Abstract
Human fertility regulation is a major way to control overpopulation. In this perspective, this study emphasized the in vitro effect of hydro-methanol extract of Tinospora cordifolia (TCHME) stem for spermicidal and reproductive hypo-functions using human and rat samples. Control, 0.5-, 1-, and 2-mg TCHME-charged groups were considered to assess the relevant parameters. Levels of spermiological parameters like sperm motility, viability, the integrity of plasma and acrosomal membrane, and nuclear chromatin decondensation were significantly reduced (p < 0.05) in the dose- and duration-dependent TCHME-charged groups compared to the control. The inhibitory concentration 50 (IC50) of TCHME on motile human and rat sperms were 0.8 and 0.4 mg/ml, respectively. Testicular androgenic key enzymes and antioxidant enzymes (human sperm pellet, testes, and epididymis of rat)’ activities were significantly diminished (p < 0.05), while antioxidant enzymes’ activities were significantly elevated (p < 0.05) in renal and insignificantly (p > 0.05) elevated in hepatic tissues of rat in TCHME-charged groups compared to the control. Significant elevation (p < 0.05) of thiobarbituric acid reactive substances (TBARS)’ level in human sperm pellet, testes, and epididymis of rats and significant diminution (p < 0.05) in TBARS levels of liver and kidney were observed in TCHME-charged groups. It focused that TCHME is more potent for stress imposition on reproductive tissues and sperm compared to the other tested tissues. Non-significant alterations (p > 0.05) in glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activities in the said organs of rat indicated its non-toxic effect. It highlighted that TCHME possesses spermicidal and reproductive tissue-specific effects which strengthen the possibilities of male contraceptive development from it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spermicides are chemical agents used as a barrier method of contraception by preventing sperm viability in the female reproductive tract. This activity can be achieved by degrading the plasma membrane of sperm or by inactivating the sperm to move forward [1]. Nonoxynol-9 (N-9), menfegol, and benzalkonium chloride (BZK) are commonly used as spermicidal chemical agents [2, 3]. N-9 is the most effective and widely used in this concern but has many adverse effects like human immunodeficiency virus (HIV) infections, urinary tract infections, vaginal and cervical attritions, disruption in genital organs, and inflammation [2].
There are varieties of medicinal plants having spermicidal action. Chloroform extract of Carica papaya seed showed an immobilization effect on human sperm [4]. NIM-76, a fraction isolated from neem oil, affects sperm motility and damages the sperm cell membrane [5]. Hexane fraction of hydro-ethanol composite extract of Achyranthes aspera and Stephania hernandifolia (1:3) possesses highly effective spermicidal action for vaginal contraception [6]. Therefore, different herbal plant–based phytoconstituents having spermicidal activity are now a demandable area of research due to safe, effective, and alternative strategies for family planning. India is one of the first countries to implement a family planning program in 1952 [7]. Throughout the world, India will be the most populous country in 2027 in this concern according to the World Bank [8]. This overgrowing population needs to be controlled for the economical, environmental, and societal benefits of the nation.
Greater than 35,000 plants are used as a phytomedicine for the well-being of different diseases [9]. Among these, many traditional herbal plants have evidence for acting as contraceptives. The Central Drug Research Institute, Central Council for Research in Ayurvedic Sciences under the Ministry of Health, has been looking for contraceptives based on traditional Ayurvedic knowledge [10].
Guduchi or Gulancha or Giloy, i.e., Tinospora cordifolia (Willd.), from the Menispermaceae family having multidimensional therapeutic actions, i.e., immune-modulatory, antidiabetic, antirheumatic, and anti-inflammatory, is used in Ayurveda and regarded as a highly praised herb due to its diverse ranges of pharmacological action [11]. There is limited information on T. cordifolia about its antifertility effect [12], and our pilot study also focuses on the same picture. The available information is unable to focus on the mechanism of male contraceptive effects of this plant in-depth. To unfold the mode of action for the hypo-testicular activity of this plant, the hydro-methanolic extract having maximum efficacy noted from our pilot study in this aspect has been considered.
Until now, there is no evidence referring to the spermicidal action of the stem of this plant. To develop an effective herbal spermicidal agent, we conducted this in vitro investigation to check whether the hydro-methanolic extract of T. cordifolia (TCHME) has any spermicidal action against rat and human spermatozoa, as well as to assess the different spermiological, androgenic, and oxidative stress markers using reproductive tissues of the rat for establishing its hypo-testicular cum contraceptive potentialities.
Materials and methods
Preparation of hydro-methanol (60:40) solvent extract
Freshly collected stems of Tinospora cordifolia (Willd.) from Gopegarh Eco Park, Paschim Midnapore, were validated (Voucher No. T. cordifolia (Willd.)/VU/BIO/15/20) by the Botany and Forestry Dept. of Vidyasagar University. Stems were washed, dried completely, and grind by using a grinder. Ground materials (30 g) were dissolved in hydro-methanolic (60:40) solvent (300 ml) for 48 h at room temperature and stirred regularly at 2-h intervals. The dissolved materials were then filtered, the solvent was evaporated in a rotary evaporator, and the remnant was allowed to shed dry. The dried extract was collected in an air-tight glass container at room temperature for use in experimental work.
Chemicals
Methanol, trichloroacetic acid (TCA), hydrogen peroxide (H2O2), sodium citrate, and fructose were purchased from Merck, Mumbai, India. Rose bengal, pyrogallol, and thiobarbituric acid (TBA) were supplied by Loba Chemie Pvt. Ltd. (Maharashtra, India). Other chemicals were purchased from Sigma-Aldrich, Bangalore, India.
