or below the 3rd percentile. Such a broad definition obviously includes normal individuals. The smaller the head circumference, the higher the chances of associated mental retardation. Prenatally, there is no consensus regarding the exact definition of abnormally small HC, some authors propose the -2SD [17] cutoff while others propose the -3SD [19] cutoff. Using the -3SD definition, Chervenak et al showed that the prenatal HC measurement was sensitive for diagnosis of microcephaly with no false negatives, -4SD was a specific test with no false positive cases[19].
The incidence of microcephaly at birth is estimated to be in the range between 1:6250 and 1:8500 deliveries. The incidence is much higher, 1.6 per 1000 after the first year of life [19] due to progressive microcephaly following perinatal insults or due to a neurodegenerative metabolic or neurogenetic process. Congenital microcephaly may present as an isolated finding, in this case it is known as primary microcephaly or microcephaly vera and be associated with a wide range of CNS and non-CNS pathologies[20].
The prenatal diagnosis of microcephaly, particularly in cases of primary microcephaly, is usually difficult before the 3rd trimester. Reece and Goldstein in a study of 9600 low risk pregnancies in which the brain was scanned for congenital anomalies failed to diagnose all 5 cases of microcephaly [21]. The authors did not state the gestational age in which the examinations were performed.
Bromely and Benacerraf [22] found that 6 out of 7 fetuses with postnatally diagnosed microcephaly had normal head size measurements before 22 weeks of pregnancy and were diagnosed only after 27 weeks of gestation.
In some cases the presence of microcephaly may be suspected based on additional sonographic findings like: small frontal lobe [23], sloping forehead [24], enlarged subarachnoid space [24, 25] and/or abnormal power Doppler demonstration of the anterior and middle cerebral arteries [24].
The accuracy of ultrasound in the diagnosis of microcephaly has not been studied prospectively. Two retrospective analyses of cases diagnosed prenatally have recently been reported. Den Hollander et al reported on 30 fetuses with prenatally diagnosed microcephaly [26]. The main referral indications were: reduced head size or suspected IUGR (16 fetuses), intracranial anomalies (5 fetuses), and extracranial anomalies (3 fetuses). Mean gestational age at the time of referral was 27 weeks and at the time of diagnosis 28 weeks. Associated anomalies were present in 83.3% of the patients: holoprosencephaly (16.7%), chromosomal anomalies (23.3%), genetic syndromes (20%) and multiple anomalies (23.3%). Only 5 patients were considered to represent "isolated microcephaly" but a careful analysis of these cases showed that 3 of them had other minor anomalies, one was probably associated with twin to twin transfusion syndrome with ventriculomegaly and one was diagnosed in a family with a previous history of microcephaly. The authors did not describe the number of fetuses with microcephaly diagnosed after delivery in their center.
Dahlgren and Wilson reviewed all cases of microcephaly diagnosed during a 10-year period at British Columbia Women's Hospital [27]. They found 45 cases; in 21, the diagnosis was made prenatally and confirmed postnatally. In 15 patients, the second trimester ultrasound was available and 12 of these patients had a normal scan between 15 and 20 weeks of gestation. In 9 patients (43%) the etiologic cause of microcephaly remained unclear: possible viral infection based on placental signs of villitis or chorioamnionitis (4), multiple malformations (1), constitutional (1) and no specific etiology identified (3).
The counseling dilemma for fetuses with a small HC remains difficult. Mental retardation can safely be predicted in cases with associated US findings, abnormal karyotype or positive test for intrauterine infection. In fetuses with isolated small HC an effort should be made to determine gyral normality in utero by US or MRI. Children with severe primary microcephaly may have a simplified gyral pattern that in the most severe cases may resemble lissencephaly. It is noteworthy that this pattern may develop late in pregnancy or even after delivery.
To illustrate this dilemma we present the HC growth curves (Figure 1) of two fetuses suspected of having microcephaly vera during pregnancy. Both fetuses were considered morphologically normal by US and MR examinations. In these cases the family history was the cornerstone for counseling. The mother of patient A has a small HC (51 cm) and is of normal intelligence. Patient B has a sibling with severe microcephaly and neurodevelopmental retardation and the parents are second cousins. While patient A is neurologically normal at 18 months of age and the HC continues to be below the 3rd percentile, patient B suffers from microcephaly with profound neurodevelopmental retardation. These examples illustrates that the evaluation of the patient's family members is crucial before counseling is attempted.
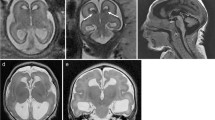
In fetuses with severe associated malformations, the diagnosis is made based on the presence of these anomalies (Figure 2).
