Abstract
Background
Stanniocalcin 2 (STC2) is a secreted protein activated by (PKR)-like Endoplasmic Reticulum Kinase (PERK) signalling under conditions of ER stress in vitro. Over-expression of STC2 in mice leads to a growth-restricted phenotype; however, the physiological function for STC2 has remained elusive. Given the relationship of STC2 to PERK signalling, the objective of this study was to examine the role of STC2 in PERK signalling in vivo.
Results
Since PERK signalling has both physiological and pathological roles in the pancreas, STC2 expression was assessed in mouse pancreata before and after induction of injury using a cerulein-induced pancreatitis (CIP) model. Increased Stc2 expression was identified within four hours of initiating pancreatic injury and correlated to increased activation of PERK signalling. To determine the effect of STC2 over-expression on PERK, mice systemically expressing human STC2 (STC2Tg) were examined. STC2Tgpancreatic tissue exhibited normal pancreatic morphology, but altered activation of PERK signalling, including increases in Activating Transcription Factor (ATF) 4 accumulation and autophagy. Upon induction of pancreatic injury, STC2Tgmice exhibited limited increases in circulating amylase levels and increased maintenance of cellular junctions.
Conclusions
This study links STC2 to the pathological activation of PERK in vivo, and suggests involvement of STC2 in responding to pancreatic acinar cell injury.
Similar content being viewed by others
Background
The stanniocalcin (STC) family of secreted glycoproteins consists of two members - STC1 and STC2 - that have 35% conserved identity and conservation of exon/intron boundaries, indicating that they are paralogs [1]. STC was first discovered in fish and shown to be involved in calcium homeostasis through effects on calcium influx [2, 3]. Although terrestrial mammals rarely experience hypercalcemia, alterations in subcellular compartmentalization of Ca2+ are found under pathological conditions such as the accumulation of improperly folded proteins. STC2 expression is upregulated upon induction of hypoxic or endoplasmic reticulum (ER) stress in N2a neuroblastoma cells [4]. This increase in Stc2 expression was reliant on the concomitant increase of Activating Transcription Factor (ATF4) [5], a protein integral to (PKR)-like endoplasmic reticulum kinase (PERK) signalling, which is part of the unfolded protein response (UPR). To date, no relationship between PERK signalling and STC2 activation has been identified in vivo.
The UPR is activated when ER homeostasis is disrupted. Alterations to proper functioning of ER machinery arise when protein load exceeds folding capacity, such as when luminal ER Ca2+ concentrations are perturbed. The UPR consists of three signalling pathways that are individually transduced by ATF6, inositol-requiring enzyme 1 (IRE1) or PERK [6, 7]. Each transducer is maintained in a repressed state by binding of the ER chaperone protein glucose regulated protein 78 (GRP78/BiP) [6, 7]. When unfolded proteins accumulate, they bind to GRP78/BiP resulting in its release from the UPR transducers [8, 9]. In the case of PERK, dissociation of GRP78/BiP leads to its homodimerization and autoactivation [10]. Activation of PERK results in phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) [11], limiting its ability to contribute to the protein translational complex, thereby inhibiting global protein translation and alleviating the protein load on the cell [12]. Phosphorylation of eIF2α also selectively upregulates translation of mRNAs from short upstream open reading frames [13]. The predominant target mRNA for peIF2α is Atf4, which subsequently increases the expression of the transcriptional repressor Atf3, and growth arrest and DNA damage-inducible (Gadd) 34 [14, 15]. GADD34 (alternatively known as Myd116; myeloid differentiation, primary response gene 116, or Ppp1r15a; protein phosphatase 1, regulatory [inhibitor] subunit 15A) negatively regulates PERK signalling by combining with protein phosphatase 1 to dephosphorylate eIF2α, thereby restoring general translation [16, 17].
PERK is expressed in the exocrine and endocrine pancreas under normal conditions [11, 18] and Perk-/-mice experience deterioration of glycemic control and exocrine insufficiency over time [19]. Exocrine specific deletion of PERK revealed cellular disorganization and degranulation, increased serum amylase levels and increased cell death [20]. Our laboratory identified rapid activation of PERK signalling following induction of pancreatitis by supramaximal secretagogue stimulation and correlated decreased activation of the pathway in mice that exhibit increased sensitivity to pancreatic injury [21]. Together, these observations indicate that PERK signalling has important roles in both the physiology and pathology of the exocrine pancreas.
Given the relationship between PERK and STC2 in vitro, the objectives of this study were to (1) determine if a similar relationship exists in the exocrine pancreas and (2) gain insight into a role for STC2 as part of the UPR. Our results revealed a correlation of Stc2 expression with PERK signalling in vivo only after initiating pancreatic injury. Transgenic over-expression of STC2 in mice (STC2Tg) [22] resulted in altered PERK signalling and decreased signs of acinar cell damage associated with cerulein-induced pancreatitis (CIP). These observations suggest that STC2 is linked to PERK signalling in acinar cells and may have a role in limiting damage during pancreatic injury.
