Abstract
It is known that the extracellular matrix structure and composition changes with aging in many organs. Despite this, knowledge on how does the extracellular part of the ovary change with increasing age in women and how those changes might be related to women’s loss of fertility is still lacking. For this, we propose that recurrent injury and repair events on the outermost layers of the ovary due to ovulation are partly responsible for those changes women experience with aging. The histological analysis of the ovaries from 18 female-to-male transgender patients revealed that the ovarian tunica albuginea (TA) increases its thickness and density correlatively with increasing age of the patient (r = 0.52 and r = 0.55, P < 0.05 respectively). The increase in thickness is independent of the total androgen dose received and occurs because of the appearance of defined fibrotic areas underneath the TA layer which increase the total distance of dense connective tissue from the ovarian surface. In conclusion, the ovarian TA increases in its thickness and density with aging because of the appearance of fibrotic areas underneath the layer in transgender patients. This fact might contribute to reduce oocyte quality and cause ovulation difficulties in older women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For women in populations which do not practice contraception, the mean age for the end of fertility is approximately 39–41 years old [1]. However, menopause generally occurs at the later age of 48–50 years old [2]. This data indicates that these women suffer from a loss of fertility while still having menstrual cycles and regular ovulation [3]. Most of the existing hypotheses for this decline in female fertility with age are centered on the study of reproductive cells, oocytes, and how their quantity and quality decreases. One of the primary reasons for the decrease in quantity is the exhaustion of the ovarian reserve by follicular atresia [4, 5]. Regarding the decline in oocyte quality, one of the catalysts is believed to be a change in the ovarian microenvironment. This is caused by the presence of an increased number of reactive oxygen species. This increase in free radicals affects the functionality of numerous oocyte organelles and proteins, causing problems in the meiotic spindle and a higher occurrence of aneuploidy, amongst other negative consequences [4,5,6].
The physical and compositional changes occurring in the ovarian stroma are known to modify the ovarian and follicular environments, thus causing a decline in oocyte quality [7]. The extracellular matrix (ECM), which is mainly secreted by fibroblasts, is a fundamental part of ovarian function and provides a suitable environment for the cells that constitute the organ [6, 7]. The ECM is primarily composed of proteins, glycosaminoglycans, and protein fibers, with the latter group including collagen, reticular, and elastic fibers [8]. Variations within the aforementioned elements which comprise the ECM affect the physical properties of the connective tissue , which in turn affect the diffusion of hormones and nutrients available for the follicles and other cells, thus modifying oocyte quality [9]. The composition of the ECM in humans and other mammals is known to change with increasing age [10]. Many tissues are generally damaged by decreasing hydration and elasticity, which renders the ECM stiffer and increasingly dense. This change in the ECM’s physical characteristics is believed to be a cause of the difficulties regarding follicular development and ovulation found in older women [11, 12].
Ovulation can be seen as an inflammatory process that injures the ovary by rupturing the outermost layers to liberate a fully mature oocyte, following follicular growth and atresia [13]. Hence, the ovarian structure requires reparation every time ovulation occurs. It is known that repeated injuries to the same organ can cause chronic inflammation, scarring, and fibrosis to the affected tissue [14]. Thus, this recurring cycle of damage and reparation in the outermost layers of the ovarian cortex may be one of the causes for consequent alteration in the structure of the ovarian stroma with increasing age.
Women with gender identity disorder (GID) undergoing female-to-male surgery could potentially provide an optimal source of healthy ovaries for research purposes. In these patients, testosterone is administered before and after genital reconstructive surgery, with target serum levels of 12–24 nM inducing virilization and suppressing secondary female sex characteristics [15]. Several studies have shown that the administration of testosterone for long periods of time can cause many side effects such as an increase of arterial stiffness [13] and visceral fat masses [14] and may even cause ovarian hyperplasia [16] which in turn may render the ovaries obtained from these transgender patients unsuitable for research regarding the effects of aging on the ovaries. It is necessary to assess whether ovaries from these patients might provide a useful model for ovarian aging. We also aim to analyze whether testosterone treatments or the pre-existence of polycystic ovary syndrome (PCOS) have any visible effects on the ovarian stromal structure, independently of the effects of aging on the ovarian ECM cortical structure of these patients.