Primary microcephaly is genetically heterogeneous, with several loci currently mapped [28]. The advances in identifying genes associated with brain development will enable future prenatal molecular diagnosis in families at risk. The recurrence risk to parents of a child with primary microcephaly is 25% [28]. The proposed prenatal investigation of fetuses with microcephaly is presented in Figure 3.
Macrocephaly
Macrocephaly is defined as a head circumference above the 98th percentile or more than 2 SD above the mean. In the absence of hydrocephaly or enlarged subarachnoid spaces it is synonymous with megalencephaly. In a study of Swedish boys it was found in 0.5% of the population and was associated with lower intelligence [29]. Other investigators found that the vast majority of children with megalencephaly have normal intelligence [30].
The most common form of megalencephaly is autosomal dominant and familial, and usually not associated with mental retardation. Yet, megalencephaly may be associated with many syndromes [20, 31].
The prenatal diagnosis of macrocephaly has been occasionally reported [16, 32, 33]. The differential diagnosis in these cases is difficult. The demonstration of an enlarged subarachnoid space, particularly in the frontal region, in a fetus with macrocephaly is suggestive of benign enlargement of the subarachnoid spaces and is usually associated with a good prognosis (when the head circumference is normal or low enlarged subarachnoid spaces may imply brain atrophy) [25].
An essential part of the evaluation is measurement of the parents' head circumference. When one parent has a large head circumference it is not immediately reassuring that the fetus has benign familial macrocephaly, since several syndromes which may combine macrocephaly and mental retardation, can be inherited in an autosomal dominant fashion while the affected parent may only present with macrocephaly. These syndromes include Neurofibromatosis type 1 (NF1) [34], Sotos syndrome [33, 35], Weaver syndrome [35], Cole-Hughes syndrome [36], and the inherited macrocephaly-hamartomas syndrome due to PTEN mutations [37]. Therefore, a careful medical history of the affected parent should be obtained regarding neurological problems, developmental milestones, skin abnormalities, endocrine disorders and propensity for the development of tumors. The parent with macrocephaly should be examined with special attention to the skin (lipomas, macules, café au lait spots, neurofibromas) and dysmorphic features. If there are specific findings in the parent, molecular diagnosis is now possible for NF1, Sotos syndrome and macrocephaly-hamartomas syndromes. A large head circumference of the fetus is even more alarming when the parents have a normal head circumference. When it is associated with overgrowth both Sotos and Weaver syndromes should be considered because most cases are sporadic. When it is observed in a fetus with short femur, skeletal dysplasias such as achondroplasia and hypochondroplasia are possible [16].
The flow chart for the investigation of fetuses with megalencephaly is presented in Figure 4.
Abnormal proliferation with abnormal cell types
According to the classification proposed by Barkovich et al [17] this category includes: non-neoplastic and neoplastic abnormal cell types. The non-neoplastic category includes 3 different entities: cortical hamartomas of tuberous sclerosis, cortical dysplasia with balloon cells and hemimegalencephaly. The neoplastic category also includes 3 different entities: dysembryoplastic neuroepithelial tumors, ganglioglioma and gangliocytoma.
Tuberous sclerosis
Tuberous sclerosis is transmitted as an autosomal dominant trait with variable expression and genetic heterogeneity, and occurs as a result of a de novo mutation in approximately 60% of the patients. Multiple organs may be involved including CNS skin, kidneys, heart and eyes. Seizures are the most common presenting symptom and are usually accompanied by mental retardation. Neuroimaging is the best diagnostic tool and is abnormal in 90% of the cases. Computed tomography demonstrates subependymal calcified nodules and cortical or subcortical areas of decreased attenuation (corresponding to tubers). Magnetic resonance does not show calcified lesions well but demonstrates the tubers better. The tubers are composed of clusters of heterotopic cells and are indicative of a migration disorder associated with abnormal cell differentiation [38].
Prenatal diagnosis of cortical tubers may be considered in affected families and in fetuses following ultrasonographic visualization of a cardiac rhabdomyoma and are therefore at risk of having tuberous sclerosis [39, 40]. Sonigo et al studied 8 fetuses with multiple cardiac tumors [39]. In all their patients the antenatal sonographic evaluation of the brain was considered normal but fetal MRI demonstrated hyperintense subependymal and cortical nodules on T1-weighted images in 5 patients while in one the nodules were only demonstrated after delivery [39]. Sgro et al reported the prenatal ultrasonographic diagnosis of tuberous sclerosis at 30 weeks of gestation with confirmation by postnatal US, CT and MRI [40].