Results
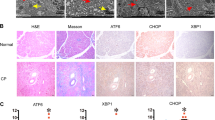
To determine if PERK signalling is active in pancreatic tissue, we performed IF with an antibody that recognizes only the phosphorylated form of PERK (pPERK). The accumulation of pPERK was restricted to a tight apical portion of the acinar cells (Figure 1A, green, arrows) typical of where Ca2+ is released during the process of regulated exocytosis. RT-PCR revealed no expression of Stc2 in control pancreata (Figure 1C). Similar results were obtained in control pancreata for Atf3, another downstream target of PERK signalling suggesting that PERK activation under resting conditions does not activate all downstream mediators of PERK signalling. Our laboratory has shown that activation of PERK signalling increases during pancreatic injury [21]. To determine if the pathological activation of PERK leads to increased STC2 expression, pancreatic injury was initiated by cerulein-induced pancreatitis (CIP). Interestingly, CIP treatment led to a change in pPERK localization within four hours, with active PERK exhibiting a more diffuse and basally-restricted pattern of accumulation (Figure 1B, green, arrow), similar to the changes observed for acinar cell exocytosis under these conditions [23]. This change in localization was accompanied by increased Stc2 and Atf3 mRNA accumulation (Figure 1C). Increased Stc2 expression was confirmed by Northern blot analysis four hours after induction of pancreatitis (Figure 1D, saline WT vs. CIP WT). While we were unable to detect STC2 protein by IF analysis (data not shown), increased accumulation of ATF3 protein was restricted to acinar cells in CIP-treated pancreatic tissue (Figure 1E, F; I = islet). To support the link between elevated Stc2 levels and injury-associated PERK activation, we examined the expression of Stc2 in Mist1-/-mice, which exhibit minimal activation of PERK during injury. Mist1 is a transcription factor required for terminal differentiation of pancreatic acinar cells, and its absence in exocrine tissue results in improper activation of UPR components both normally and during injury [21]. In sharp contrast to wild type mice, Stc2 mRNA was virtually undetectable upon CIP treatment of Mist1-/-mice (Figure 1D; Saline Mist1-/-vs. CIP Mist1-/-).
STC2 accumulation increases in pancreatic tissue following induction of pancreatic injury. (A, B) Immunofluorescence (IF) for pPERK (green) before (A) and 4 hours after (B) initiation of CIP. pPERK (arrowhead) localizes to the apical end of acinar cells under physiological conditions and more basally during CIP. Magnification bar = 13 μM. DAPI was used to counter stain nuclei. (C) RT-PCR of whole pancreatic RNA extracts from saline (S) and cerulein (C) treated mice showed increased accumulation of Stc2 and Atf3 mRNA after initiation of CIP in pancreatic tissue. Amplification of Amylase (Amy) was used as a positive control for RT reactions. (D) Northern blot analysis revealed elevated Stc2 mRNA four hours after initial cerulein (CIP) or saline (S) injection. No increases in Stc2 were observed in Mist1-/-(M-/-) pancreatic tissue under similar conditions. (E, F) IF for ATF3 expression revealed limited accumulation before CIP (E) and acinar-specific expression afterwards (F). Magnification bar = 63 μM. I = islet.
These findings suggest that STC2 is co-induced with PERK in acinar cells only after injury and may protect the pancreas in response to CIP. To determine if STC2 could alter the acinar cell response to pancreatic injury, we examined a mouse model in which STC2 is constitutively expressed (STC2Tg; [22]). IHC confirmed increased accumulation of STC2 in STC2Tgpancreata compared to wild type (WT) tissue, with STC2 accumulation in islets and exocrine tissue (Figure 2A, B). H&E histology (Figure 2C, D) indicated that morphology was not overtly altered in STC2Tgpancreatic tissue. IF staining for pancreatic differentiation markers MIST1 (exocrine, Figure 2E, F) and PDX1 (endocrine, Figure 2G, H) showed similar spatial localization and GLUT-2 accumulation was localized to the cell membrane of endocrine cells in both genotypes (Figure 2I, J). Western blot analysis confirmed equivalent levels of these markers, as well as amylase, between WT and STC2Tgtissue (Figure 2K).
Overall morphology and differentiation does not appear to be affected in STC2Tgmice. (A, B) IHC for STC2 in WT (A) and STC2Tg(B) pancreatic islets shows that STC2 accumulates in both acinar (*) and islet (I) tissue only in the STC2Tgmice. Magnification bar = 27 μm. Tissue was counterstained with hematoxylin to reveal tissue morphology. (C, D) Hematoxylin and eosin staining comparing general histology of pancreatic tissue in WT (C) or STC2Tg(D) pancreata. Magnification bar = 60 μM; I - islet. IF analysis for MIST1 (E, F), PDX1 (G, H) or GLUT2 (I, J) in WT (E, G, I) or STC2Tg(F, H, J) pancreata. Magnification bar = 54 μm. (K) Representative Western blot analysis for pancreatic differentiation markers amylase, MIST1, PDX1 and GLUT2.
