Abstract
In women undergoing chemotherapy, it is inevitable that infertility risk will increase because of impaired reproductive functions. Premature ovarian insufficiency (POI), which occurs as a devastating result of chemotherapy, is the complete depletion or dysfunction of ovarian follicles. Adipose-derived mesenchymal stem cells (ADMSCs) transplantation is among the alternative treatment methods for POI, which currently do not have an effective treatment method. Apoptosis of granulosa cells in POI is seen as the main mechanism of the disease. It is also reported that in addition to molecules directly associated with apoptosis, connexins, and pannexins are also potential effector molecules in apoptosis. The roles of these molecules in POI, which are known to play a role in many important mechanisms in the ovary, are unknown. In this study, it was aimed to analyze the expressions of Connexin43 and Pannexin1, which are thought to be effective in the formation of POI, and to show the relationship between the antiapoptotic effects of ADMSCs transplantation and these molecules in POI. For this purpose, Caspase3, Connexin43, Pannexin1 proteins, and mRNA expressions were analyzed by immunohistochemistry and RT-qPCR, and AMH levels were measured by ELISA. It was determined that Pannexin1, Caspase3 proteins, and mRNA levels increased in the POI, while Pannexin1 and Caspase3 expressions decreased in the ADMSCs treated group. While Connexin43 level decreased in POI, Connexin43 protein and mRNA levels increased in ADMSCs group. Consequently, this study demonstrated for the first time that Connexin43 and Pannexin1 were associated with apoptosis in POI. In addition, it was revealed that ADMSCs transplantation could produce antiapoptotic effects by modulating these molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Premature ovarian insufficiency (POI) is a condition of amenorrhea and hypergonadotropic hypogonadism based on loss of ovarian function in women under the age of 40. It is characterized by complete depletion or dysfunction of the follicles in the ovaries before menopause [1]. Although the incidence of the disease increases by age, its overall prevalence is between 1 and 2% [2]. Chemotherapy is among the most common iatrogenic reasons of POI. Studies have reported that anticancer drug therapy causes various levels of ovarian damage and fertility suppression [3]. The gonadotoxic effect of chemotherapy on ovarian function varies depending on age, dose, type of chemotherapeutic agent, and amount of exposure [4].
Cyclophosphamide (CTX), also known as cytophosphane, is a widely used chemotherapeutic agent and also an immune suppressor drug. In particular, it is used as antineoplastic in lymphoma, leukemia, multiple myeloma, breast and ovarian cancers, as well as an immunosuppressant in organ transplantation and in the treatment of certain diseases such as nephrotic syndrome. CTX, which is a chemotherapeutic in the alkylating group, causes DNA fragmentations or crosslinking of DNA in the cell by alkylating nucleic acids. Thus, it inhibits the cell division [5, 6]. Unfortunately, in women treated with CTX, the risk of persistent amenorrhea and early menopause is high [7]. Because the CTX is not a cell cycle specific drug, resting and developing follicles, as well as the oocyte, are damaged by CTX [8]. Even acute exposure to CTX induces DNA double-strand breaks, and by triggering apoptotic death of primordial follicles within 12–24 h, it causes a massive loss of ovarian reserve [9].
Due to the complexity of POI, there is currently no specific treatment method for it. Although hormone replacement therapy (HRT) is often recommended in the clinic, restoring reproductive ability with this treatment is seen as unlikely. Therefore, there is a need to develop effective treatments for POI. In regenerative medicine, the therapeutic approach of mesenchymal stem cells (MSCs) is used in many areas. Adipose-derived mesenchymal stem cells (ADMSCs) are one of the multipotent adult stem cell types. ADMSCs are unique cells that exhibit self-renewal, high proliferation rates, and multiple differentiation capacity [10]. These cells, which have low immunogenic properties, are capable of secreting many important growth factors, cytokines, trophic factors, and regenerative factors. In terms of repair and regeneration of autologous cells, ADMSCs offer great advantages with their ease of access and repeatable applicability [11, 12]. ADMSCs, which have been also started to be studied in POI, are seen as an alternative treatment method. But in these recent studies too, it is not known how and with which mechanism ADMSCs are effective in POI. However, since the main mechanism in POI is apoptosis, which occurs in follicles and oocytes, studies have focused on molecules associated with apoptosis [13,14,15].
By directly connecting the cytoplasmic compartments of neighboring cells with intercellular channels to each other, gap junctions provide direct intercellular passage of substances such as ions smaller than 1kD, metabolites, secondary messengers, Ca2 + , inositol phosphate, and ATP [16]. These intercellular channels consist of connexin (Cx) and pannexin in vertebrates, and innexin family proteins in invertebrates [17]. When forming gap junctions, the half channels called connexons formed by the connexins between two adjacent cells combine mutually and thus a complete channel is formed [18]. It is known that Cx43 and Cx37 are the most abundant gap junction proteins that is found in the ovary. While Cx37 is expressed on the oocyte and is responsible for communication between oocyte granulosa cells, Cx43 is expressed in granulosa cells and is responsible for communication between granulosa [19]. The main functions of connexins in the ovary are taking charge in folliculogenesis, oogenesis, meiotic arrest, steroidogenesis, and apoptosis [16]. In particular, it has been shown that Cx43 is inversely proportional to apoptosis and necessary for cell survival. Also, in ovarian follicles, increased apoptosis decreases Cx43 expression [20]. The pannexin (Panx) family, which is one of the channel proteins, consists of 3 members: Pannexin 1, Pannexin 2, and Pannexin 3. The fact that Panx1 is expressed in organs such as the brain, heart, skin, testicles, thymus, and liver has been shown by the Northern blot analyses [21]. Similar to the connexin oligomer connexons, pannexin oligomers are called pannexons. However, while connexons are called half-channels that constitute half of a cell channel, pannexons are a single membrane channel in the cell membrane [22, 23]. Panx1 is considered as part of the P2X7 receptor complex specifically required for ATP release. P2X7 purinergic receptors are part of the signaling network involved in many physiological and pathological processes. In vivo, P2X7 receptors can mediate apoptotic cell death [24]. It has been reported that purinergic receptors in the ovary have very important roles in ovarian function [25]. Therefore, it has been thought that the Panx1 molecule would also have important roles in the ovary. But until this time, any study in the literature has not presented data on the expression of Panx1 in the ovary [26]. There is not enough information about the expressions of Cx43 and Panx1 in POI, which are shown to have important functions in apoptosis.