In affected families and in fetuses with cardiac tumors visualization of brain nodules should be attempted by MRI. However, failure of visualization does not provide complete reassurance that the newborn will not suffer from tuberous sclerosis.
Cortical dysplasia with balloon cells
The diagnosis of cortical dysplasia with balloon cells (CDBC) is based on the presence of focal cortical dysplasia with balloon cells without concurrent manifestations of TS. It is not clear if CDBC and TS represent different entities or if they are different clinical and radiological expressions of the same disease [41]. Most of the patients present with seizures and are often refractory to medical treatment [41].
The prenatal diagnosis of cortical dysplasia with balloon cells has not been reported but must be considered in cases with suspected focal cortical anomalies as demonstrated by US or MRI.
Hemimegalencephaly
Hemimegalencephaly (HME) is a rare congenital malformation characterized by unilateral enlargement of the cerebral hemisphere. It may present as an isolated finding or in conjunction with neurocutaneous disorders [17]. HME is considered a primary disturbance in cellular lineage, differentiation and proliferation, interacting with a disturbance in gene expression of body symmetry [41].
All patients have seizures that are usually intractable and most are mentally retarded [42]. HME has characteristic imaging findings that include: asymmetry of the hemispheres with a pathologically enlarged side, abnormal gyral pattern, ventriculomegaly and abnormally thickened white matter [43]. Associated CNS pathologies (agenesis of the corpus callosum, Dandy-Walker malformation or abnormal cerebellum) may be present.
The prenatal diagnosis of HME has been reported in at least 4 occasions [16, 44–46] at 32 [44], 20 [45] and 22 weeks [46]. The diagnosis was suspected due to unilateral ventricular dilatation with a shift of the falx cerebri. We recently reported an additional fetus with HME [16]. The referral diagnosis at 25 weeks was asymmetric ventriculomegaly, but during the transvaginal neurosonographic examination abnormal sulci and gyri and abnormal periventricular echogenicity on the affected side were observed. An MRI established the diagnosis of HME at 29 weeks and final confirmation was achieved by the pathological examination after the termination of pregnancy.
Neoplastic cell proliferation disorders
These entities are extremely rare. Chung et al reported on the prenatal diagnosis of an enlarging intracranial mass that proved to be a gangliocytoma [47]. Even with the use of neurosonography or MRI the differential diagnosis of fetal intracranial tumors will remain difficult and in most cases the diagnosis will be made postnatally.
Conclusion
Obstetricians often encounter the diagnostic dilemma presented by abnormal fetal head growth. Although the developmental outcome in most cases is good, a maximal effort should be made to reduce the chances of misdiagnosis of one of the multiple syndromes that can present with fetal microcephaly or macrocephaly.
References
Campbell S, Johnstone FD, Holt EM, May P: Anencephaly: early ultrasonic diagnosis and active management. Lancet. 1972, 2: 1226-1227. 10.1016/S0140-6736(72)92273-8.
Romero R, Pilu G, Jeanty P, Ghidini A, Hobbins JC: Prenatal diagnosis of congenital anomalies. 1988, Norwalk: Appleton & Lange
Kratochwil A, Stoger H, Schaller A: Obstetrical ultrasonic diagnosis of hydrocephalus. Geburtshilfe Frauenheilkd. 1973, 33: 322-325.
Michell RC, Bradley-Watson PJ: The detection of fetal meningocoele by ultrasound B scan. J Obstet Gynaecol Br Commonw. 1973, 80: 1100-1101.
Karp LE, Smith DW, Omenn GS, Johnson SL, Jones K: Use of ultrasound in the prenatal exclusion of primary microcephaly. Gynecol Invest. 1974, 5: 311-316.
Bartolucci L: Biparietal diameter of the skull and fetal weight in the second trimester: an allometric relationship. Am J Obstet Gynecol. 1975, 122: 439-445.
Kurtz AB, Wapner RJ, Kurtz RJ, Dershaw DD, Rubin CS, Cole-Beuglet C, Goldberg BB: Analysis of biparietal diameter as an accurate indicator of gestational age. J Clin Ultrasound. 1980, 8: 319-326.
Johnson ML, Dunne MG, Mack LA, Rashbaum CL: Evaluation of fetal intracranial anatomy by static and real-time ultrasound. J Clin Ultrasound. 1980, 8: 311-318.
Hadlock FP, Deter RL, Harrist RB, Park SK: Fetal head circumference: relation to menstrual age. AJR Am J Roentgenol. 1982, 138: 649-653.