We next examined PERK signalling in STC2Tgpancreatic tissue. PERK activation results in phosphorylation of eIF2α and translation of ATF4 [24, 25]. ATF4 is required for expression of Gadd34, Atf3 and Stc2 [5, 14, 16]. Western blot analysis of WT and STC2Tgpancreatic tissue revealed decreased phosphorylation of both PERK and eIF2α (Figure 3A, B; n = 4 animals; p < 0.01) in STC2Tgtissue. Surprisingly, ATF4 accumulated to higher levels in the STC2Tgpancreas, suggesting dysregulation of PERK signalling. We next examined XBP1, another mediator of the UPR that is outside of the PERK signalling pathway. Xbp1 mRNA is targeted by the endonuclease activity of IRE1 leading to production of an active, spliced (s) form of XBP1 [26]. Western blot analysis revealed a consistent decreased in sXBP1 accumulation in STC2Tgmice indicating that elevated STC2 levels may also affect the other UPR pathways.
Systemic over-expression of STC2 ( STC2Tg) alters the PERK signalling pathway in pancreatic tissue. (A) Representative Western blot analysis for mediators of PERK signalling: pPERK, peIF2α, total (t) eIF2α, ATF4 and sXBP1 in wild type (WT) and STC2Tg(Tg), revealed decreased levels of pPERK, peIF2α and sXBP1, and increased amounts of ATF4 in STC2Tgmice. (B) Densitometry revealed that the ratio of peIF2α to total eIF2α is decreased in STC2Tgextracts relative to WT tissue (n = 3 animals; *p < 0.05). (C) Similar western blot analysis revealed increased accumulation of GADD34 and decreased accumulation of calcineurin in STC2Tgpancreatic protein extracts. No difference in calregulin accumulation was observed. Molecular weights (kDa) are provided.
To understand why activation of PERK and eIF2α appear reduced in STC2Tgtissue, we examined regulators of these two proteins. The phosphorylation of eIF2α is reversed by GADD34 through its interaction with protein phosphatase 1 (PP1) and recent evidence indicates that calcineurin binds PERK and stimulates its autophosphorylation [27]. In STC2Tgpancreatic tissue, the levels of GADD34 protein were significantly higher than wild type tissue (Figure 3C). Conversely, STC2Tgcalcineurin levels were considerably lower than WT counterparts. No changes in the ER resident protein calregulin were observed between genotypes (Figure 3C). Based on these results, it appears that over-expression of STC2 affected expression of mediators and feedback mechanisms in PERK signalling.
Alterations to PERK/ATF4 signalling should have an effect on cell autophagy, a long-term response to ER stress, which is regulated by ATF4 during hypoxia [28]. Western blot analysis followed by densitometry revealed a significant increase in the cleaved form of the autophagy marker myosin associated protein 1 light chain 3 (LC3) in STC2Tgpancreatic tissue (Figure 4A, B; n = 4 animals p < 0.01) consistent with increased accumulation of ATF4 in STC2Tgmice. IF analysis corroborated increased autophagy as punctate LC3 staining is notably increased in STC2Tgexocrine tissue (Figure 4D) when compared to WT tissue (Figure 4C).
STC2Tgacinar cells have increased autophagy. (A) Representative Western blot analysis for LC3 I and LC3 II accumulation in wild type (WT) and STC2Tg(Tg) pancreatic extracts revealed increased accumulation of cleaved LC3 II which was (B) quantified by densitometry (n = 3 animals; *p < 0.05). IF for LC3 cellular accumulations in (C) WT and (D) STC2Tgpancreatic sections showed increased autophagic vesicles (arrowhead) in STC2Tgpancreatic tissue. Magnification bar = 10 μm.
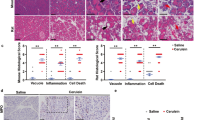
The alterations in PERK signalling and cell autophagy suggested that STC2Tgmice should have altered sensitivity to pancreatic injury. Four hours after the induction of pancreatitis, we compared circulating serum amylase levels and tissue edema between WT and STC2Tgmice (Figure 5). While serum amylase levels were moderately higher in saline-treated STC2Tgmice, the proportional increase in serum amylase levels following CIP was significantly lower in STC2Tgmice when compared to WT mice, indicative of decreased sensitivity to pancreatic insult (Figure 5A; n = 4 animals; p < 0.05). However, no difference was observed in tissue edema after CIP between genotypes (Figure 5B). We next examined the effects of pancreatic injury at the cellular level by examining enzyme activation and cell structures. Western blot analysis of procarboxypeptidase (CPA) activation revealed reduced levels of active CPA in CIP STC2Tgpancreatic tissue when compared to WT cerulein-treated tissue (Figure 5C, D). Interestingly, analysis of cell junction proteins showed continued expression in STC2Tgtissue following CIP that was not evident in WT tissue. In WT acinar cells, the expression of β-catenin decreased to negligible levels, indicative of a loss in adherens junctions between acinar cells as expected (Figure 5E, F). However, β-catenin remained readily detectable in STC2Tgpancreatic tissue after induction of CIP (Figure 5G, H). Similarly, punctate connexin32 (Cx32) accumulation was lost in WT (Figure 5I, J) but not in STC2Tgacini (Figure 5K, L) suggesting a greater disruption in gap junction complexes in WT acinar cells. Western blot analysis corroborated IF data with CIP leading to a more dramatic loss of β-catenin and Cx32 accumulation in WT extracts compared to STC2Tgextracts after four hours (Figure 5M) indicating that intercellular complexes were protected in STC2Tgmice, suggestive of reduced cellular damage. Combined, these findings suggest that acinar cell integrity is maintained to a higher degree when acini are exposed to higher than normal levels of STC2.