In this study, for the first time, it was aimed to analyze the expressions of Cx43 and Panx1, which were considered effective in POI formation, at the level of protein and mRNA. In addition, it was also aimed to demonstrate the relationship of the antiapoptotic effects of ADMSCs transplantation in POI with these molecules.
Materials and Methods
Animals
In the study, 22 adult Wistar female rats whose weights ranging from 180 ± 50 g and which were supplied from Manisa Celal Bayar University Experimental Animal Application and Research Center were used. Rats were kept for 3 days (for adaptation) against any signs of health problems. Throughout the study, the animals were kept under stable conditions (22 °C temperature, 30–70% humidity, light/dark cycle 12/12 h), and their unrestricted access to food and water was ensured.
Induction of POI Models
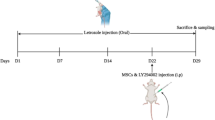
Rats were divided into 3 groups: control (n = 5), POI (n = 7), and POI + ADMSCs (n = 7). No treatment was administered to the control group rats. One hundred twenty mg/kg CTX i.p injection was administered to POI group rats on the first day and 8th day of the experiment. For POI + ADMSCs group, after injection of CTX as i.p on the first and 8th day, 1 × 106 ADMSCs was administered as i.p 3 consecutive days from the 15th day of the experiment. On the 24th day of the experiment, all rats were sacrificed. The left ovaries of the rats were fixed by using 10% formalin for H&E and immunohistochemistry staining. The right ovaries, on the other hand, were taken for RT-qPCR analysis.
ADMSCs Isolation, Culture, Characterization
Adipose tissue-derived mesenchymal stem cells were obtained from subcutaneous inguinal fat of a 6–8-week-old non-diabetic rat according to the protocol [27]. Briefly, the obtained adipose tissue was washed several times in PBS against contamination of any cell from the blood. Fat tissue was cut into thin pieces and incubated in 0.1% type 1 collagenase. For the inhibition of enzyme activity, DMEM / F12 medium and containing 10% FBS were added in an equal volume and the mixture was centrifuged. Then the cell suspension was passed through a 100 µm filter and centrifuged again. After centrifugation, the supernatant was discarded and the pellet was cultured in DMEM/F12 medium,10% FBS, 1% penicillin / streptomycin-amphotericin B and the cells were allowed to adhere to the culture dish and proliferate. Cells from passage 3 and 4 were used for the experiment. To characterize the cells, immunohistochemical staining was performed and scored according to specific surface antigen criteria [28].
Hormone Assay
The amount of serum AMH in rats was measured by ELISA Assay. Serum was obtained after the centrifugation of cardiac blood just taken before sacrification. Based on the manufacturer protocol, ELISA analysis was performed in serums by using the Bioassay Technology Laboratory AMH ELISA kit (BT-Lab Cat#E0456Ra, China). Then at 450Nm, values were read in the microplate reader and OD values were calculated.
Morphologic Evaluation of Ovary
Left ovary tissues were fixed in 10% formalin. Tissues kept in running tap water for 1 night were passed through increasing alcohol series for dehydration. After the tissues were kept in toluene for transparency, they were embedded in paraffin. By taking sections with a thickness of 5 µm from paraffin blocks, H&E staining was performed. Serial sections were taken from all ovaries belonging to each group and primordial, primary, secondary, and antral follicles and atretic follicles in the ovary were examined by a light microscopy (DM 750 Leica, Heerbrugg, Switzerland) and photographed with a camera (DP71 Olympus, Germany).
Immunohistochemical Analysis
After the sections were deparafinized, they were rehydrated. To block the activation of endogenous peroxide, 3% hydrogen peroxide was used. After the sections were incubated for 10 min at 37 °C with trypsin for antigen retrieval, they were washed with PBS. By adding blocking solution on the sections, nonspecific binding sites were blocked. Sections were incubated at + 4 °C for 1 night with anti-Cx43 (Santa Cruz Biotechnology Cat# sc-271837, RRID: AB_10707826, 1:100), anti Panx1 (Abcam Cat# ab124131, RRID: AB_10972168, 1:300), and anti Casp3 (Santa Cruz Biotechnology Cat# sc-56053, RRID: AB_781826, 1:100) which were primary antibodies. On the next morning, the tissues were washed out by PBS and biotinylated secondary antibodies were added on slides. The sections were then washed back with PBS and incubated with DAB to observe the immune reaction. Nuclear staining of the sections was done with Mayer's hematoxylin. Finally, tissues washed and passed through increasing alcohol series were kept in xylene for 30 min and covered with the entellan. Negative and positive controls of antibodies for immunohistochemical experiments are shown in Fig. S1a.
Immunofluorescence Staining
For immunofluorescence (IF) staining, the sections were blocked with 5% bovine serum albumin (BSA) and, incubated with primary antibodies anti Cx43 (anti Cx43, sc-271837, Santa Cruz, Germany, 1:100) and anti Panx1 (ab139715, Abcam, UK, 1:300) overnight at 4 °C. Then incubated with a secondary antibody (anti-rabbit IgG Alexa Fluor 488 Conjugate Thermo, CST #4412) for 60 min at room temperature. Nuclei were counterstained using DAPI. Slides were visualized under a fluorescence microscope. Negative controls of immunofluorescent experiments are shown in Fig. S1b.
RNA Extraction and RT-qPCR Analysis
Left ovarian tissues taken from all subjects were used for qPCR processes. For RNA extraction, RiboEX solution was added on each ovarian tissue and homogenization was done with homogenizer (TissueLyser LT, Qiagen, USA). RNA isolation was performed according to the RiboEX manufacturer’s protocol. The amount and purity of the obtained RNAs were measured using the Nanodrop (ND-1000 Spectrophotometer, Thermo Fisher Scientific, USA). cDNA synthesis from RNAs was performed using the thermal cycler (Applied Biosystems® Veriti® 96-Well Thermal Cycler, USA) according to the protocol of the Wizscript cDNA synthesis kit (w2211, Wizbiosolutions, South Korea). After cDNA synthesis, mRNA expressions of caspase 3, connexin 43 and pannexin 1 were measured using the WizPureTM qPCR master kit (SYBR) (W1711, Wizbiosolutions). The used primer sequences are given in Table 1 [29,30,31,32]. qPCR analysis was performed with the rotor gene-Q (Qiagen, USA). At the end of the experiment, Ct values were obtained for each sample. GAPDH was used as the housekeeping gene and ΔCt values were calculated by normalizing the obtained Ct values according to the GAPDH level. The resulting values were shown in the graph as gene expression and fold-change values by using the formula 2−ΔΔCt. Each sample of each group was studied as three repetitions.