Filly RA, Cardoza JD, Goldstein RB, Barkovich AJ: Detection of fetal central nervous system anomalies: a practical level of effort for a routine sonogram. Radiology. 1989, 172: 403-408.
Malinger G, Katz A, Zakut H: Transvaginal fetal neurosonography. Supratentorial structures. Isr J Obstet Gynecol. 1993, 4: 1-5.
Timor-Tritsch IE, Monteagudo A: Transvaginal fetal neurosonography: standardization of the planes and sections by anatomic landmarks. Ultrasound Obstet Gynecol. 1996, 8: 42-47. 10.1046/j.1469-0705.1996.08010042.x.
Girard N, Raybaud C, Gambarelli D, Figarella-Branger D: Fetal brain MR imaging. MRI Clin N Am. 2001, 9: 19-56.
Lev D, Lerman-Sagie T, Zakalkah N, Vinkler C, Yanoov-Sharav M, Ben-Sira L, Kidron D, Glezerman M, Malinger G: The fetal neurology clinic. A multidisciplinary approach for the treatment of fetuses with suspected nervous system pathology. Eur J Hum Genet. 2002, 10 (S1): 274-
Volpe JJ: Neurology of the newborn. 1995, Philadelphia: WB Saunders
Malinger G, Lerman-Sagie T, Watemberg N, Rotmensch S, Lev D, Glezerman M: A normal second-trimester ultrasound does not exclude intracranial structural pathology. Ultrasound Obstet Gynecol. 2002, 20: 51-56. 10.1046/j.1469-0705.2002.00743.x.
Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB: Classification system for malformations of cortical development: update 2001. Neurology. 2001, 57: 2168-2178.
Sarnat HB: Molecular genetic classification of central nervous system malformations. J Child Neurol. 2000, 15: 675-687.
Chervenak FA, Jeanty P, Cantraine F, Chitkara U, Venus I, Berkowitz RL, Hobbins JC: The diagnosis of fetal microcephaly. Am J Obstet Gynecol. 1984, 149: 512-517.
Jones KL: Smith's recognizable patterns of human malformation. 1997, WB Saunders Company, Philadelphia, 776-777.
Reece EB, Goldstein I: Three-level view of fetal brain imaging in the prenatal diagnosis of congenital anomalies. J Matern Fetal Med. 1999, 8: 249-252. 10.1002/(SICI)1520-6661(199911/12)8:6<249::AID-MFM3>3.0.CO;2-2.
Bromley B, Benacerraf BR: Difficulties in the diagnosis of microcephaly. J Ultrasound Med. 1995, 14: 303-306.
Goldstein I, Reece EA, Pilu G, O'Connor TZ, Lockwood CJ, Hobbins JC: Sonographic assessment of the fetal frontal lobe: a potential tool for prenatal diagnosis of microcephaly. Am J Obstet Gynecol. 1988, 158: 1057-1062.
Pilu G, Falco P, Milano V, Perolo A, Bovicelli L: Prenatal diagnosis of microcephaly assisted by vaginal sonography and power Doppler. Ultrasound Obstet Gynecol. 1998, 11: 357-360. 10.1046/j.1469-0705.1998.11050357.x.
Malinger G, Lerman-Sagie T, Achiron R, Lipitz S: The subarachnoid space: normal fetal development as demonstrated by transvaginal ultrasound. Prenatal Diagn. 2000, 20: 890-893. 10.1002/1097-0223(200011)20:11<890::AID-PD945>3.0.CO;2-Z.
den Hollander NS, Wessels MW, Los FJ, Ursem NT, Niermeijer MF, Wladimiroff JW: Congenital microcephaly detected by prenatal ultrasound: genetic aspects and clinical significance. Ultrasound Obstet Gynecol. 2000, 15: 282-287. 10.1046/j.1469-0705.2000.00092.x.
Dahlgren L, Wilson RD: Prenatally diagnosed microcephaly: a review of etiologies. Fetal Diagn Ther. 2001, 16: 323-326. 10.1159/000053935.
Dobyns WB: Primary microcephaly: new approaches for an old disorder. Am J Med Genet. 2002, 112: 315-317. 10.1002/ajmg.10580.
Petersson S, Pedersen NL, Schalling M, Lavebratt C: Primary megalencephaly at birth and low intelligence level. Neurology. 1999, 53: 1254-1259.
Lorber J, Priestley BL: Children with large heads: a practical approach to diagnosis in 557 children, with special reference to 109 children with megalencephaly. Dev Med Child Neurol. 1981, 23: 494-504.