STC2 over-expression reduces severity of acinar cell damage during cerulein-induced pancreatic injury. (A) Analysis of serum amylase levels four hours into CIP treatment show that serum amylase levels rise approximately 3 fold in WT mice when compared to saline (•) whereas the increase in STC2Tgmice is significantly lower at 2-fold (■; * p < 0.01). (B) Tissue edema analysis revealed significant increases for both WT and STC2Tgtissue 4 hours into CIP (black bars) when compared to saline controls (white bars; n = 4 animals). No difference between genotypes was observed. (C) Representative Western blot for procarboxypeptidase (47 kDa) and carboxypeptidase (CPA; 33 kDa) or trypsinogen four hours after initial saline (Sal.) or cerulein (CIP) injections showed decreased accumulation of activate CPA in STC2Tgmice four hours into CIP. Trypsinogen levels do not differ between genotypes. (D) Quantification by densitometry (graph) comparing the ratio of cleaved (active; 33 kDa) to total CPA revealed a significant increase in WT tissue and, to a significantly lower extent, in STC2Tgtissue (n = 4 animals, letters represent statistically different values; p < 0.05). IF analysis for β-catenin (E-H) or Cx32 (I-L) in wild type (WT) or STC2Tg(Tg) mice four hours after initial injection of saline (E, G, I, K) or cerulein (F, H, J, L) showing decreased accumulation only in WT tissue during CIP. (M) Representative Western blot analysis on pancreatic protein extracts 4 hours after initial saline or cerulein (CIP) injection confirm decreased β-catenin and Cx32 accumulation only in WT-CIP treated tissue (n = 4).
As a final marker for CIP severity, we assessed the amount of acinar cell necrosis and apoptosis within the two genotypes. The ratio of necrosis to apoptosis correlates to CIP severity [29]. Visualization of apoptosis using TUNEL staining revealed increased apoptosis in both WT (Figure 6A, B; arrowheads) and STC2Tg(Figure 6C, D; arrowheads) pancreatic tissue after induction of pancreatitis. Quantification of TUNEL positive cells revealed slight decreases in acinar cell apoptosis in STC2Tgtissue (Figure 6E). We next examined necrosis through IF for High mobility group protein b1 (HMGB1), a protein that interacts with DNA, but upon necrotic stimulus, is released from the nucleus and the cell to participate in inflammatory responses [30, 31]. Cytoplasmic staining of HMGB1 is routinely used as an indicator of cell necrosis. HMGB1 is localized to the nucleus in both WT (Figure 6F) and STC2Tg(Figure 6H) saline treated tissue. After induction of pancreatitis, punctate, cytoplasmic HMBG1 staining was observed in distinct patches of cells only in WT tissue (Figure 6G). All STC2Tgtissue analysed was devoid of punctate cytoplasmic HMGB1 staining (Figure 6I). This analysis reveals that the levels of both apoptosis and necrosis are decreased in STC2Tgtissue, indicative of less tissue damage after CIP.
Cell death is decreased during CIP in STC2Tgtissue. TUNEL analysis revealed apoptosis (arrowheads) during CIP (B, D) but not saline injections (A, C) in WT (A, B) and STC2Tg(C, D) pancreata. Magnification bar = 45 μM. (E) Quantification of TUNEL positive cells in WT and STC2Tgtissue (n = 4 animals; letters indicate significant differences). (F-I) HMGB1 IF to identify necrosis revealed nuclear localization in saline conditions for WT (F; n = 3 animals) and STC2Tg(H; n = 3 animals) tissue. After induction of pancreatitis, patches of cells with cytoplasmic, punctate HMGB1staining, indicative of necrosis, were observed in WT tissue (G; n = 4 animals) but not in STC2Tgtissue (I; n = 4 animals). Magnification bar = 34 μM
Discussion
STC2 is a secreted protein that, when expressed to high levels in mice, has profound effects leading to growth restriction [22]. However, ablation of Stc2 in mice results in limited phenotypic alterations suggesting that STC2 functions may fall outside normal physiology [32]. In support of such a hypothesis, in vitro analysis has identified increased Stc2 accumulation following treatment with thapsigargin or tunicamycin, agents that activate ER stress pathways [5]. STC2 is also up-regulated in response to hypoxic insult and is expressed to higher levels in many tumor-derived cell lines [4, 33]. This suggests that STC2 may be involved in responding to external challenges that activate cell stress pathways. In this study, we have identified increased Stc2 accumulation within four hours of inducing pancreatic injury that correlated with activation of the PERK signalling. Importantly, forced expression of STC2 in mice altered PERK signalling and reduced cellular damage in response to pancreatic injury. These results suggest a role for STC2 responding to and mediating the effects of cell stress in pancreatic tissue.