Results
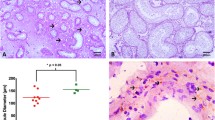
When the effect of the patients’ age on the thickness and the density of the ovarian TA was analyzed, a moderate significant positive linear correlation between the thickness of the TA and the age of the patient was identified (Fig. 1a; r = 0.52, P < 0.05). The findings were further confirmed by a second analysis of the same samples stained following Masson’s trichrome method (Supplementary Information 1).
a A moderate positive linear correlation was detected between the thickness of the TA layer and the age of the patients (r = 0.52, P < 0.05). b A second moderate positive linear correlation was detected between the density of the TA and the age of the patients (r = 0.55, P < 0.05). TA tunica albuginea, r Spearman coefficient
Furthermore, a second moderate positive linear correlation was also detected between the density of the TA and the patient’s age (Fig. 1b; r = 0.55, P < 0.05). The presence of these two positive correlations indicates that the area comprised of the ovarian TA expands and becomes more compact in older individuals. This is further confirmed by the presence of a third moderate positive correlation between the TA thickness and density (Fig. 2; r = 0.54, P < 0.05). The increase in the thickness of the TA appears to be mainly due to the development of areas of fibrosis underneath this layer, which increases the total volume of the dense connective tissue under the ovarian surface. Groups of primordial follicles can be observed below or surrounded by these fibrotic areas (Fig. 3), indicating that the ovaries from these older patients remain active and are not atrophic.
a Ovarian section from a young transgender donor (21 years old). There is a clear separation between the area comprised of dense connective tissue of the TA and the rest of the cortical area. Groups of ovarian follicles (circled area) can be seen just underneath the layer. b Ovarian section from an old transgender donor (38 years old) presenting a pronounced fibrotic growth underneath the TA layer. The fibrotic area comprised of dense connective tissue is devoid of ovarian follicles. TA tunica albuginea. Scale bars represent 200 μm
When the effect of hormonal treatment on the TA parameters was studied, no correlation was detected between the patient’s total dosage of androgens and the thickness (P = 0.76) or density (P = 0.14) of the ovaries’ TA (Fig. 4a and b, respectively). The presence of PCOS or of a high concentration of serum testosterone (> 54 IU) amongst the patients was also analyzed (Table 1). A total of five patients (27.8%) were diagnosed with PCOS. Of those diagnosed, only one patient (5.6%) was diagnosed before the onset of hormonal treatment and four patients (22.2%) were diagnosed after. When both parameters were further analyzed against the density and thickness of the TA, no correlation was detected. Having a high dose of testosterone in serum was not related to the posterior development of PCOS.
Discussion
In this study, we demonstrated that in GID patients, the ovarian cortical area, comprised of dense connective tissue, grows in thickness and density with increasing chronological age. Neither the total androgen dosage received nor the length of the androgen treatment appears to have any evident impact on this development.
It is a known fact that the ovarian surface breaks every time ovulation occurs, due to the action of proteolytic enzymes and the thinning and degradation of the aforementioned layer, injuring the ovary and requiring reparation every time it occurs [17, 18]. It is to be expected that the older an individual is, the more cycles of injury and repair the ovaries will have suffered. This constant remodeling causes an increase in the quantity of dense connective tissue just under the TA layer, due to excessive fibrillary collagen deposition. This is most likely due to a loss of the enzymatic regulation for collagen degradation, thus creating the appearance of areas with fibrosis on the tissues, replacing the normal, functional tissue [19,20,21]. Numerous studies mention that constant cycles of damage and repair within an organ cause permanent damage in the form of fibrosis and the scarring of the tissues [22, 23]. In studies involving ovaries from cancer patients receiving chemotherapy treatment, areas of fibrosis in the cortical area just beneath the TA have been detected. This phenomenon also occurs in some patients who have not received any treatment [24] and in postmenopausal ovaries [25]. These changes in the ovarian stroma are considered to be one of the reasons contributing to the fertility problems these patients experience after chemotherapy treatments or as they age. Further supporting this hypothesis for fertility loss with aging, recent studies in mice have shown that older mice tend to ovulate fewer oocytes than younger mice, while their ovaries possess an aberrant ovarian surface epithelium with increased thickness [26]. It has also previously been reported that the oncogenic behavior of the ovarian surface epithelium is most likely related to the loss of oocytes in the ovary which occurs with aging [27].
In our study, we have detected that the older an individual is, the more the ovarian TA increases in density. Intracellular water loss accompanies the aging process of numerous organs, due to the decline in space between the cells and the elements of the extracellular matrix [28]. However, the possible contribution of water loss due to aging on the increase in tissue compaction that has been observed could not be assessed with certainty, as the water content of the samples were not directly evaluated. In tissues displaying fibrosis, the high deposition of fibrillary collagen types I and III adds stiffness to the tissues [29]. This excessive deposition and diminished degradation of the ECM can cause areas of fibrosis with little vasculature and a reduced number of fibroblasts, thus resulting in a more compacted ECM structure [30]. Biomechanical studies of the ovary suggest that specific characteristics of rigidity in the cortex are necessary to maintain an adequate primordial follicular pool quality. Excessive stiffness and rigidity of the follicular environment can cause oocytes to secrete high quantities of androgens and progesterone and have diminished quality [11, 31].