Sperm and tissue sample preparation
Sixteen adult healthy normospermic male individuals (25–30 years of age) were chosen for the conduction of this experiment on human sperm samples. Semen samples were collected after 5 days of abstinence according to standard guidelines [13]. Semen analysis was done after spontaneous coagulation followed by liquefaction at 37 °C.
To conduct the in vitro study on human sperm, experimental rat’s sperm, and other reproductive and metabolic tissues, safety guidelines were followed according to the Institutional Ethics Committee (IEC). To conduct the experiment on rat samples, twelve healthy fertile male rats were selected for this study. They were kept for an acclimatization period of 15 days before the animal sacrifice in connection with tissue sample collection to be used in the in vitro study. Caudal epididymis was collected and washed in 0.9% normal saline (1 ml) to prepare the sperm suspension. Testes, epididymis, liver, and kidney were dissected and washed properly with normal saline. Metabolic and reproductive organs were considered here to determine whether the extract has any organ-specific differential cum selective activity for this purpose.
In vitro experimental design
In vitro solvent, i.e., Krebs-Ringer Buffer (KRB) solution was prepared with 5% CO2 and 95% O2 gaseous mixture (30 bubbles/min) to maintain the normal physiological homeostasis of tissue samples during incubation throughout in vitro experimentation. KRB solution (pH − 7.4) was made with 125 mM NaCl, 2.5 mM KCl, 1.25 mM Na2HPO4, 2 mM CaCl2, 1 mM MgCl2, 25 mM NaHCO3, and 25 mM glucose. For this study, 10 ml KRB solution was transferred to all the test tubes. Epididymal-washed sperm of rat and human semen samples were diluted in KRB solution at the ratio of 1:1. After that, 500 μl of diluted sample was added into the test tube containing 10 ml KRB solution considering as an in vitro medium.
Spermiological sensors were evaluated at different incubation time intervals like 0 min (within 20 s), 15 min, and 30 min after charging the different doses of TCHME. To assess the androgenic, oxidative stress, and toxicity markers, all dissected organs were incised at different poles to favoring of infusion of extract and in vitro media properly. The prepared and processed samples with all contents in test tubes allotted for specific tissue samples were incubated. Biochemical estimations of androgenic key enzyme activities, oxidative stress, and toxicity markers were evaluated after the completion of 2-h incubation period considering the time of charging of TCHME at different doses. Human sperm, rat sperm and tissue samples were divided into the following four groups.
Control group
Incised tissues (liver, kidney, testes, and epididymis), epididymal-washed sperm of rat, and human semen samples were taken in six different test tubes allotted for each sample separately in in vitro media (KRB with 5% CO2 and 95% O2 gaseous mixture). All the test tubes were incubated at 37 °C for 2 h in the case of tissue samples, but for 20 s, 15 min, and 30 min in the case of sperm samples of rat and human.
0.5-mg TCHME-charged group
All incised organs, epididymal-washed sperm of rat, and human semen samples were put separately into the six different test tubes containing the above-said in vitro media. Then, 0.5 mg/ml TCHME was charged in all the test tubes and incubation was done at 37 °C for the sample-specific period as control group.
1-mg TCHME-charged group
Pre-mentioned incised organs, rat epididymal-washed sperm, and semen samples of human were placed in six different test tubes maintaining the media, i.e., KRB with 5% CO2 and 95% O2, gaseous mixture and allowed to charge with 1 mg/ml TCHME and placed in incubation at 37 °C for sample-specific different periods with parity of control group.
2-mg TCHME-charged group
In this group, 2 mg/ml TCHME was charged in six separate test tubes containing human semen, rat epididymal-washed sperm, or incised organs. Above-mentioned in vitro media was applied and the incubation was performed at 37 °C for tissue or sperm sample-specific time exposure as the control.
Sperm motility
Sperms from control and charged groups, i.e., 0.5-, 1-, and 2-mg TCHME-charged groups, were taken at different incubation periods and allowed to check the percentage of motile sperm [14]. A drop of in vitro solvent with sperm samples exposed to TCHME for a specific duration was placed on separate glass slides covered by coverslip immediately and viewed under a microscope (400×). The percentage of motile sperm was expressed in all the groups.
IC50 value
By using the various concentrations (0.5, 1, 1.5, and 2 mg/ml) of TCHME, an inhibitory concentration of this extract was determined that causes 50% immobilization of motile sperm [15] which was considered as IC50 value. Immobilization was checked instantly within 20 s after TCHME application on human sperm and rat epididymal-washed sperm. Motile sperm percentage was assessed in the control group and noted as motility of sperm at 0 mg/ml concentration.
Sperm viability
The viability test of sperm was done by the eosin-nigrosin staining [16]. A thin smear was prepared on a glass slide using the mixture of the semen sample (50 μl), 1% eosin (50 μl), and 10% nigrosine (150 μl) at a ratio of 1:1:3. This slide was observed under the light microscope (400×) and the number of dark pink–stained (dead) head of sperm and unstained head of spermatozoa (alive) were counted. The values were expressed in terms of percentage.
Hypo-osmotic swelling (HOS) test
Human semen samples incubated with TCHME in in vitro media for different durations were mixed with pre-warmed HOS solution at the ratio of 1:10, prepared by dissolving 0.735 g of sodium citrate and 1 g of fructose in 100 ml of distilled water. The percentage of numbers of curled-tail sperm was noted after incubation of the mixture at 37 °C for 2 h. Epididymal-washed rat sperm was also used for HOS assessment according to the same protocol [17].