Studies assessing the expression of Stc2 have revealed a limited expression pattern in adult tissues. In the human pancreas, Stc2 is specifically expressed in alpha cells of the islets of Langerhans and not in acinar tissue [1]. Our mRNA analysis revealed murine pancreatic expression of Stc2 only after induction of pancreatitis by supramaximal secretagogue stimulation. Unfortunately, IHC on CIP-treated tissue sections did not detect STC2 protein (data not shown) indicating that STC2 may be expressed at lower than detectable levels or that it is rapidly secreted into the circulation. While we have not definitively identified acini as the origin of Stc2 expression, Atf3, a downstream mediator of PERK signalling, accumulated only in acinar cells during CIP, suggesting that CIP treatment leads to a cell autonomous activation of Stc2. As observed in HEK293 and N2a neuroblastoma cells, the increased accumulation of Stc2 is consistent with activation of PERK signalling in response to injury [5]. Induction of Stc2 mRNA levels was not observed after CIP in Mist1-/-mice, which do not activate PERK or ATF3, and experience increased severity of CIP [21]. These findings support the activation of Stc2 as a protective mechanism, potentially as a mediator of the UPR.
While previous studies have correlated increased expression of STC2 as part of the UPR, the effects of STC2 on PERK signalling have not been examined. We have now shown that systemically high levels of STC2 reduce PERK activation. STC2Tgmice showed decreased phosphorylation of PERK and eIF2α in pancreatic tissue suggesting that continued exposure to STC2 may lead to activation of negative feedback mechanisms. Since no change in total eIF2α is observed, this difference is peIF2α is specific to the phosphorylation event and not decreased eIF2α accumulation. While we showed a decrease in pPERK, total PERK levels were not assessed and this could account for possible differences in activation of the pathways as well.
We have identified at least two effectors of the PERK signalling pathway that were changed in STC2Tgmice. First, GADD34, which combines with protein phosphatase 1 to reduce eIF2α phosphorylation [17], accumulated to higher levels. Second, we observed significantly decreased accumulation of calcineurin, which stimulates PERK autophosphorylation, thereby increasing activity [27]. Whether these changes are reflective of a direct role for STC2 in their regulation or a response to other events caused by over exposure to STC2 is unclear. However, the altered expression of these factors suggests a role for STC2 in regulating Ca2+ homeostasis since both calcineurin and PERK are affected by altered Ca2+ sequestration in the ER. These data suggest that constitutive exposure to STC2 results in depression of the activity of key factors regulating the UPR.
In light of decreased PERK and eIF2α activation, it was surprising that increased accumulation of ATF4 was observed in the pancreas of STC2Tgmice. Phosphorylation of eIF2α is known to stimulate translation of Atf4 mRNA, leading to increased ATF4 protein accumulation. However, increased ATF4 expression, and the subsequent increase in GADD34 could be mediated by increased stability of the ATF4 protein. Recent studies have shown that ATF4 is stabilized by interaction with p300 through inhibition of ubiquitination [34], and that association with p300/CBP enhances ATF4 transcriptional ability [34, 35]. It is possible that in STC2Tgmice, stabilization of ATF4 is enhanced regardless of decreased phosphorylation of eIF2α, accounting for its increased expression. Although there is little known about non-peIF2α related transcriptional activation of ATF4, increased transcriptional activation by a yet to be identified factor cannot be ruled out.
Figure 7 summarizes a potential role for STC2 in the UPR, where over-expression of STC2 negatively affects calcineurin expression, (Figure 7, dotted line), which would lead to inhibition of calcineurin-dependent phosphorylation of PERK. Decreased phosphorylation of PERK results in decreased phosphorylation of eIF2α, as observed in STC2Tgpancreatic tissue. Although the increased expression of ATF4 in STC2Tgtissue is not intuitive considering the phosphorylation of both PERK and eIF2α, it reveals a potential role for STC2 in ATF4 regulation (Figure 7, dotted arrow). This increase in ATF4 expression leads to increased GADD34 expression, which acts as a negative feedback mechanism to contribute to decreased phosphorylation of eIF2α [16].
Schematic pathway showing a possible role for STC2 in affecting the PERK signalling pathway. The increased accumulation of ATF4 leads to increased expression of GADD34, ATF3 and STC2. Elevated levels of STC2 can alter both PERK phosphorylation and ATF4 levels through as yet undetermined mechanisms (dashed arrows), as well as promote reduced damage during pancreatic injury. Whether this protective effect of STC2 is a normal function of the protein needs to be clarified.
As would be expected from increased ATF4 expression, STC2Tgmice also exhibited an increased level of autophagy, a downstream consequence of elevated ATF4 levels. Autophagy is important for maintenance of cellular homeostasis through balancing synthesis and degradation or recycling of cellular components. Independent studies have indicated that either phosphorylation of eIF2α or expression of ATF4 is required for induction of autophagy in vitro [28, 36, 37]. The increased expression of ATF4 in STC2Tgpancreatic tissue may be the cause of increased autophagy. Interestingly, we did not observe increased acinar cell apoptosis (data not shown), which is another downstream result of increased UPR. Therefore, it appears that only certain parts of the UPR have been activated in STC2Tgmice.
Whether STC2 is protective in nature is still unclear since studies have shown it has both protective [38] and detrimental roles [4, 33] in cancer progression in vitro. Our results suggest a protective advantage following exocrine pancreatic injury in vivo. STC2Tgmice exhibit a decreased proportional increase in serum amylase levels, increased maintenance of cellular junctions, decreased apoptosis and necrosis and decreased activation of CPA in exocrine pancreatic tissue, all suggestive of decreased pancreatitis severity. In addition, the enhanced autophagy observed in STC2Tgacinar tissue would facilitate more rapid degradation of resident digestive enzymes upon injury, thereby limiting damage to cellular contents. This would account for increased maintenance of cellular junctions. However, to truly understand the role of STC2 in pancreatic injury, further experiments in mice lacking Stc2 mice should be performed.