Scoring and Statistical Analysis
For all antibodies stained, tissues were evaluated in 10 ovarium sections belonging to each animal by taking into account the staining intensity in the 30 areas × 40 objective. This evaluation was carried out by accepting 0 as no staining, 1 as weak staining, 2 as moderate staining, and 3 as strong/intense staining. All data obtained at the end of the experiment were analyzed using the GraphPad Prism 8.3.1 program. First, the groups were tested for normality. Then, all groups were compared using the one-way ANOVA test. The value of P ≤ 0.05 was considered significant in statistical analysis results. Tukey's test was used as a post hoc test for multiple comparisons.
Results
Cultivation and Characterization of Rat ADMSCs
It was shown with marker that the cultivated cells were MSCs. In the IHC results, ADMSCs were positive for CD90, CD44, CD73, and Stro-1, while they were negative for CD34, CD45. In addition, immunohistochemical staining was performed for MSC characterization (Fig. 1).
Characterization of rat adipose derived mesenchymal stem cells (ADMSCs). A–B Representative microscopic images showing morphology of cultured ADMSCs. Immunohistochemical analysis of ADMSCs surface markers. C–F Cells were positive for CD44, CD90, CD73 and Stro-1. G–H Cells were negative for CD34, CD45
Chemotherapy Reduced the Number of Healthy Follicles in Ovary
H&E staining was performed in the ovary for morphological evaluation of CTX exposure. Ovarian follicles in all groups were counted (Fig. 2B). In the control group ovaries, the cortex and medulla had a regular organization. Numerous primordial follicles were observed in the cortex, consisting of an inner primary oocyte and a single layer of flat follicle cells around it. (Fig. 2A-a). Early and late primary follicles consisting of single or multilayered cubic cells around the primary oocyte were seen less in number than primordial follicles. In primary follicles, theca follicle surrounding the zona pellucida and granulosa cells was observed. (Fig. 2A-b). Secondary and antral follicles were observed in normal morphology, which is characterized by the formation of antrum cavity between stratified granulosa cells. (Fig. 2A-c, d). It was seen that some of the primordial follicles in the ovarian tissue of the POI group were degenerate (Fig. 2A-e). The decrease in the number of primordial follicles in the POI group was significant compared to the control (Fig. 2B, p < 0.001). Atresia was observed especially in primary, secondary, and antral follicles. In atretic follicles, degenerated oocytes, and zona pelusida were seen (Fig. 2A-f, g). While the decrease in the number of healthy primary follicles in the POI group was not statistically significant compared to the control group (Fig. 2B, p = 0.112), there was a significant decrease in secondary and antral follicles compared to the control (Fig. 2B, p < 0.001). When the control group and POI group were compared in terms of the number of atretic follicles, a significant increase in the number of atretic follicles was observed in the POI group (Fig. 2B, p < 0.001).
The number of ovarian follicles and H&E staining for all groups A Morphologically normal primordial, primary, secondary, graafian follicles showing with black arrow in control groups (a, b, c, d), Scale bar: 10, 10, 20, 20 µm. Abnormal, degenerated primordial follicle (e), atretic primary, secondary, graafian follicles showing with black arrow in POI group (f, g, h), Scale bar: 10, 10, 10, 10 µm. Morphologically normal primordial, primary, secondary, graafian follicles showing with black arrow in POI + ADMSCs group (i, j, k, l), Scale bar: 10, 20, 20, 20 µm. B Data are presented as mean + standard deviation (SD). ***p < 0.001, **p < 0.002, *p < 0.033. a: significant compared to control, b: significant compared to POI
Chemotherapy Reduced AMH Level in Ovary
Since the AMH level is one of the clinical biomarkers of POI, the AMH level was evaluated in the study by the ELISA method as an indicator of CTX inducing POI. It was observed that AMH levels in the POI group decreased significantly compared to the control group (Fig. 3, p = 0.008).
Chemotherapy Induced Apoptosis in Ovarian Follicles
The immunoreactivity of Casp3, which was the effector enzyme of apoptosis, was evaluated by h-score analysis in the primordial, primary, secondary, and antral follicles of all groups. In the control group follicles, a small number of cells showing mild Casp3 immunoreactivity were encountered. In the POI group, moderate Casp3 immunoreactivity was observed in primordial follicles, while strong Casp3 immunoreactivity was observed in primary, secondary, and antral follicles (Fig. 4A). In the h-score analysis, in the CTX group compared to the control group; The increase in Casp3 expression in primordial follicles was statistically less significant than in primary, secondary and antral follicles (Fig. 4B, p < 0.033, p < 0.001). qRT-PCR results were also supporting IHC results. It was found that Casp3 mRNA levels increased more than 2 times in the POI group compared to the control group (Fig. 4C, p < 0.001).
ADMSCs transplantation decreased the Casp3 expression in ovary of POI groups. A Representative images for immunohistochemical staining of Casp3 in ovarian follicles for each group. The arrows represent Casp3 immunopositive cells. B H-score analysis of Casp3 immunoreactivity among each group. Data are presented as mean ± standard deviation (SD). C The mRNA levels of Casp3. Statistical analysis was done by one-way ANOVA test and Tukey’s test was used for multiple group comparison. ***p < 0.001, **p < 0.002, *p < 0.033. a: significant compared to control, b: significant compared to POI
Chemotherapy Reduced Cx43 Expression in Ovary
The gap junction protein Cx43 immunoreactivity was evaluated by h-score analysis in primordial, primary, secondary, and antral follicles of all groups. Cx43 immunoreactivity was observed at moderate-strong in primordial, primary, secondary, and graaf follicles in the control group. In the POI group, on the other hand, Cx43 immunoreactivity was observed at a mild level in primordial, primary, secondary, antral follicles (Fig. 5A). Compared to the control group, the decrease in Cx43 expression in the POI group was found statistically significant in all follicle types (Fig. 5B, p < 0.001). In addition, IF staining was performed for the immunolocalization of Cx43 (Fig. 7). As a result of IF staining, it was observed that Cx43 expression decreased in the POI group compared to the control. According to qRT-PCR results too, Cx43 mRNA levels were significantly decreased in the POI group compared to control (Fig. 5C, p < 0.001).