Acrosome intactness status (AIS)
Glass slides were prepared with a gelatin coat, and after 24 h the slides were fixed with 0.05% glutaraldehyde to assess the acrosomal status of sperm. Human semen, and rat epididymal-washed samples were incubated in in vitro media for different times and then 100 μl of such samples were diluted with phosphate-buffered saline D-glucose solution (1000 μl) at the ratio of 1:10. To make the phosphate-buffered saline D-glucose solution, 1 g of glucose was added in 10 ml phosphate-buffered saline. Smear was made with the diluted semen on a gelatin pre-coated glass slide and incubated at 37 °C for 2 h. Percentages of the number of haloes in gelatin-coated slides surrounding the head part of sperm were counted. Haloes were formed by sperm acrosomal enzymes [18].
Nuclear chromatin decondensation (NCD) test
Sperm samples from in vitro media after charging of TCHME for different duration were used for this study. The NCD test was done according to the standard procedure [19]. The sperm pellet was washed twice with 0.05 M borate buffer (pH, 9.0). Then, 6mM EDTA and 1% SDS mixture (900 μl) were prepared by adding these two ingredients at the ratio of 1:1. This mixture was added to that suspended sperm pellet in 0.05 M borate buffer (100 μl). The reaction was stopped by adding an equal amount of 2.50% glutaraldehyde. From that mixture, 15 μl was taken in an Eppendorf tube, and 5 μl of rose bengal (0.80%) was added. This mixture was placed on a glass slide and covered with a cover slip. Percentages of sperm were counted having uncoiled chromatin viewed under a microscope at 400×.
Assay of testicular ∆5, 3β-hydroxysteroid dehydrogenase (HSD) and 17β-HSD activities
Androgenic key enzyme activities, i.e., ∆5, 3β-HSD, and 17β-HSD, in testis were assessed in accordance to the standard protocols [20, 21]. Testicular homogenization (tissue conc. 100 mg/ml) was performed in 20% spectroscopic grade glycerol containing 5 mM of potassium phosphate and 1 mM EDTA and allowed to centrifuge for 30 min at 10,000 rpm at 4 °C. Supernatant of 500 μl was taken to check both enzyme activities as per the standard method. Absorbances were observed in a spectrophotometer at 340 nm for 180 s at 30-s intervals.
Estimation of antioxidant enzyme activity
Tissue (liver, kidney, testes, and epididymis of rat) homogenization was prepared at a concentration of 50 mg/ml in 0.05 M Tris HCl buffer solution for the estimation of catalase (CAT) activity. Human sperm pellet was also prepared with the above-mentioned buffer solution. All were allowed to centrifuge at 10,000 rpm for 10 min at 4 °C. Reading was taken after 20 s with the addition of 0.00035 M H2O2 in a spectrophotometric cuvette. Six subsequent absorbances were recorded at 240 nm for 180 s at 30-s intervals [22].
Superoxide dismutase (SOD) activity was measured in the pre-mentioned tissues according to the standard procedure [23]. Pyrogallol (20 μl) was used for auto-oxidation, and absorbance was noted in a spectrophotometer for 180 s at 420 nm with 30-s intervals.
Quantification of thiobarbituric acid reactive substances (TBARS)
For the quantification of end products of lipid peroxidation, TBARS level was measured in human sperm pellet, liver, kidney, testes, and epididymis of rats [24] of the control and extract charged groups. Absorbance was recorded at 535 nm in a spectrophotometer.
Estimation of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activities
For toxicity assessment, GOT and GPT activities in the testes, epididymis, liver, and kidney were evaluated according to the standard protocol [25]. The substrates L-aspartic acid (1.33 gm) and α-ketoglutaric acid (15 mg) were used for GOT activities’ assessment, whereas substrates DL-alanine (1.78 g) and α-ketoglutaric acid (30 mg), dissolved in PBS separately, were used for the estimation of GPT activities. The reaction was stopped by adding the DNPH reagent (1 ml). Color was developed by the addition of 0.4 (N) NaOH, and reading was taken in a spectrophotometer at 540 nm against the blank.
Phytochemical analysis through qualitative assay
Qualitative screening of phytomolecules in TCHME was conducted as per standard protocol [26].
Liquid chromatography-mass spectrometry (LC-MS)
The QuattroMicroTMAPI mass spectrometer (Waters, Milford, Massachusetts, USA) was used to conduct the LC-MS studies. The liquid chromatographic apparatus consists of a quaternary pump, an online vacuum degasser, an autosampler, and a thermostatic column compartment that were linked in line to a photodiode array detector (Waters 2998). Using the Waters MASS LYNX 4.1 software, data was collected and analyzed. The method was developed and modified based on existing literature [27, 28]. An autosampler was used to inject the sample (10 μl), and an XTerra MS C18 reversed-phase column (2.1 × 100 mm, i.e., 25-μm particle size; Waters) was used for separation. The mobile phase consisted of solvent A, i.e., 0.1% formic acid in the water, and the other one was solvent B, i.e., 6% methanol:acetonitrile (2:1). The solvent gradient started with 5% B and 95% A. Gradient elution of solvent B was initiated by 40% of solvent B for 0–9 min, and that elution was hold up to 12 min. Again, the gradient elution of 100% solvent B continued for the next 12–24 min, and hold up to 27 min. Column equilibration was maintained by restoring solvent B with 5% at the 28th min. The column was kept at a constant temperature of 25 °C, and the flow rate was 0.3 ml/min. All of the chemicals were found using a PDA detector at the wavelength range of 190–690 nm. The MS spectra were acquired in the positive and negative ion mode (source block temperature 130 °C, desolvation temperature 300 °C, capillary voltage 4 kV, cone voltage 35 V). The desolvation and cone gas were 450 and 80 l h−1, respectively. The data was obtained using MS scanning with a 100–1000 (m/z) scan range, a 0.5-s scan time, and a 0.1-s interscan delay period.