Conclusions
In conclusion, this is the first study to correlate PERK signalling and STC2 in vivo, and suggests two novel roles for STC2, as a downstream effector of PERK signalling and possible protective factor during pancreatic injury. This is also the first time that STC2 has been shown to affect proteins that regulate Ca2+-mediated response in the cell.
Methods
Mouse handling
For characterization of Stc2 expression during cerulein-induced pancreatitis (CIP), wild type and Mist1-/-mice were maintained on a C57/Bl6 background. For analysis of pancreatitis severity, STC2Tgand wild type mice are maintained on a C57/Bl6 × CBA background. Mice carrying a targeted ablation of the Mist1 gene (Mist1-/-) [39] and transgenic mice that express STC2 (STC2Tg) from the CMV promoter + chicken β-actin promoter [22], have previously been described. All experiments were approved by the Animal Care Committee at the University of Western Ontario (Protocol #116-2008) and mice handled according to regulations stipulated by the Canadian Council on Animal Care.
Induction of pancreatitis
To induce pancreatic injury, 2-4 month-old female mice were given up to seven hourly intraperitoneal injections of cerulein (50 μg/kg body weight; Sigma-Aldrich). As a control, mice were injected with 0.9% saline. Mice were sacrificed 1, 4 or 8 hours after initial cerulein injection and pancreatic tissue from each was immediately processed to isolate RNA, protein, and tissue sections for histological or immunohistochemical analysis. To assess serum amylase, blood was obtained via cardiac puncture, placed on ice for 20 minutes and centrifuged at 4°C, 2500 rpm for 15 minutes. Serum amylase was quantified using a Phadebas amylase assay (Magle Life Sciences, Lund, Sweden) as per manufacturer's instructions.
Tissue preparation and histology
Pancreatic tissue was either directly embedded in OCT or incubated in formalin for 24 hours followed by paraffin embedding. For morphological analysis, paraffin embedded tissue was sectioned to 6 μM and stained with hematoxylin and eosin. Immunofluorescent (IF) analysis was performed on fresh frozen sections as described previously [40]. Primary antibodies and their dilutions are listed in Table 1. Following primary antibody incubation and PBS washes, sections were incubated for 1 hour with the secondary antibodies of either anti-rabbit FITC or anti-mouse TRITC (1:250; Sigma-Aldrich). After further washing, sections were incubated with 4',6-diamidino-2-phenylindole (DAPI; 1:1000) and slides were mounted with Vectashield (Vector Laboratories, Burlingame CA, USA). Histological staining was visualized with a Leica DMIOS upright microscope and images were captured using OpenLab 4.0.3 Software (PerkinElmer, Waltham, MA, USA).
TUNEL analysis
TUNEL analysis was used to identify apoptotic cells using the In situ Cell Death Detection kit (Roche, Laval, QC, Canada) following manufacturer's instructions. Briefly, frozen sections were fixed, washed and permeabilized before labelling with TUNEL reaction mixture for 60 minutes. After washing, sections were counterstained with DAPI, coverslipped and visualized using a Leica DMIOS upright microscope and images were captured using OpenLab 4.0.3 Software (PerkinElmer, Waltham, MA, USA). Analysis of percent apoptotic cells was carried out by comparing TUNEL positive cells to total cell number. Cell counts based on DAPI were done using Image J software, and TUNEL positive cells were determined manually. Ten random fields of view from 4 sections per animal were used for analysis.
RNA isolation, Northern blotting and Real-Time RT-PCR
RNA was isolated from the splenic portion of the pancreas and processed using TRIzol (Invitrogen, Burlington, ON, Canada) as described [21]. Northern blotting was performed as previously described [39]. Thirty μg of RNA was resolved by electrophoresis on a 1% agarose/formaldehyde gel and blotted onto Hybond membrane (GE Healthcare, Baie d'Urfe, QC, Canada). Membranes were hybridized overnight at 42°C with an α-32PdCTP radiolabelled probe for Stc2 or for 18S rRNA as a loading control.
For RT-PCR, two μg of RNA was reverse transcribed using Improm-II reverse transcriptase and random primers (Promega, Madison WI, USA). RT-PCR for Stc2, Atf3, amylase and β-actin was performed with 1 μL of cDNA and Taq DNA polymerase (Promega, Madison WI, USA).
Protein isolation and immunoblot analysis
Protein extraction was carried out to obtain both nuclear and cytoplasmic fractions. Briefly, 5 volumes of buffer CE pH 7.6 (10 mm Hepes, 60 mM KCl, 1 mM EDTA, 0.075% v/v NP-40, 1 mM DTT, 1 mM PMSF) were added to whole pancreatic tissue prior to homogenization. Homogenized cells were centrifuged at 1000 rpm, 4°C for 4 minutes. The supernatant was removed and saved as cytoplasmic extract. The nuclear pellet was gently washed with CE buffer lacking NP-40 and centrifuged again. After removal of the supernatant, the nuclear pellet was covered with 1 pellet volume of NE buffer (20 mm Tris-HCl, 420 mM NaCl, 1.5 mm MgCl2, 0.2 mM EDTA, 1 mM PMSF, 25% w/v glycerol) and the NaCl concentration was adjusted to 400 mM for the entire volume. An additional pellet volume of NE buffer was added followed by vortexing for resuspension. The extract was incubated on ice for 10 minutes with intermittent vortexing. Both cytoplasmic and nuclear extracts were centrifuged at 14,000 rpm, 4°C, for 10 minutes to pellet any debris. Nuclear extracts were used for analysis of MIST1, PDX1, XBP1 and ATF4 levels, whereas cytoplasmic extracts were used for all other immunoblot analysis.