ADMSCs transplantation increased the Cx43 expression in ovary of POI groups. A Representative images for immunohistochemical staining of Cx43 in ovarian follicles for each group. The arrows represent Cx43 immunopositive cells. B H-score analysis of Cx43 immunoreactivity among each group. Data are presented as mean ± standard deviation (SD). C The mRNA levels of Cx43. Statistical analysis was done by one-way ANOVA test and Tukey’s test was used for multiple group comparison. ***p < 0.001, **p < 0.002, *p < 0.033. a: significant compared to control, b: significant compared to POI
Chemotherapy Increased Panx1 Expression
Immunoreactivity of Panx1 which is among channel proteins was evaluated by h-score analysis in primordial, primary, secondary, and antral follicles of all groups. Panx1 immunoreactivity was observed at a mild level in ovarian tissue, cortex stroma, and medulla of the control group. Panx1 expression was almost nonexistent in primordial follicles. Panx1 immunoreactivity was also very low in primary, secondary and antral follicles (Fig. 6A). Compared to the control group, strong Panx1 immunoreactivity was observed in all follicle types and ovarian surface epithelium in the ovarian tissue of the POI group, (Fig. 6A). This increase in the Panx1 expression was found to be statistically significant compared to the control group (Fig. 6B, p < 0.001). By IF staining, it was able to be observed that there was a significant increase in Panx1 expression in ovarian follicles (Fig. 7). According to the qRT-PCR results, Panx1 mRNA level was significantly increased in the POI group compared to the control (Fig. 6C, p < 0.001).
ADMSCs transplantation decreased the Panx1 expression in ovary of POI groups. A Representative images for immunohistochemical staining of Panx1 in ovarian follicles for each group. The arrows represent Panx1 immunopositive cells. B H-score analysis of Panx1 immunoreactivity among each group. Data are presented as mean ± standard deviation (SD). C The mRNA levels of Panx1. Statistical analysis was done by one-way ANOVA test and Tukey’s test was used for multiple group comparison. ***p < 0.001, **p < 0.002, *p < 0.033. a: significant compared to control, b: significant compared to POI
ADMSCs Transplantation Restored Ovarian Function and Increased the Number of Healthy Follicles
In the POI + ADMSCs group, the number of healthy follicles in the cortex was higher compared to the POI group. Healthy primordial and primary follicles were observed in areas close to the surface under the tunica albuginea (Fig. 2A-i, j). In the POI + ADMSCs group, compared to the POI group, whereas the increase in the number of primordial follicles was statistically significant (Fig. 2B, p = 0.049), no significant increase in the number of primary follicles was observed (Fig. 2B, p = 0.205). Although atretic follicles were observed in some areas, the morphology of the developing follicles was observed as normal (Fig. 2A-k, l). When looking at the number of secondary and antral follicles in the POI + ADMSCs group, significant increases were found in both types of follicles compared to the POI group (Fig. 2B, p = 0.018, p = 0.031). Finally, there was also a decrease by half in the number of atretic follicles in the POI + ADMSCs group compared to the POI group (Fig. 2B, p < 0.001).
ADMSCs Transplantation Increased AMH Level in Ovary
In the POI + ADMSCs group, the level of AMH increased significantly compared to the POI group (Fig. 3, p = 0.027). When the POI + ADMSCs group was compared with the control, no significant difference was found (Fig. 3, p > 0.999).
ADMSCs Transplantation Repressed Apoptosis Through the Cx43 and Panx1
It was observed that ADMSCs transplantation reduced apoptosis through decreasing Casp3 expression in all follicle types. In POI + ADMSCs group Casp3 immunoreactivity was found to be mild in all follicle types (Fig. 4A). Also, h-score analyses showed that Casp3 immunoreactivity decreased statistically significantly (Fig. 4B, p < 0.001). In qRT-PCR analyses, on the other hand, Caps3 mRNA levels decreased significantly in this group (Fig. 4C, p < 0.001). In the POI + ADMSCs group, Cx43 immunoreactivity was moderate to strong in primary, secondary, antral follicles (Fig. 5A). This increase in all follicle types was found significant when compared to the POI group (Fig. 5B, p < 0.001). By IF staining, a significant increase in Cx43 expression was observed in ADMSCs groups (Fig. 7). In Cx43 mRNA expression, an increase correlated with IHC results was observed (Fig. 5C, p = 0.001). Panx1 immunoreactivity in ovarian follicles of POI + ADMSCs group was observed at mild/moderate. Rather than follicles, Panx1 expression was observed in stroma and theca externa cells (Fig. 6A). In the POI + ADMSCs group, Panx1 expression in all follicle types decreased significantly compared to the POI group (Fig. 6B, p < 0.001). Also, IF staining showed the increasing of Panx1 expression in ADMSCs group (Fig. 7). A significant decrease in Panx1 mRNA level was also found in the POI + ADMSCs group compared to the POI group (Fig. 6C, p = 0.005).
Discussion
Until recently, little was known about POI. Despite many studies, there is no clear treatment for the disease. The biggest reason for this is that the mechanism for the formation of the disease has not been fully revealed. In this study, POI was induced in rats with cyclophosphamide, one of the most common triggers of the disease. Since it was considered that Cx43 and Panx1 molecules were directly related to follicular cells’ apoptosis which is the main mechanism of the disease, the association of these molecules with POI was evaluated. In addition, the effect of ADMSCs transplantation, suggested in the regenerative treatment of POI, on these molecules was shown. In our study, a decrease in all ovarian follicles was detected after CTX administration. In the POI group, the number of primordial and primary follicles decreased less than the number of secondary and antral follicles. In a study conducted by Badaway et al., after a single dose of CTX injection in BALB/C rats, a decrease was found in primordial and primary follicles from day 4; but at the end of the 28th day, the number of primordial and primary follicles decreased too much (almost disappeared) [33]. In our study, on the other hand, because the experimental process was terminated earlier, a less decrease in the primordial and primary follicles of the POI group was determined. After ADMSCs transplantation, an increase in all follicle types was observed. In a study conducted by Sun et al. [14], an increase in the number of primordial and primary follicles was found in both groups as a result of ADMSCs administration, which they gave via in situ and intravenous ways in POI that they created with CTX in rats. These researchers also reported that this condition reduced apoptosis in the granulosa cells of stem cells and thereby stimulated the development of healthy follicles.