We also found that the androgen treatment received did not influence the TA changes caused by aging in the GID population of this study. Multiple studies of the transgender population have shown that the cortical layer of the ovary becomes highly collagenized and much thicker than that of ovaries from non-transgender women, due to the effects of exogenous testosterone administration [16, 32, 33]. However, the age of the patients in these studies was not taken into account, so it is unclear if this thickening of the cortical layer might also be further intensified by chronological aging. Focal cortical fibrosis was detected in women over 37 years old without ovarian neoplasia which increased the areas with dense collagen and therefore depleted ovarian follicles on the ovarian surface [24]. In the case of our study, we found that an increase in the thickness and density of the tissue in GID patients was not caused by the length nor the dosage of the androgen treatment as our study included some older patients who started the treatment just a few years before their oophorectomy surgeries, but rather, that it was related to the age of the patient. These findings might imply that when the ovary receives constant high doses of androgens for extended periods of time, the outermost cortical layers thicken, with the appearance of subcortical fibrotic areas. This is most likely due to continuous follicular growth and atresia and also the constant cycles of injury-repair to the TA caused by ovulations.
Previous studies of female GID patients have shown a high incidence of PCOS. In consequence, we also studied the incidence of this disorder in the population comprised in our study. We detected that 27.7% of the patients were affected by this pathology, which is consistent with the findings of a previous study of the Japanese GID population [16]. However, in our case, the development of PCOS seemed to be independent from the presence of a high circulating testosterone concentration, which coincides with previous findings amongst the transgender population [34].
We must indicate that our study has several limitations. We did not have access to healthy ovaries from women who did not receive androgen treatment as a control population. Neither did we have access to the complete medical histories of the patients involved in this study. In consequence, any information regarding the patients’ menstrual cycle stages or their use of oral contraceptives was unknown. Moreover, the number of patients in the study was limited. The low numbers of histological samples analyzed per patient and their high heterogeneity due to patient-specific characteristics could help to explain why we could not detect any effects of the length of the androgenic treatment, nor of the presence of PCOS on the values regarding the TA thickness and density.
In conclusion, this prospective study assessed the occurrence of structural changes in the ECM of the outermost layers of the ovaries from GID patients. The TA appears to become increasingly enlarged and compacted with age, due to the appearance of fibrotic areas underneath the aforementioned outermost layer. The nature of this phenomenon requires further investigation to assess whether these ovaries could serve as a reliable source for the study of the loss of fertility occurring in women with aging.
Materials and Methods
Subjects
The participants in this study consisted of 18 Japanese women diagnosed with GID with ages ranging from 21 to 46 years old (mean age ± SD; 30.1 ± 7.5 years old) who underwent operations for genital reconstruction at the Okayama University Hospital between April 2014 and April 2016. All of the patients in the study received testosterone treatment until 6 weeks prior to the sex re-assignment surgery, at which time the ovaries were collected for the study.
The use of human ovaries for this study was approved by the local Institutional Review Board (K1807-035) and (K1810-006), and the informed written consent was received prior to the reconstructive surgeries from all the patients.
Histology
The ovaries were kept at a constant temperature of 25 °C while they were transported to the laboratory within 1 h of collection. After several washes in PBS to remove excess blood, the ovaries were trimmed of excess tissue, cut into fragments of 3 mm in thickness, and immediately fixed in a 4% (w/v) PFA in PBS solution for 24 h at room temperature (RT). The ovarian fragments were dehydrated in a series of ethanol of increasing concentration, cleared in xylene, and embedded in paraffin following a routine protocol. The paraffin blocks were then cut with a manual microtome into 5 μm sections, mounted on glass slides (Platinum PRO, Matsunami Glass Ind., Osaka, Japan), and stored until staining.