Electrophoresis and immunoblotting were carried out as previously described [41]. For immunoblot analysis, 40 to 60 μg of pancreatic protein was resolved by SDS/PAGE and transferred to PVDF membrane (Biorad, Mississauga, ON, Canada). Blots were blocked with 5% non-fat dry milk (NFDM) and probed with primary antibodies diluted in 5% NFDM overnight at 4°C. For primary antibodies and their dilutions, see Table 1. Blots were washed three times in PBS supplemented with Tween-20 (PBS-T) then incubated with anti-rabbit or anti-mouse horseradish peroxidase conjugated secondary antibody (1:10,000 or 1:2000, Jackson Labs, Bar Harbor, ME, USA) in 5% NFDM for one hour. Blots were subjected to another series of washes in PBS-T, incubated with Western Lightning chemiluminescence substrate (Perkin Elmer, Waltham MA, USA), exposed to X-ray film (Fisher Scientific, Ottawa, ON, Canada) and developed. Denistometry was performed on immunoblot autoradiographs by analysis with a FluorChem 8800 documentation system and accompanying FluorChem 8800 software (Alpha Innotech, San Leandro CA, USA).
Statistical Analyses
All statistical analyses were performed using Graphpad Prism 4.02 (Graphpad Software, San Diego CA, USA). Carboxypeptidase cleavage and serum amylase levels were analyzed using a two-way ANOVA followed by a Bonferroni post-hoc test. Levels of phosphorylated eIF2α and LC3 cleavage were statistically analyzed using a Mann-Whitney test.
References
Moore EE, Kuestner RE, Conklin DC, Whitmore TE, Downey W, Buddle MM, Adams RL, Bell LA, Thompson DL, Wolf A, Chen L, Stamm MR, Grant FJ, Lok S, Ren H, De Jongh KS: Stanniocalcin 2: characterization of the protein and its localization to human pancreatic alpha cells. Horm Metab Res. 1999, 31 (7): 406-414. 10.1055/s-2007-978764.
Flik G, Labedz T, Neelissen JA, Hanssen RG, Wendelaar Bonga SE, Pang PK: Rainbow trout corpuscles of Stannius: stanniocalcin synthesis in vitro. The American journal of physiology. 1990, 258 (5 Pt 2): R1157-1164.
Sundell K, Bjornsson BT, Itoh H, Kawauchi H: Chum salmon (Oncorhynchus keta) stanniocalcin inhibits in vitro intestinal calcium uptake in Atlantic cod (Gadus morhua). J Comp Physiol B. 1992, 162 (6): 489-495.
Law AY, Wong CK: Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp Cell Res. 2009, 316 (3): 466-476.
Ito D, Walker JR, Thompson CS, Moroz I, Lin W, Veselits ML, Hakim AM, Fienberg AA, Thinakaran G: Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties. Molecular and cellular biology. 2004, 24 (21): 9456-9469. 10.1128/MCB.24.21.9456-9469.2004.
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D: Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000, 2 (6): 326-332. 10.1038/35014014.
Okamura K, Kimata Y, Higashio H, Tsuru A, Kohno K: Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochemical and biophysical research communications. 2000, 279 (2): 445-450. 10.1006/bbrc.2000.3987.
Freiden PJ, Gaut JR, Hendershot LM: Interconversion of three differentially modified and assembled forms of BiP. Embo J. 1992, 11 (1): 63-70.
Laitusis AL, Brostrom MA, Brostrom CO: The dynamic role of GRP78/BiP in the coordination of mRNA translation with protein processing. The Journal of biological chemistry. 1999, 274 (1): 486-493. 10.1074/jbc.274.1.486.
Liu CY, Kaufman RJ: The unfolded protein response. J Cell Sci. 2003, 116 (Pt 10): 1861-1862.
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC: Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Molecular and cellular biology. 1998, 18 (12): 7499-7509.
Harding HP, Zhang Y, Ron D: Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999, 397 (6716): 271-274. 10.1038/16729.
Hinnebusch AG: Translational regulation of yeast GCN4. A window on factors that control initiator-trna binding to the ribosome. The Journal of biological chemistry. 1997, 272 (35): 21661-21664. 10.1074/jbc.272.35.21661.
Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC: Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Molecular and cellular biology. 2004, 24 (3): 1365-1377. 10.1128/MCB.24.3.1365-1377.2004.
Ma K, Vattem KM, Wek RC: Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. The Journal of biological chemistry. 2002, 277 (21): 18728-18735. 10.1074/jbc.M200903200.
Ma Y, Hendershot LM: Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. The Journal of biological chemistry. 2003, 278 (37): 34864-34873. 10.1074/jbc.M301107200.