Hormonal evaluations are the most important of POI's clinical diagnostic criteria. In studies, it has been reported that AMH level is more sensitive than FSH level, and it is an important indicator for early diagnosis of POI since it can be measured at any stage of the menstrual cycle [34]. In our study, a statistically significant decrease was found in the POI group compared to the control group. In a study conducted on rats, Liu et al. observed a significant decrease in AMH and estradiol 2 (E2) levels at the 1st and 2nd weeks in POI induced by CTX and busulfan. They reported that the decrease in AMH levels was due to the fact that CTX exposure caused the transformation of normal follicles into atretic follicles by suppressing the PI3K/AKT pathway, which was effective in cell growth and proliferation in granulosa cells [35]. In another study, Zhang et al. reported that as a result of suppression of the PI3K/AKT/mTOR pathway, the cytochrome c release from mitochondria started with the translocation of the BAX protein to mitochondria in the cytoplasm, it triggered apoptosis in the ovary follicles, and caused the level of AMH to decrease [36]. In their meta-analysis study examining the results of 45 studies conducted on a total of 5607 young and adult women who were subjected to chemotherapy, Overbeek et al. noted that there were high levels of FSH, low levels of AMH, inhibin, and E2 in women who received chemotherapy compared to control groups. They also stated that this situation reflects decreased ovarian function in patients undergoing chemotherapy [37]. In addition, it is thought that due to the presence of estrogen-sensitive regions of the AMH gene, decreased E2 levels in POI affect AMH levels [38]. In this study, an increase in AMH level was observed because of ADMSCs transplantation for therapeutic purposes in the POI group. It is thought stem cells stimulate the development, proliferation, and cell cycle in the ovary and suppress cell death proteins. In a study conducted by Yang et al., AMH levels increased as a result of umbilical cord mesenchymal stem cells transplantation in POI rats. Related to this result, the researchers noted that increasing the proliferation marker Ki67 in granulosa cells in the stem cell group triggered angiogenesis in the ovary and ensured improvement of ovarian function and follicle development [39]. Since there are few studies showing the effectiveness of ADMSCs in POI, the molecular mechanisms they affect are not much known. Huang et al. gave the exosomes they obtained from ADMSCs to rats exposed to CTX. They reported that by increasing the expression of SMAD2, 3 and 5 proteins, ADMSCs exosomes provided an improve in ovarian function and thus an increase in AMH levels [40].
In this study, the amount of Casp3, the effector enzyme of apoptosis, was demonstrated in POI by qPCR, IF and IHC methods. It is thought that one of the most basic mechanisms underlying the gonadotoxicity caused by CTX in the ovary is the triggering of the apoptotic pathway. Pascuali et al. [41] observed high Casp3 expression in developing ovarian follicles in POI induced by a single dose of CTX exposure in mice, but not Casp3 expression in primordial follicles. They suggested that CTX could trigger apoptosis in the developing follicles to destroy them and trigger the activation of new reserve primordial follicles to replace the destroyed developing follicles, causing the ovarian “burnout” phenomenon, that is, the depletion of the ovarian reserve. In addition, they supported this idea by demonstrating the activation of the PI3K/PTEN/Akt pathway in the CTX group primordial follicles [41]. Luo et al. showed increased apoptosis in granulosa cells in POI induced by CTX and busulfan with TUNNEL assay [42]. In a study they conducted, Xicong et al. reported that CTX induces activation of the p53-p66Shc pathway in the POI model. They also emphasized that this pathway also reduced resistance to reactive oxygen species (ROS) and triggered apoptosis in the ovary by inducing Casp3 activation [43]. In our study, Casp3 mRNA and protein expression decreased in all follicle types in the POI group as a result of ADMSCs transplantation. The most important reason for this is the antiapoptotic properties that ADMSCs have. There are few studies in the literature showing the effect of ADMSCs on apoptosis in POI. Sun et al. administered ADMSCs directly to the ovaries in rats and they showed decreased apoptosis in ADMSCs grous by using Tunnel method [14]. Su et al. also found that when they administered ADMSCs to rats, in which they created POI, together with collagen scaffolds, the amount of Casp3 decreased in the ovary of rats [13]. Huang et al. found that when they administered exosomes obtained from ADMSCs to rats with POI, there was a decrease in Casp3 expression [40].
In this study, significant decreasing in protein and mRNA expression of Cx43, which was among the gap junction molecules, were found in the POI group. It has been emphasized in many studies that Cx43 is effective not only as a channel protein that enables intercellular signal transduction, but also in important processes such as folliculogenesis, oogenesis, steroidogenesis, and apoptosis [16, 44]. It has been noted that Cx43, which is known to play a critical role in fertility in women, is correlated with both Cx43 expression and pregnancy rate in IVF applications (18). But there are very few studies regarding the POI-related state of such an important protein that is responsible for the functioning of the ovary. In a study conducted by Prunskaite et al., when they knocked out the WNT4 gene, which has important functions in female fertility and folliculogenesis, they found that POI was triggered in rats; they also noted that as a result of WNT4 knockout, the Cx43 protein expression of the cell adhesion proteins decreased [45]. Similar to our results, Besikcioglu et al. encountered decreased Cx43 expression in ovarium tissue of POI group rats and they thought that the decrease in Cx43 might be responsible for the decrease in BMP6 and BMP15 [46]. There are important studies indicating that Cx43 is an effective protein in apoptosis. In their study, Krysko et al. reported that Cx43 expression in granulosa cells was inversely proportional to the apoptotic index and that Cx43 had important roles in terms of the survival of granulosa cells [20]. In the same study, researchers reported that intracellular apoptotic signals were transmitted between cells through gap junctions, so gap junction protein expression increased in apoptosis, but Cx43 regulated cell survival rather than channel function where death signals were transmitted [20]. In another study, it was reported that estrogen in the ovary increased Cx43 expression and low estrogen levels caused a decrease in Cx43. Since this condition reduced the passage of signals, nutrients, and growth factors between granulosa cells, case apoptosis was triggered in granulose cells [47]. In studies conducted on different tissue types and cells, it has also been noted that with the reduction or inhibition of Cx43 expression, the amount of ROS increases in the cells, and as a result, apoptosis is induced [48]. In our study too, we believe that a decreased Cx43 amount in the POI group may be associated with hypogonadism, which is the main clinical indicator of the disease. Low estrogen levels lead to decreased in Cx43 expression in granulosa cells, and this also triggers the increased in Casp3 expression. In our study, increased Cx43 protein and mRNA expression were detected as a result of ADMSCs administration in the POI group. In the literature, no studies related to Cx43 expression in POI after ADMSCs transplantation were encountered. As a result of bone marrow-derived mesenchymal stem cells (BM-MSCs) administration, Beşikcioğlu et al. found an increase in follicle maturation markers and Cx43 expression in POI group rats [46]. Hwangbo et al. observed that Cx43 expression increased in the heart tissue of rats when they transplant ADMSCs to rats in which they created myocardial infarction (MI) [49]. Similarly, Yi Li et al. also reported that Cx43 expression that decreased as a result of MI was reorganized and increased as a result of stem cell administration [50]. In conclusion, in also our study, as in other tissues, Cx43 expression increased in POI with ADMSCs administration in ovarian tissue, and we believe that this increase is due to the healing effects provided by stem cells.