Statistical analysis
Mean and standard error of the mean (SEM) were computed from all the experimental data. Statistical analysis was performed by the software Origin Lab, Version 8.5, using ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Significant differences between the groups were expressed at a level of p < 0.05. Data were expressed as mean ± SEM.
Results
Sperm motility
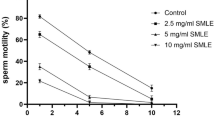
According to WHO, the lower limit of total motile sperm for fertility is 40% [16]. Here, in 0.5- and 1-mg TCHME-charged groups, the human motile sperm after 15 min of incubation were below the WHO lower border, but in 2-mg TCHME-charged group, at 0 min of incubation, human motile sperm was also below the lower limit of WHO value. Complete immobilization was observed in the 2-mg TCHME-charged group after 30 min of incubation (Fig. 1A).
Dose- and duration-dependent in vitro effect of TCHME on (A) human sperm and (B) rat epididymal-washed sperm motility. Values were expressed as mean ± SEM (n = 6). Statistical analysis was conducted through ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Values with different superscripts (a, b, c, d, e, f, g, h) on each bar and point on lines differ from each other significantly, p < 0.05. (C) IC50 value determination of the TCHME on the motility of human sperm. (D) IC50 value determination of the TCHME on the motility of rat epididymal-washed sperm after immediate exposure (within 20 s)
Considering the lower cut-off value of motile sperm of human as per WHO and translating this value for rat’s sperm assessment, it has been noted that epididymal washed sperm of rats showed this value below the cut-off level in the 0.5 TCHME-charged group at 15 min of incubation. At 0 min of incubation, the motile sperm percentages were also below this range at the exposure of 1- and 2-mg TCHME-charged groups. But, 100% immobilization was observed at 30 min of incubation in both 1- and 2-mg TCHME-charged groups (Fig. 1B).
When the results of all the TCHME-charged groups were compared to the control group, a significant increment of immotile sperm percentage was observed which is supported by low percentages (p < 0.05) of motile human and epididymal washed rat sperms.
IC50 value
Fifty percent immobilization of human and epididymal washed sperm of rat were noted immediately (0 min) at a concentration of 0.8 mg and 0.4 mg/ml, respectively (Fig. 1C, 1D).
Sperm viability
As per the WHO reference limit, the percentage of viable sperm (alive) for fertility is at least 58% [16]. In 0.5- and 1-mg TCHME-charged groups, viable human sperm percentages were below the cut-off value after 15 min of incubation. In 2-mg TCHME-charged group, the viable sperm percentage was below that limit in all three durations of exposure (Fig. 2A).
Direct effect of TCHME at dose- and duration-dependent fashion on the viability of (A) human sperm, (B) rat epididymal-washed sperm, hypo-osmotic swelled (C) human sperm percentage, and (D) epididymal-washed sperm percentage in rat. Values were expressed as mean ± SEM (n = 6). Statistical analysis was conducted through ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Values with different superscripts (a, b, c, d, e, f, g) on each bar and point on line differ significantly, p < 0.05
On the other hand, viable epididymal washed sperms of rats were below that WHO level in 0.5-mg TCHME-charged group after 15 min of incubation, while in all three durations, viable epididymal-washed sperm percentages were below the reference limit in 1- and 2-mg TCHME-charged groups (Fig. 2B).
Considering the percentages of diminution of viable sperm in charged groups compared to the control group, the significant diminution (p < 0.05) of viable human and epididymal-washed rat sperm was noted as dose-specific and duration of exposure-specific fashions.
Hypo-osmotic swelled (HOS) sperm
WHO reported that the lower limit of hypo-osmotic swelled sperm is 58% [16]. The percentage of HOS-positive human sperm showed below the WHO cut-off in 0.5- and 1-mg TCHME-charged groups after 15-min incubation and in 2-mg TCHME-charged group at all three-incubation periods (Fig. 2C).
On the other hand, below this cut-off, the HOS epididymal-washed sperms of rat was noted after 15 min of incubation in 0.5-mg TCHME-charged group, whereas in 1- and 2-mg TCHME-charged groups, these HOS-positive sperm were below the limit at all three durations of exposure (Fig. 2D). When comparing all TCHME-charged groups to the control group, significant reductions (p < 0.05) in the percentage of HOS-positive rat and human sperm were seen in 0.5-, 1-, and 2-mg TCHME-charged groups.
Acrosome intactness status (AIS)
Less than 40% of AIS is reported for male factor infertility [29]. Below this level, acrosome intact human spermatozoa were observed after 15 min of incubation in 2-mg TCHME-charged group. But less than 40% AIS was not observed at any incubation period of 0.5- and 1-mg TCHME-charged groups (Fig. 3A).
Diminution in percentages of acrosome intactness status (AIS) of (A) human sperm, (B) epididymal-washed sperm of rat, nuclear chromatin decondensed (C) human sperm, and (D) rat epididymal-washed sperm after the direct exposure of TCHME in dose- and duration-dependent manner. Values were represented as mean ± SEM, n = 6. ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Bars and point on lines with different superscripts (a, b, c, d, e, f) differ from each other significantly, p < 0.05
In the case of rat epididymal-washed sperm, ≤ 40% intact acrosome was found after 30 and 15 min of incubation in 1- and 2-mg TCHME-charged groups, respectively. At any incubation period using the dose of 0.5-mg TCHME, the values were not below the pre-mentioned cut-off value (Fig. 3B).
Significant diminution (p < 0.05) of AIS of human and epididymal-washed sperm of rat at all three incubation periods in 0.5-, 1-, and 2-mg TCHME-charged groups were noted compared to the control group.