Novoa I, Zeng H, Harding HP, Ron D: Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001, 153 (5): 1011-1022. 10.1083/jcb.153.5.1011.
Sood R, Porter AC, Ma K, Quilliam LA, Wek RC: Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem J. 2000, 346 (Pt 2): 281-293.
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D: Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001, 7 (6): 1153-1163. 10.1016/S1097-2765(01)00264-7.
Iida K, Li Y, McGrath BC, Frank A, Cavener DR: PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007, 8: 38-10.1186/1471-2121-8-38.
Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL: Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. American journal of physiology. 2007, 292 (4): G1123-1132.
Gagliardi AD, Kuo EY, Raulic S, Wagner GF, DiMattia GE: Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005, 288 (1): E92-105.
Cosen-Binker LI, Lam PP, Binker MG, Gaisano HY: Alcohol-induced protein kinase Calpha phosphorylation of Munc18c in carbachol-stimulated acini causes basolateral exocytosis. Gastroenterology. 2007, 132 (4): 1527-1545. 10.1053/j.gastro.2007.01.042.
Vattem KM, Wek RC: Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004, 101 (31): 11269-11274. 10.1073/pnas.0400541101.
Lu PD, Harding HP, Ron D: Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004, 167 (1): 27-33. 10.1083/jcb.200408003.
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D: IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002, 415 (6867): 92-96. 10.1038/415092a.
Bollo M, Paredes RM, Holstein D, Zheleznova N, Camacho P, Lechleiter JD: Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS One. 2010, 5 (8): e11925-10.1371/journal.pone.0011925.
Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL: Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010, 29 (31): 4424-4435. 10.1038/onc.2010.191.
Bhatia M: Apoptosis versus necrosis in acute pancreatitis. American journal of physiology. 2004, 286 (2): G189-196. 10.1152/ajprenal.00224.2003.
Fortunato F, Burgers H, Bergmann F, Rieger P, Buchler MW, Kroemer G, Werner J: Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009, 137 (1): 350-360. 10.1053/j.gastro.2009.04.003. 360 e351-355
Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002, 418 (6894): 191-195. 10.1038/nature00858.
Chang AC, Hook J, Lemckert FA, McDonald MM, Nguyen MA, Hardeman EC, Little DG, Gunning PW, Reddel RR: The murine stanniocalcin 2 gene is a negative regulator of postnatal growth. Endocrinology. 2008, 149 (5): 2403-2410. 10.1210/en.2007-1219.
Law AY, Wong CK: Stanniocalcin-2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells. Exp Cell Res. 2010, 316 (20): 3425-3434. 10.1016/j.yexcr.2010.06.026.
Lassot I, Estrabaud E, Emiliani S, Benkirane M, Benarous R, Margottin-Goguet F: p300 modulates ATF4 stability and transcriptional activity independently of its acetyltransferase domain. The Journal of biological chemistry. 2005, 280 (50): 41537-41545. 10.1074/jbc.M505294200.
Cherasse Y, Maurin AC, Chaveroux C, Jousse C, Carraro V, Parry L, Deval C, Chambon C, Fafournoux P, Bruhat A: The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-regulated transcription of CHOP. Nucleic Acids Res. 2007, 35 (17): 5954-5965. 10.1093/nar/gkm642.
Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T: ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007, 14 (2): 230-239. 10.1038/sj.cdd.4401984.
Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, Harris AL: The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009, 69 (10): 4415-4423. 10.1158/0008-5472.CAN-08-2839.
Raulic S, Ramos-Valdes Y, DiMattia GE: Stanniocalcin 2 expression is regulated by hormone signalling and negatively affects breast cancer cell viability in vitro. J Endocrinol. 2008, 197 (3): 517-529. 10.1677/JOE-08-0043.
Pin CL, Rukstalis JM, Johnson C, Konieczny SF: The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol. 2001, 155 (4): 519-530. 10.1083/jcb.200105060.
Fazio EN, Pin CL: Mist1-null mice are resistant to streptozotocin-induced beta cell damage. Biochemical and biophysical research communications. 2007, 353 (3): 823-828. 10.1016/j.bbrc.2006.12.110.
Johnson CL, Kowalik AS, Rajakumar N, Pin CL: Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev. 2004, 121 (3): 261-272. 10.1016/j.mod.2004.01.003.
Acknowledgements
The authors would like to thank members of the Pin and DiMattia laboratories, especially Charis Johnson, Christin Giller and Yudith Ramos-Valdes for their support and suggestions in these studies. This work was funded by operating grants from the Canadian Institutes of Health Research (CP - MOP 53083; GD - MOP 53128). C.P. is supported by a salary award from the CIHR. E.F. is supported by a doctoral research award from the CIHR/CDHF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
EF carried out the animal studies, protein and gene expression analysis, performed the statistical analysis and drafted the manuscript. KK carried out some immunofluorescent analysis and SC performed Northern blot analysis. GD provided mouse lines and participated in its design and coordination and helped draft the manuscript. CP conceived the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fazio, E.N., DiMattia, G.E., Chadi, S.A. et al. Stanniocalcin 2 alters PERK signalling and reduces cellular injury during cerulein induced pancreatitis in mice. BMC Cell Biol 12, 17 (2011). https://doi.org/10.1186/1471-2121-12-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2121-12-17