In this study, the expression of Panx1 in the ovary was shown for the first time by immunohistochemical immunofluorescence and RT-qPCR analyses. Panx1 channels are often represented as non-selective channels that release ATP. Extracellular ATP has important roles in many physiological events [24]. Extracellular ATP controls intracellular ATP signaling via purinergic receptors. Panx1 channels also continue to be activated by ATP via purinergic receptors [51]. In the ovary, in response to gonadotropic hormones, purinergic signals regulate the processes of cell proliferation, steroidogenesis, and apoptosis [25]. In the ovary, activation of P2 receptors, which are among purinergic receptors, by ATP affects both proliferation and cell death of human granulosa-luteal cells. These antagonistic responses to ATP increase the expression of proliferative response-associated genes, including EGR1 and RAF1, by inducing activation and nuclear translocation of ERK 1/2 via P2YR receptors. On the other hand, it has been reported that P2RX7 receptors that have long-term exposure to high ATP concentrations are able to promote apoptosis by providing activation of pro-caspase-3 and proteolysis of PARP [52]. Since there are no studies related to Panx1 expression in the ovarium until this time, it is thought that Panx1 may control ovarium functions through purinergic receptors. Moreover, there are some studies on the function and localization of Panx1 in the male reproductive system. By taking into account the well-known role of ATP in sperm maturation and the role of Panx channels in ATP secretion, a study reported that Panxs played a role in the secretion of ATP out of the cell in luminal and basal cells in the epididymis [53]. In the same study, it was also reported that Panx1 was controlled by testosterone and other testicular factors. Based on the male reproductive system as well, it is thought that Panx1 may also have a role in the process of steroidogenesis and folliculogenesis in the ovary. As with the ovary, there is not any study showing Panx1 expression in POI.
As a result of our study, it was found that in the POI model produced with CTX in rats, Panx1 expression increased compared to the control group. In a study conducted by Zhou et al., Panx1 expression in intracerebral hemorrhage (ICH) was examined, and it was reported that Panx1 expression significantly increased in rat brain tissue after ICH, apoptotic neurons significantly decreased in the group to which Panx1 inhibitor carbenoxolone was given, and Casp3 protein expression significantly decreased in the western blot analysis performed [54]. In a study conducted by Benabou et al., it was revealed that Casp3 expression and neuronal cell death reduced significantly with the addition of a Panx1 inhibitor to the medium in hippocampal slice cultures [55]. This suggests that an underlying cause of increased apoptosis and Casp3 expression occurring in granulosa cells in POI may also be Panx1 activation in the ovary via P2X7R receptors. It is thought that another underlying cause of increased Panx1 expression in the CTX group may also be inflammation. Among the ovotoxic effects of CTX, there is also triggering inflammation in granulosa cells [56, 57]. Makarenkova et al. reported that Panx1 expression and activation increased with inflammation [58]. Dahl et al. also said in their study that Panx1 might be the main target in the suppression of inflammation associated with secondary cell death. Panx1 inhibitor has been suggested as a potential therapeutic to limit secondary cell death of probenecid [51]. In our study, a statistically significant decrease in Panx 1 expression was achieved as a result of ADMSCs transplantation in POI. It is believed that ADMSCs provide this effect through reducing apoptosis and inflammation. In the literature, we could not find any study mentioning the effects of ADMSCs on Panx1 expression. In this sense, it was shown for the first time in our study that ADMSCs also had effects on Panx1 expression.
Conclusion
In this study, the association of Cx43 and Panx1 molecules with apoptosis in granulosa cells in chemotherapy-induced POI was shown for the first time. Several agents are available to channels built up by connexin and pannexin proteins, including Alcoholic substances, glycyrrhetinic acid, anesthetics and fatty acids inhibit connexin hemichannels and pannexin channels. Mimetic peptides, which reproduce specific amino acid sequences in connexin or pannexin primary protein structure, need to be investigated for clinical use. Because of their therapeutic potential and translational relevance as target, both Cx and Panx channels should be studied in terms of mechanisms in ovary. Therefore, the situation of these molecules in the POI has been wondered. The fact that the role of ADMSCs transplantation on these molecules has also been reported thanks to this study is in character that will guide the methods to be developed for POI treatment in the future. Also, in future studies ADMSCs transplantation with inhibitors or activators of these molecules can be recommended as a suggestion for the treatment of POI.
References
Santoro NF, Cooper AR. Primary ovarian insufficiency: a clinical guide to early menopause. Springer; 2016.
ESHRE. Management of women with premature ovarian insufficiency. Guideline of the European Society of Human Reproduction and Embryology; 2015.
Bath L, Wallace W, Shaw M, Fitzpatrick C, Anderson R. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Müllerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–74. https://doi.org/10.1093/humrep/deg473.
Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–43. https://doi.org/10.1093/humupd/7.6.535.
Hao X, Anastácio A, Liu K, Rodriguez-Wallberg KA. Ovarian follicle depletion induced by chemotherapy and the investigational stages of potential fertility-protective treatments-a review. Int J Mol Sci. 2019;20(19):4720. https://doi.org/10.3390/ijms20194720.