Nuclear chromatin decondensed (NCD) sperm
In the case of in vitro, below 70% of decondensed spermatozoa are unable to fertilize oocytes and are regarded as infertile [19]. In this in vitro experiment, below this value was observed at 0 min of incubation in 2-mg TCHME-charged group only (Fig. 3C). But at any incubation period of 0.5- and 1-mg TCHME-charged groups, less than 70% NCD was not noted.
While in epididymal-washed sperm of rat, below 70% NCD sperms were found in 1 mg (15 min) and 2 mg (0 min) TCHME-charged group (Fig. 3D). Insignificant alteration (p > 0.05) of NCD sperms (human and rat) were observed among the control group, 0.5-mg (0, 15 min), and 1-mg (0 min) TCHME-charged groups. Compared to the control group, a significant reduction (p < 0.05) in percentages of NCD of rat epididymal-washed sperm was noted only after 30 min of incubation in the 0.5-mg TCHME-charged group.
∆5, 3β-hydroxysteroid dehydrogenase (HSD) and 17β-HSD activities
Significant decrease (p < 0.05) in ∆5, 3β-HSD, and 17 β-HSD activities of rat testicular tissue were noted after the direct charge of 0.5, 1, and 2 mg in different TCHME-charged groups compared to the control group. But there was no significant alteration (p > 0.05) when a comparison was made between the 1- and 2-mg TCHME-charged groups (Fig. 4).
Activities of testicular Δ5, 3β-HSD, and 17β-HSD in experimental rats of control and different TCHME-charged groups after 2-h incubation. Bars were represented as mean ± SEM, n = 6. ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Bars with different superscripts (a, b, c) differ from each other significantly, p < 0.05
Antioxidant enzymes activities
Activities of CAT (Fig. 5A) and SOD in human sperm pellet (Fig. 5B) were reduced significantly (p < 0.05) in 1- and 2-mg TCHME extract–charged groups compared to the control group. But there was no significant change (p > 0.05) in CAT and SOD activities between the 0.5-mg and control groups (Fig. 5A, 5B).
Effects of direct exposure of TCHME (after 2 h) on oxidative stress markers, i.e., (A) on CAT activity, (B) SOD activity, and (C) TBARS level of human spermatozoa in various conditions. Bars were represented as mean ± SEM, n = 6. ANOVA followed by multiple comparisons Student’s two-tailed “t”-test. Bars with different superscripts (a, b, c) differ from each other significantly, p < 0.05
Activities of CAT and SOD in testis and epididymis of experimental rats were decreased significantly (p < 0.05) in all TCHME-charged groups, i.e., 0.5, 1, and 2 mg, with respect to the control group. But insignificant alterations (p > 0.05) of CAT and SOD activities were noted in between 1- and 2-mg TCHME-charged groups (Table 1).
There was an insignificant elevation (p > 0.05) in hepatic CAT activity, but a significant elevation (p < 0.05) of renal CAT activity was noted in all TCHME-charged groups compared to the control group. Hepatic and renal SOD activities were increased significantly (p < 0.05) in 0.5-, 1-, and 2-mg TCHME-charged groups compared to the control group (Table 1).
Level of TBARS
Thiobarbituric acid reactive substances (TBARS)’ level in human sperm pellet showed significant elevation (p < 0.05) after charging 1- and 2-mg TCHME compared to the control group (Fig. 5C). Insignificant alteration (p > 0.05) was noted between the 0.5-mg TCHME-extract charged and the control group.
In the case of testicular and epididymal TBARS levels in rats, significant upward elevation (p < 0.05) was noted in all the extract-charged groups in respect to the control group. But insignificant changes (p > 0.05) were observed when a comparison was made between 1-mg and 2-mg TCHME-charged groups (Table 1).
The quantity of TBARS in hepatic and renal tissues decreased significantly (p < 0.05) in all TCHME-charged groups in respect to the control group (Table 1).
GOT and GPT activities
Insignificant changes (p > 0.05) were noted in GOT and GPT activities in different tissue samples in experimental rats, i.e., liver, kidney, testes, and epididymis of all extract-charged groups compared to the control group (Table 2).
Qualitative screening of phytochemical
Hydro-methanol extract of T. cordifolia (TCHME) showed positive results for alkaloids, flavonoids, and terpenoids in the qualitative screening (Table 3).
Liquid chromatography-mass spectrometry (LC-MS) studies
The LC-MS spectrums’ interpretation was performed using a spectrum analysis for organic compounds by manual literature survey [30, 31]. The LC-MS analysis of the stem of TCHME detected mass spectrum peaks with candidate mass (m/z) at positive ions 341.43, 337.22, and 352.22 and negative ions with candidate mass having 406.07, 433.75, and 328.37. Each positive peak was fragmented, resulting in fragmentation spectrums tentatively indicating that there were substances of corydine (fragment ions at m/z 310.12, and 279.10), berberine (fragment peaks at m/z 320.19, and 292.34), and palmatine (fragment ions at m/z 336.12, and 322.10), respectively. After the fragmentation of the negative peak tentatively identified as tinosporin (fragment peaks at m/z 406.16, and 407.17), dihydrokaempferol-3-O-rhamnoside (fragment ions at m/z 432.10, and 285.03), and boldine (fragment peaks at m/z 282.09, and 265.08), respectively [30, 31]. The retention time of each mass spectrum, product ions, and nature of the identified compounds are given in Table 4. Mass spectrums of compounds obtained from LC-MS studies are given in Fig. 6.