For the staining of the samples’ connective tissue, Picrosirius Red (PSR), a selective dye for fibers of collagen I and III, was used. The samples were deparaffinized in xylene, rehydrated in a series of ethanol baths with decreasing concentration ending in a final bath with distilled water; after which, they were stained with a Picrosirius Red solution prepared by dissolving Sirius Red F3BA (Direct Red 80, C.I. 357.80, Waldeck GmbH & Co KG, Münster) in a saturated aqueous solution of picric acid (Millipore-Sigma, St. Louis, MO) at 0.1% w/v. The slides were incubated with the PSR staining solution for 60 min at RT. The slides were next incubated in 0.5% glacial acetic acid (Nacalai Tesque, Kyoto, Japan) for a total of three, 2 min washes. The excess acidified water was removed from the tissue sections and the tissue was rapidly dehydrated in 2 washes of 99% ethanol and 2 washes of 100% ethanol (1 min per each incubation). The slides were cleared in xylene for 10 min and mounted with Mount-Quick (Daido Sangyo Co. Ltd., Kyoto, Japan). For each replication of the experiment, the samples were processed together in batch form to minimize variations in the staining intensity.
Image Analysis
For each ovarian section examined, 2 non-overlapping bright field images were taken with a fluorescence microscope and the attached viewer software (Keyence BZ-X710, Keyence Corp., Osaka, Japan; Supplementary Information 2). The imaging software ImageJ (National Institutes of Health Bethesda, MD, USA) was used to quantify the thickness and the density of the dense ovarian connective tissue (Fig. 5). To assess the thickness, 5 random points were manually selected from each of the two images obtained. The average distance from the selected points to the ovarian surface was then calculated. To quantify the density of the tissue, two non-overlapping areas of 100 μm2 were selected from each of the pictures. Consequently, the area for PSR staining above a determined threshold was kept constant for all the images analyzed. For the interpretation of the density data, a value of 0 was considered not to contain any tissue and a value of 10,000 indicated that all the area was composed uniquely of tissue. The higher the value obtained by the threshold selected area, the denser the tissue was considered to be.
Androgen Dose Received, Serum Levels, and Incidence of PCOS
The mean duration of the hormonal treatment for the patients was 55.9 ± 41.5 (mean ± SD) months. As older individuals might have received hormonal treatment for a longer period of time than younger ones, the total dose of androgen received by all of the patients in this study was calculated as the dosage in IU received since the start of the treatment to 6 weeks prior to the sex-rearrangement operation according to the following formula: Total dose received (IU) = [(androgen dose (IU)/week) × (length of the treatment (in weeks))]. In one case, the exact starting date of the hormonal treatment was unknown, so that sample was removed for the evaluation of the androgen effect on the ovarian TA thickness and density. Regarding the concentration of androgen in serum, values less than 54 IU were considered to be within the normal range, whereas values equal to or over 54 IU were considered to be high.
Data regarding the presence of PCOS was obtained from each of the patients’ medical histories. In Japan, the criteria used for the diagnosis of PCOS differ slightly from that used in Europe or the USA. The patients were diagnosed according to the Rotterdam Consensus criteria and considered as having PCOS only if they fulfilled all 3 of the criteria [35]. Patients were classified as manifesting initial PCOS if they had already been diagnosed before starting the androgen hormonal treatment, as PCOS after treatment if they were diagnosed with PCOS after starting the hormonal treatment, or as not having PCOS if they were never diagnosed with the disease.
Statistical Analysis
IBM SPSS Statistics 21 for Windows was used for the statistical analysis of the experimental data. All of the data on the thickness and density of the TA was obtained from 3 replications per patient. The effect of the patient’s age, hormonal treatment, or PCOS presence on the thickness and density of the TA was analyzed by a Pearson’s correlation test. A P value < 0.05 was considered to be statistically significant in all cases.
Abbreviations
- GID:
-
gender identity disorder
- ECM:
-
extracellular matrix
- TA:
-
tunica albuginea
- RT:
-
room temperature
- PSR:
-
picrosirius red
References
Bongaarts J, Potter RG. 2 - natural fertility and its proximate determinants. In: Fertility, biology, and behavior. San Diego: Academic Press; 1983. p. 21–51. https://doi.org/10.1016/B978-0-08-091698-9.50007-5.
Gray RH. Biological and social interactions in the determination of late fertility. J Biosoc Sci Suppl. 1979;6:97–115.
Frank O, Bianchi PG, Campana A. The end of fertility: age, fecundity and fecundability in women. J Biosoc Sci. 1994;26(3):349–68.
Jirge PR. Poor ovarian reserve. J Hum Reprod Sci. 2016;9(2):63–9. https://doi.org/10.4103/0974-1208.183514.
Subrat P, Santa SA, Vandana J. The concepts and consequences of early ovarian ageing: a caveat to women’s health. J Reprod Infertil. 2013;14(1):3–7.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne). 2018;9:327. https://doi.org/10.3389/fendo.2018.00327.
Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152(3):245–60. https://doi.org/10.1530/REP-16-0129.