Cohen MM: Mental deficiency, alterations in performance, and CNS abnormalities in overgrowth syndromes. Am J Med Genet. 2003, 117C (1): 49-56. 10.1002/ajmg.c.10013.
DeRosa R, Lenke RR, Kurczynski TW, Perssutte WH, Nemes JM: In utero diagnosis of benign fetal macrocephaly. Am J Obstet Gynecol. 1989, 161: 690-692.
Chen CP, Lin SP, Chang TY, Chiu NC, Shih SL, Lin CJ, Wang W, Hsu HC: Perinatal imaging findings of inherited Sotos syndrome. Prenat Diagn. 2002, 22: 887-892. 10.1002/pd.433.
Clementi M, Milani S, Mammi I, Boni S, Monciotti C, Tenconi R: Neurofibromatosis type 1 growth charts. Am J Med Genet. 1999, 87: 317-323. 10.1002/(SICI)1096-8628(19991203)87:4<317::AID-AJMG7>3.0.CO;2-X.
Opitz JM, Weaver DW, Reynolds JF: The syndromes of Sotos and Weaver: reports and review. Am J Med Genet. 1998, 79: 294-304. 10.1002/(SICI)1096-8628(19981002)79:4<294::AID-AJMG12>3.0.CO;2-M.
Naqvi S, Cole T, Graham JM: Cole-Hughes macrocephaly syndrome and associated autistic manifestations. Am J Med Genet. 2000, 94: 149-152. 10.1002/1096-8628(20000911)94:2<149::AID-AJMG7>3.0.CO;2-#.
DiLiberti JH: Inherited macrocephaly-hamartoma syndromes. Am J Med Genet. 1998, 79: 284-290. 10.1002/(SICI)1096-8628(19981002)79:4<284::AID-AJMG10>3.0.CO;2-N.
Aicardi J: Neurocutaenous diseases and syndromes. In Diseases of the nervous system in childhood. Edited by: Aicardi J. 1998, London: Mac Keith Press, 131-153.
Sonigo P, Elmaleh A, Fermont L, Delezoide AL, Mirlesse V, Brunelle F: Prenatal MRI diagnosis of fetal cerebral tuberous sclerosis. Pediatr Radiol. 1996, 26: 1-4.
Sgro M, Barozzino T, Toi A, Johnson J, Sermer M, Chitayat D: Prenatal detection of cerebral lesions in a fetus with tuberous sclerosis. Ultrasound Obstet Gynecol. 1999, 14: 356-359. 10.1046/j.1469-0705.1999.14050356.x.
Mackay MT, Becker LE, Chuang SH, Otsubo H, Chuang NA, Rutka J, Ben-Zeev B, Snead OC, Weiss SK: Malformations of cortical development with balloon cells: Clinical and radiologic correlates. Neurology. 2003, 60: 580-587.
Flores-Sarnat L: Hemimegalencephaly: part 1. Genetic, clinical, and imaging aspects. J Child Neurol. 2002, 17: 373-384.
Sasaki M, Hashimoto T, Shimada M, Iinuma K, Fushiki S, Takano T, Oka E, Kondo I, Miike T: Nation-wide survey on hemimegalencephaly in Japan. No To Hattatsu. 2000, 32: 255-260.
Sandri F, Pilu G, Dallacasa P, Foschi F, Salvioli GP, Bovicelli L: Sonography of unilateral megalencephaly in the fetus and newborn infant. Am J Perinatol. 1991, 8: 18-24.
Ramirez M, Wilkins I, Kramer L, Slopis J, Taylor SR: Prenatal diagnosis of unilateral megalencephaly by real-time ultrasonography. Am J Obstet Gynecol. 1994, 170: 1384-1385.
Hafner E, Bock W, Zoder G, Schuchter K, Rosen A, Plattner M: Prenatal diagnosis of unilateral megalencephaly by 2D and 3D ultrasound: a case report. Prenat Diagnosis. 1999, 19: 159-162. 10.1002/(SICI)1097-0223(199902)19:2<159::AID-PD470>3.3.CO;2-7.
Chung SN, Rosemond RL, Graham D: Prenatal diagnosis of a fetal intracranial tumor. J Ultrasound Med. 1998, 17: 521-523.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Malinger, G., Lev, D. & Lerman-Sagie, T. Assessment of fetal intracranial pathologies first demonstrated late in pregnancy: cell proliferation disorders. Reprod Biol Endocrinol 1, 110 (2003). https://doi.org/10.1186/1477-7827-1-110
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-110