Discussion
Less involvement of males in family planning, adverse effects of synthetic steroidal contraceptives, and limited choice of male birth control options all are motivating factors for the development of alternate and safe male contraceptives. Saponins, alkaloids, flavonoids, triterpenes, and tannins found in traditional medicinal plants contribute to the spermicidal and hypo-testicular effects [32, 33]. Here, TCHME focusing the existence of alkaloids, flavonoids, and terpenoids in the qualitative screening and LC-MS study highlight the confirmation of four alkaloids (corydine, berberine, boldine, palmatine), one flavonol (dihydrokaempferol-3-O-rhamnoside), and one terpenoid (tinosporin) with observed and calculated m/z along the retention time as per Table 4.
One of the important sensors for male fertility assessment is sperm motility, and for the evaluation of male contraceptive efficacy of TCHME, this spermiological sensor was considered. The motility of human and epididymal-washed rat spermatozoa was decreased after TCHME exposure at different doses and duration of exposure, possibly due to low adenosine triphosphate (ATP) production [34]. This may be due to the mitochondrial malfunction of spermatozoa that may also prevent the phosphorylation of adenosine diphosphate (ADP) to ATP through uncoupling of oxidative phosphorylation which is the main energy source for sperm motility in comparison to glycolysis [35]. The sperm mitochondrial dysfunction by TCHME may be due to oxidative stress imposition [36] reflected here by oxidative stress sensor analysis. Marked elevation in TBARS level in reproductive organs and reduction in TBARS quantity in metabolic organs, as well as decreased activity of CAT and SOD in reproductive organs along with the increment of CAT and SOD activities in metabolic organs, support the induction of oxidative stress to reproductive organs in selective or differential aspect than metabolic organs by the direct charge of TCHME. Loss of membrane integrity, increased membrane permeability, a reduction in nuclear chromatin decondensation, and reduced antioxidant enzyme activity all are supporting in favor of high lipid peroxidation in sperm membrane architecture [37]. All such spermiological deteriorating effects were supported here by IC50 value of TCHME. The IC50 value of TCHME for spermicidal activity is far less than extract of other plant [6] which strengthen it as promising spermicidal agent.
As sperm motility is not the only prime parameter to assess the fertilizing ability of sperm, so, other sperm parameters like sperm viability, HOS, and acrosomal cap status were determined in this concern [16]. In TCHME extract–charged groups, sperm cells failed to maintain the plasma membrane architecture which was confirmed by the reduction of alive (viable) sperm percentage in the viability test, lower percentage of curled tail observed in the HOS test, and substantial decrease of AIS of sperm. Membrane integrity is deteriorated by reactive oxygen species–induced membrane phospholipid oxidation peroxidation chain reaction [38]. As the sperm membrane has a high level of phospholipid than other cell membranes [39], so TCHME-induced disruption in the membrane integrity of sperm can be explained.
The two most significant enzymes in the fertilization process are acrosin, and hyaluronidase, unique to the acrosome, are severely inhibited by plant-based derivatives [40]. Changes in the acrosomal cap status of sperm following TCHME-charging may suggest damage to the outer acrosomal membrane. Besides this, the fertilizing capacity of sperm also depends on some functional characteristics like NCD. Chromatin is highly packaged and compact in structure in sperm, but decondensation of this chromatin is very much important for the formation of pro-nucleus and successful fertilization [41]. Charging of TCHME at different concentrations reduced the ability to decondense the sperm nuclear chromatins probably by modifying the DNA bonding and cross-link formation patterns [41, 42].
Epididymis is the site of sperm maturation as well as capacitation [43], and as the extract is more specific for reproductive tissue, so epididymal tissue was considered here besides testicular tissue. The most interesting result in this observation is that the TCHME induces antioxidative activity slightly and has no pro-oxidant activity in metabolic tissues like the liver and kidney but has prominent pro-oxidant activity in reproductive organs like the testis and epididymis. Such tissue-specific effects of TCHME especially for male reproductive organs unfold the possibility for male contraceptive efficacy without any general oxidative stress imposition.
Testosterone is very important for male fertility as it maintains spermatogenesis [44]. Two key androgenic enzymes, i.e., ∆5, 3β-HSD, and 17β-HSD activities, were measured here. It may be explained by observing other phytochemicals having potent anti-androgenic activity like flavonoids, terpenoids, and alkaloids. Significant diminution of these enzyme activities in TCHME-charged groups may be due to the inhibition in androgenic key enzyme activity by phyto-ingredients present in the extract through competitive inhibition with substrate and or non-competitive inhibition with co-enzyme (NAD) binding with the enzyme [45].
Insignificant changes of the GOT and GPT activities in the said tissues in different TCHME-charged groups indicated the non-toxic nature of this stem extract. This may be due to no effect of TCHME on lysozyme of these cells, and so such enzymes are not elevated.
Dose-specific contraceptive efficacy of phytomolecules present in TCHME may be explained by the ligand binding model to the orphan receptor distributed in target tissues. Low dose, i.e., 0.5-mg of TCHME may result in fractional receptor occupancy due to partial saturation of orphan receptors by phytomolecule cum ligand. At the dose of 1 mg of TCHME, maximum responses were indicated, which may be due to total receptor occupancy by ligand cum phytomolecules that execute optimum effect. Two mg dose of TCHME may be considered as super saturation dose due to the presence of excess ligand phytomolecules remain in an unbound state and so, no further modulatory effects were exhibited [46].
Conclusion
It has been shown from the perspective of this in vitro findings that TCHME has spermicidal action as well as more specific inhibitory effects on testis and epididymis where spermatozoa are generated and stored respectively without imposing any metabolic toxicity. Overall results focus on TCHME as a promising male contraceptive where more study is needed for isolation and characterization of the specific one.