Jeelani R, Khan SN, Shaeib F, Kohan-Ghadr H-R, Aldhaheri SR, Najafi T, et al. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med. 2017;110:11–8. https://doi.org/10.1016/j.freeradbiomed.2017.05.006.
Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–14. https://doi.org/10.1056/NEJMcp0808697.
Nguyen Q, Zerafa N, Liew S, Findlay JK, Hickey M, Hutt K. Cisplatin-and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod. 2019;25(8):433–44. https://doi.org/10.1093/molehr/gaz020.
Szymanska KJ, Tan X, Oktay K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol Hum Reprod. 2020;26(8):553–66. https://doi.org/10.1093/molehr/gaaa043.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8. https://doi.org/10.1016/j.jcyt.2013.02.006.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–4. https://doi.org/10.1016/j.tibtech.2006.01.010.
Ntege EH, Sunami H, Shimizu Y. Advances in regenerative therapy: A review of the literature and future directions. Regener Ther. 2020;14:136–53. https://doi.org/10.1016/j.reth.2020.01.004.
Su J, Ding L, Cheng J, Yang J, Li XA, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31(5):1075–86. https://doi.org/10.1093/humrep/dew041.
Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. https://doi.org/10.1186/scrt231.
Bao R, Xu P, Wang Y, Wang J, Xiao L, Li G, et al. Bone marrow derived mesenchymal stem cells transplantation rescues premature ovarian insufficiency induced by chemotherapy. Gynecol Endocrinol. 2018;34(4):320–6. https://doi.org/10.1080/09513590.2017.1393661.
Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol. 2008;282(1–2):18–25. https://doi.org/10.1016/j.mce.2007.11.001.
Hervé J-C, Phelan P, Bruzzone R, White TW. Connexins, innexins and pannexins: bridging the communication gap. Biochem Biophys Acta. 2005;2005(1719):3–5. https://doi.org/10.1016/j.bbamem.2005.11.013.
Winterhager E, Kidder GM. Gap junction connexins in female reproductive organs: implications for women’s reproductive health. Hum Reprod Update. 2015;21(3):340–52. https://doi.org/10.1093/humupd/dmv007.
Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM. Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J Cell Sci. 2005;118(1):113–22. https://doi.org/10.1242/jcs.01587.
Krysko DV, Mussche S, Leybaert L, D’Herde K. Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. J Histochem Cytochem. 2004;52(9):1199–207. https://doi.org/10.1369/jhc.3A6227.2004.
Panchina Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10(13):R473–4. https://doi.org/10.1016/s0960-9822(00)00576-5.
Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, et al. Pannexin channels are not gap junction hemichannels. Channels. 2011;5(3):193–7. https://doi.org/10.4161/chan.5.3.15765.
Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta Biomembr. 2013;1828(1):15–22. https://doi.org/10.1016/j.bbamem.2012.01.017.
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett. 2007;581(3):483–8. https://doi.org/10.1016/j.febslet.2006.12.056.
Martínez-Ramírez AS, Vázquez-Cuevas FG. Purinergic signaling in the ovary. Mol Reprod Dev. 2015;82(11):839–48. https://doi.org/10.1002/mrd.22537.
Kibschull M, Gellhaus A, Carette D, Segretain D, Pointis G, Gilleron J. Physiological roles of connexins and pannexins in reproductive organs. Cell Mol Life Sci. 2015;72(15):2879–98. https://doi.org/10.1007/s00018-015-1965-4.
Lotfy A, Salama M, Zahran F, Jones E, Badawy A, Sobh M. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells. 2014;7(2):135. https://doi.org/10.15283/ijsc.2014.7.2.135.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. https://doi.org/10.1080/14653240600855905.
Cai X-J, Wang L, Hu C-M. Effects of GABAB receptor activation on spatial cognitive function and hippocampal neurones in rat models of type 2 diabetes mellitus. Biosci Rep. 2018;38(1).https://doi.org/10.1042/BSR20171184.
Burns AR, Phillips SC, Sokoya EM. Pannexin protein expression in the rat middle cerebral artery. J Vasc Res. 2012;49(2):101–10. https://doi.org/10.1159/000332329.
Rajamanickam GD, Kastelic JP, Thundathil JC. The ubiquitous isoform of Na/K-ATPase (ATP1A1) regulates junctional proteins, connexin 43 and claudin 11 via Src-EGFR-ERK1/2-CREB pathway in rat Sertoli cells. Biol Reprod. 2017;96(2):456–68. https://doi.org/10.1095/biolreprod.116.141267.
Mosadegh M, Hasanzadeh S, Razi M. Nicotine-induced damages in testicular tissue of rats; evidences for bcl-2, p53 and caspase-3 expression. Iran J Basic Med Sci. 2017;20(2):199. https://doi.org/10.22038/ijbms.2017.8249.
Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Women’s Health. 2017;9:441. https://doi.org/10.2147/IJWH.S134074.
Alipour F, Rasekhjahromi A, Maalhagh M, Sobhanian S, Hosseinpoor M. Comparison of specificity and sensitivity of AMH and FSH in diagnosis of premature ovarian failure. Dis Mark. 2015;2015:585604. https://doi.org/10.1155/2015/585604.
Liu M, Qiu Y, Xue Z, Wu R, Li J, Niu X, et al. Small extracellular vesicles derived from embryonic stem cells restore ovarian function of premature ovarian failure through PI3K/AKT signaling pathway. Stem Cell Res Ther. 2020;11(1):3. https://doi.org/10.1186/s13287-019-1508-2.
Zhang H, Qin F, Liu A, Sun Q, Wang Q, Li Q, et al. Kuntai capsule attenuates premature ovarian failure through the PI3K/AKT/mTOR pathway. J Ethnopharmacol. 2019;239: 111885. https://doi.org/10.1016/j.jep.2019.111885.
Overbeek A, van den Berg MH, van Leeuwen FE, Kaspers GJ, Lambalk CB, van Dulmen-den BE. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: a systematic review. Cancer Treat Rev. 2017;53:10–24. https://doi.org/10.1016/j.ctrv.2016.11.006.
Lee J-H, Lee M, Ahn C, Kang HY, Tran DN, Jeung E-B. Parabens accelerate ovarian dysfunction in a 4-vinylcyclohexene diepoxide-induced ovarian failure model. Int J Environ Res Public Health. 2017;14(2):161. https://doi.org/10.3390/ijerph14020161.