Data availability
Authors can show the data of the study if there is any valid request from the editorial board of the journal.
Code Availability
Software Origin Lab, Version 8.5 for Windows 10.
References
Chappell BT, Brooke LG, Brandon H. Mechanisms of action of currently available woman-controlled, vaginally administered, non-hormonal contraceptive products. Ther Adv Reprod Health. 2022;16:1–6. https://doi.org/10.1177/11795581211107120.
Xu M, Zhao M, Li RHW, Lin Z, Chung JPW, Li TC, Lee TL, Chan DYL. Effects of nonoxynol-9 (N-9) on sperm functions: systematic review and meta-analysis. Reprod Fertil. 2022;3(1):R19–33. https://doi.org/10.1530/RAF-21-0024.
D’Cruz OJ, Erbeck D, Uckun FM. A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol Pathol. 2005;33(4):465–76. https://doi.org/10.1080/01926230590959866.
Ghaffarilaleh V, Fisher D, Henkel R. Carica papaya seed extract slows human sperm. J Ethnopharmacol. 2019;241:111972. https://doi.org/10.1016/j.jep.2019.111972.
Sharma SK, SaiRam M, Ilavazhagan G, Devendra K, Shivaji SS, Selvamurthy W. Mechanism of action of NIM-76: a novel vaginal contraceptive from neem oil. Contraception. 1996;54(6):373–8. https://doi.org/10.1016/s0010-7824(96)00204-1.
Paul D, De D, Ali KM, Chatterjee K, Nandi DK, Ghosh D. Comparative study on the spermicidal activity of organic solvent fractions from hydroethanolic extracts of Achyranthes aspera and Stephania hernandifolia in human and rat sperm. Contraception. 2010;81(4):355–61. https://doi.org/10.1016/j.contraception.2009.09.001.
Anand AK, Prasad V, Alam M. Herbal or modern methods of contraception! Choice is yours. Int J Reprod Contracept Obstet Gynecol. 2015;4(4):947–53. https://doi.org/10.18203/2320-1770.ijrcog20150405.
UN Population Division. World population prospects 2019. In: Department of Economic and Social Affairs. 2019. Retrieved from http://www.ncbi.nlm. nih.gov/pubmed/12283219. Accessed 04.09.2022.
Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK, Sun JN, Ma DL, Han YF, Fong WF, Ko KM. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med. 2013;2013:1–25. https://doi.org/10.1155/2013/627375.
Bhatt N, Deshpande M. A Critical review and scientific prospective on contraceptive therapeutics from ayurveda and allied ancient knowledge. Front Pharmacol. 2021;12:629591. https://doi.org/10.3389/fphar.2021.629591.
Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) – validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1(2):112–21. https://doi.org/10.4103/0974-7788.64405.
Gupta RS, Sharma A. Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J Exp Biol. 2003;41(8):885–9.
Chaudhury K, Bhattacharyya AK, Guha SK. Studies on the membrane integrity of human sperm treated with a new injectable male contraceptive. Hum Reprod. 2004;19(8):1826–30. https://doi.org/10.1093/humrep/deh332.
Zemjanis R. Diagnostic and therapeutic techniques in animal reproduction. 2nd ed. Baltimore: the Williams & Wilkins Company; 1970. p. 88–96.
Ratnasooriya WD, Amarasekera AS, Perera NSD, Premakumara GAS. Sperm antimotility properties of a seed extract of Abrus precatorius. J Ethnopharmacol. 1991;33(1-2):85–90. https://doi.org/10.1016/0378-8741(91)90166-B.
World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5th ed. Geneva: World Health Organization press. New York, NY: Cambridge University Press; 2010. p. 7–271.
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Reproduction. 1984;70(1):219–28. https://doi.org/10.1530/jrf.0.0700219.
Gopalkrishnan K. Standardized procedures in human semen analysis. Curr Sci. 1995;68(4):353–62. https://www.jstor.org/stable/24096433
Gopalkrishnan K, Hinduja IN, Anand Kumar TC. In vitro decondensation of nuclear chromatin of human spermatozoa: assessing fertilizing potential. Arch Androl. 1991;27(1):43–50. https://doi.org/10.3109/01485019108987650.
Jarabak J, Adams JA, Williams-Ashman HG, Talalay P. Purification of 17β-hydroxysteroid dehydrogenase of human placenta and studies on its trans dehydrogenase function. J Biol Chem. 1962;237(2):345–57.
Talalay P. Hydroxysteroid dehydrogenase. In: Colowic S, editor. Methods in Enzymology. New York, NY: Academic Press; 1962. p. 512–6.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–40.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
Jagadeesan G, Kavitha AV. Recovery of phosphatase and transaminase activity of mercury intoxicated Mus musculus (Linn.) liver tissue by Tribulus terrestris (Linn.) (Zygophyllaceae) extract. Trop Biomed. 2006;23(1):45–51.
Hossain MA, KAS AL-R, ZH AL-M, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–10. https://doi.org/10.1016/S2221-1691(13)60142-2.
Gauri SS, Mandal SM, Atta S, Dey S, Pati BR. Novel route of tannic acid biotransformation and their effect on major biopolymer synthesis in Azotobacter sp. SSB81. J App Microbiol. 2013;114(1):84–95. https://doi.org/10.1111/jam.12030.
Li M, Wang H, Huan X, Cao N, Guan H, Zhang H, Cheng X, Wang C. Simultaneous LC-MS/MS bioanalysis of alkaloids, terpenoids, and flavonoids in rat plasma through salting-out-assisted liquid-liquid extraction after oral administration of extract from Tetradium ruticarpum and Glycyrrhiza uralensis: A sample preparation strategy to broaden analyte coverage of herbal medicines. Anal Bioanal Chem. 2021;2021(413):5871–84. https://doi.org/10.1007/s00216-021-03568-1.
Chan PJ, Corselli JU, Jacobson JD, Patton WC, King A. Spermac stain analysis of human sperm acrosomes. Fertil Steril. 1999;72(1):124–8. https://doi.org/10.1016/s00150282(99)00201-0.
Bajpai V, Singh A, Chandra P, Negi MPS, Kumar N, Kumar B. Analysis of phytochemical variations in dioecious Tinospora cordifolia stems using HPLC/QTOF MS/MS and UPLC/QqQLIT-MS/MS. Phytochem Anal. 2016;27(2):92–9. https://doi.org/10.1002/pca.2601.
Goufo P, Singh RK, Cortez I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants. 2020;9(5):398. https://doi.org/10.3390/antiox9050398.
Sefrioui MR, El Othmani IS, Filali H, Derfoufi S, Derraji S, Benmoussa A, Said AAH. Evaluation of spermicidal activity of saponosides from Saponaria officinalis/Caryophyllaceae, Glycyrrhizia glabra/Fabaceae and Herniaria glabra/Caryophyllaceae. Med Pharm Rep. 2021;94(2):239–47. https://doi.org/10.15386/mpr-1879.
Okon UA, Etim BN. Citrus aurantifolia impairs fertility facilitators and indices in male albino wistar rats. Int J Reprod Contracept Obstet Gynecol. 2014;3(3):640–6. https://doi.org/10.5455/2320-1770.ijrcog20140949.
Davila MP, Muñoz PM, Bolaños JG, Stout TAE, Gadella BM, Tapia JA, Silva CB, Ferrusola CO, Pena FJ. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction. 2016;152(6):683–94. https://doi.org/10.1530/REP-16-0409.
Bennison C, Hemmings N, Brookes L, Slate J, Birkhead T. Sperm morphology, adenosine triphosphate (ATP) concentration and swimming velocity: unexpected relationships in a passerine bird. Proc Biol Sci. 2016;283(1837):20161558. https://doi.org/10.1098/rspb.2016.1558.
Castellini C, D’Andrea S, Cordeschi G, Totaro M, Parisi A, Di Emidio G, Tatone C, Francavilla S, Barbonetti A. Pathophysiology of mitochondrial dysfunction in human spermatozoa: focus on energetic metabolism, oxidative stress and apoptosis. Antioxidants. 2021;10(5):695. https://doi.org/10.3390/antiox10050695.
Walczak–Jedrzejowska R, Wolski JK, Slowikowska–Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66(1):60–7. https://doi.org/10.5173/ceju.2013.01.art19.
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22(9):1–21. https://doi.org/10.3390/ijms022094642.
Shan S, Xu F, Hirschfeld M, Brenig B. Sperm lipid markers of male fertility in mammals. Int J Mol Sci. 2021;22(16):1–21. https://doi.org/10.3390/ijms22168767.
Chakrabarti K, Pal S, Bhattacharyya AK. Sperm immobilization activity of Allium sativum L. and other plant extracts. Asian J Androl. 2003;5(2):131–6.
Ribas-Maynou J, Garcia-Bonavila E, Hidalgo CO, Catalán J, Miró J, Yeste M. Species-specific differences in sperm chromatin decondensation between eutherian mammals underlie distinct lysis requirements. Front Cell Dev Biol. 2021;9:669182. https://doi.org/10.3389/fcell.2021.669182.
Mondal P, Maity R, Mallick C. In vitro spermicidal effect of Thevetia peruviana leaves on human spermatozoa. Andrologia. 2022;54(2):e14323. https://doi.org/10.1111/and.14323.
Gervasi MG, Visconti PE. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology. 2017;5(2):204–18. https://doi.org/10.1111/andr.12320.
Guido C, Santoro M, De Amicis F, Perrotta I, Panza S, Rago V, Cesario MG, Lanzino M, Aquila S. Human sperm anatomy and endocrinology in varicocele: role of androgen receptor. Reproduction. 2014;147(5):589–98. https://doi.org/10.1530/REP-13-0542.
Hu GX, Zhao BH, Chu YH, Zhou HY, Akingbemi BT, Zheng ZQ, Ge RS. Effects of genistein and equol on human and rat testicular 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase 3 activities. Asian J Androl. 2010;12(4):519–26. https://doi.org/10.1038/aja.2010.18.
Salahudeen MS, Nishtala PS. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm J. 2017;25(2):165–75. https://doi.org/10.1016/j.jsps.2016.07.002.
Acknowledgements
The authors are grateful to the UGC for providing the funding necessary to complete this work. University Grants Commission (UGC), Govt. of India. NTA Ref No.: 200510431209.
Author information
Authors and Affiliations
Contributions
Puja Das conducted the project, methodology, and drafted the original manuscript. Data analysis and data curation were performed by Kuladip Jana, and Dipanwita Mitra handled the animals and collected the human semen samples. Debidas Ghosh supervised, conceptualized the study design, and edited and validated the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Ethics Committee of Vidyasagar University with reference no. VU/IAEC/CPCSEA/6/7/2022 dt.22.11.2022.
Consent to participate
Each participant signed the informed consent.
Consent for publication
All authors agree to publish the article in this journal after acceptance.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, P., Mitra, D., Jana, K. et al. In Vitro Study on Spermicidal Action of Hydro-methanol Extract of Tinospora cordifolia (Willd.) Stem in Rat and Human Sperm: a Comparative Analysis. Reprod. Sci. 30, 3480–3494 (2023). https://doi.org/10.1007/s43032-023-01327-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01327-4