Yang Y, Lei L, Wang S, Sheng X, Yan G, Xu L, et al. Transplantation of umbilical cord–derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. Vitro Cell Dev Biol Anim. 2019;55(4):302–11. https://doi.org/10.1007/s11626-019-00337-4.
Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):1–12. https://doi.org/10.1186/s13287-018-0953-7.
Pascuali N, Scotti L, Di Pietro M, Oubiña G, Bas D, May M, et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod. 2018;33(5):844–59. https://doi.org/10.1093/humrep/dey045.
Luo Q, Liu R, Wang L, Hou Y, Zhang H. The effects of inhibin B in the chemotherapy drug-induced premature ovarian insufficiency mice and hPMSCs treatment. Reprod Sci. 2020;27(5):1148–55. https://doi.org/10.1007/s43032-019-00128-y.
Xiong Y, Liu T, Wang S, Chi H, Chen C, Zheng J. Cyclophosphamide promotes the proliferation inhibition of mouse ovarian granulosa cells and premature ovarian failure by activating the lncRNA-Meg3-p53-p66Shc pathway. Gene. 2017;596:1–8. https://doi.org/10.1016/j.gene.2016.10.011.
Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction-Cambridge. 2002;123(5):613–20. https://doi.org/10.1530/REP.0.1230613.
Prunskaite-Hyyryläinen R, Shan J, Railo A, Heinonen KM, Miinalainen I, Yan W, et al. Wnt4, a pleiotropic signal for controlling cell polarity, basement membrane integrity, and antimüllerian hormone expression during oocyte maturation in the female follicle. FASEB J. 2014;28(4):1568–81. https://doi.org/10.1096/fj.13-233247.
Besikcioglu HE, Sarıbas GS, Ozogul C, Tiryaki M, Kilic S, Pınarlı FA, et al. Determination of the effects of bone marrow derived mesenchymal stem cells and ovarian stromal stem cells on follicular maturation in cyclophosphamide induced ovarian failure in rats. Taiwan J Obstet Gynecol. 2019;58(1):53–9. https://doi.org/10.1016/j.tjog.2018.11.010.
Sun X-F, Li Y-P, Pan B, Wang Y-F, Li J, Shen W. Molecular regulation of miR-378 on the development of mouse follicle and the maturation of oocyte in vivo. Cell Cycle. 2018;17(18):2230–42. https://doi.org/10.1080/15384101.2018.1520557.
Shin K-T, Nie Z-W, Zhou W, Zhou D, Kim J-Y, Ock SA, et al. Connexin 43 knockdown induces mitochondrial dysfunction and affects early developmental competence in porcine embryos. Microsc Microanal. 2020:1–10. https://doi.org/10.1017/S1431927620000033.
Hwangbo S, Kim J, Her S, Cho H, Lee J. Therapeutic potential of human adipose stem cells in a rat myocardial infarction model. Yonsei Med J. 2010;51(1):69–76. https://doi.org/10.3349/ymj.2010.51.1.69.
Li J-Y, Ke H-H, He Y, Wen L-N, Xu W-Y, Wu Z-F, et al. Transplantation of mesenchymal stem cells modulated Cx43 and Cx45 expression in rats with myocardial infarction. Cytotechnology. 2018;70(1):225–34. https://doi.org/10.1007/s10616-017-0136-x.
Dahl G, Keane RW. Pannexin: from discovery to bedside in 11±4 years? Brain Res. 2012;1487:150–9. https://doi.org/10.1016/j.brainres.2012.04.058.
Tai C-J, Chang S-J, Chien L-Y, Leung PC, Tzeng C-R. Adenosine triphosphate induces activation of caspase-3 in apoptosis of human granulosa-luteal cells. Endocr J. 2005;52(3):327–35. https://doi.org/10.1507/endocrj.52.327.
Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, et al. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78(2):124–38. https://doi.org/10.1002/mrd.21280.
Zhou L, Liu C, Wang Z, Shen H, Wen Z, Chen D, et al. Pannexin-1 is involved in neuronal apoptosis and degeneration in experimental intracerebral hemorrhage in rats. Mol Med Rep. 2018;17(4):5684–91. https://doi.org/10.3892/mmr.2018.8624.
de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, et al. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36(10):2232–7. https://doi.org/10.1161/01.STR.0000182239.75969.d8.
Abolaji AO, Adedara IA, Abajingin AO, Fatunmibi OJ, Ladipo EO, Farombi EO. Evidence of oxidative damage and reproductive dysfunction accompanying 4-vinylcyclohexene diepoxide exposure in female Wistar rats. Reprod Toxicol. 2016;66:10–9. https://doi.org/10.1016/j.reprotox.2016.09.009.
Elkady M, Shalaby S, Fathi F, El-Mandouh S. Effects of quercetin and rosuvastatin each alone or in combination on cyclophosphamide-induced premature ovarian failure in female albino mice. Hum Exp Toxicol. 2019;38(11):1283–95. https://doi.org/10.1177/0960327119865588.
Makarenkova HP, Shestopalov VI. The role of pannexin hemichannels in inflammation and regeneration. Front Physiol. 2014;5:63. https://doi.org/10.3389/fphys.2014.00063.
Acknowledgements
This study was supported by the Scientific Research Committee of Manisa Celal Bayar University (Project number: 2019-021). The authors would like to thank Dr. Suna Karadeniz Saygili and Dr. Tuna Onal.
Funding
This study was supported by the Scientific Research Committee of Manisa Celal Bayar University (Project number: 2019–021).
Author information
Authors and Affiliations
Contributions
Busra SEN HALICIOGLU carried out experiment, design of study, analysis of data, and final approval of the version to be published. Khandakar A. S. M. SAADAT carried out q-RT-PCR analysis. Mehmet Ibrahim TUGLU contributed to the conception and design of the study, final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics Approval
This study was conducted by the approval of Manisa Celal Bayar University Faculty of Medicine Animal Experiments Local Ethics Committee (Approval number: 77.637.435; Date: 13/03/2018).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sen Halicioglu, B., Saadat, K.A.S.M. & Tuglu, M.I. Adipose-Derived Mesenchymal Stem Cell Transplantation in Chemotherapy-Induced Premature Ovarian Insufficiency: the Role of Connexin and Pannexin. Reprod. Sci. 29, 1316–1331 (2022). https://doi.org/10.1007/s43032-021-00718-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00718-9