Exbrayat J-M (2013) Histochemical and Cytochemical Methods of Visualization. Boca Raton: CRC Press. https://doi.org/10.1201/b14967.
Smith RM, Woodruff TK, Shea LD. Designing follicle-environment interactions with biomaterials. Cancer Treat Res. 2010;156:11–24. https://doi.org/10.1007/978-1-4419-6518-9_2.
Phillip JM, Aifuwa I, Walston J, Wirtz D. The mechanobiology of aging. Annu Rev Biomed Eng. 2015;17:113–41. https://doi.org/10.1146/annurev-bioeng-071114-040829.
West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–48. https://doi.org/10.1016/j.biomaterials.2007.07.001.
Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75(6):916–23. https://doi.org/10.1095/biolreprod.106.054833.
Emi Y, Adachi M, Sasaki A, Nakamura Y, Nakatsuka M. Increased arterial stiffness in female-to-male transsexuals treated with androgen. J Obstet Gynaecol Res. 2008;34(5):890–7. https://doi.org/10.1111/j.1447-0756.2008.00857.x.
Elbers JM, de Jong S, Teerlink T, Asscheman H, Seidell JC, Gooren LJ. Changes in fat cell size and in vitro lipolytic activity of abdominal and gluteal adipocytes after a one-year cross-sex hormone administration in transsexuals. Metabolism. 1999;48(11):1371–7.
Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132–54. https://doi.org/10.1210/jc.2009-0345.
Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28(2):453–61. https://doi.org/10.1093/humrep/des385.
Espey LL. Ovarian proteolytic enzymes and ovulation. Biol Reprod. 1974;10(2):216–35. https://doi.org/10.1095/biolreprod10.2.216.
Hanna MD, Chizen DR, Pierson RA. Characteristics of follicular evacuation during human ovulation. Ultrasound Obstet Gynecol. 1994;4(6):488–93. https://doi.org/10.1046/j.1469-0705.1994.04060488.x.
Colwell AS, Longaker MT, Lorenz HP. Fetal wound healing. Front Biosci. 2003;8:s1240–8. https://doi.org/10.2741/1183.
Kular JK, Basu S, Sharma RI. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:2041731414557112. https://doi.org/10.1177/2041731414557112.
Jones MG, Andriotis OG, Roberts JJ, Lunn K, Tear VJ, Cao L, et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife. 2018;7. https://doi.org/10.7554/eLife.36354.
Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128(1):97–107. https://doi.org/10.1172/JCI93563.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. https://doi.org/10.1002/path.2277.
Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–33. https://doi.org/10.1093/humrep/dem027.
McCloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, et al. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res. 2020;26(3):632–42. https://doi.org/10.1158/1078-0432.CCR-19-0603.
Mara JN, Zhou LT, Larmore M, Johnson B, Ayiku R, Amargant F, et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging (Albany NY). 2020;12(10):9686–713. https://doi.org/10.18632/aging.103237.
Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005;322(1):117–24. https://doi.org/10.1007/s00441-005-1100-1.
Edmonds CJ, Jasani BM, Smith T. Total body potassium and body fat estimation in relationship to height, sex, age, malnutrition and obesity. Clin Sci Mol Med. 1975;48(5):431–40. https://doi.org/10.1042/cs0480431.
Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013;1832(7):884–90. https://doi.org/10.1016/j.bbadis.2013.02.007.
Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–94. https://doi.org/10.1007/s00432-015-1974-6.
Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28(1):3–6. https://doi.org/10.1007/s10815-010-9478-4.
Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab. 1989;69(1):151–7. https://doi.org/10.1210/jcem-69-1-151.
Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, et al. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19(5):445–52. https://doi.org/10.1111/j.1365-2559.1991.tb00235.x.
Caanen MR, Schouten NE, Kuijper EAM, van Rijswijk J, van den Berg MH, van Dulmen-den Broeder E, et al. Effects of long-term exogenous testosterone administration on ovarian morphology, determined by transvaginal (3D) ultrasound in female-to-male transsexuals. Hum Reprod. 2017;32(7):1457–64. https://doi.org/10.1093/humrep/dex098.
Kubota T. Update in polycystic ovary syndrome: new criteria of diagnosis and treatment in Japan. Reprod Med Biol. 2013;12(3):71–7. https://doi.org/10.1007/s12522-013-0145-1.
Funding
This research was partially supported by the Sasakawa Scientific Research Grant from The Japan Science Society (Grant number: 2020-4052).
Author information
Authors and Affiliations
Contributions
PF contributed in the experimental design, performed all the histology experiments and image analysis, analyzed and interpreted all the datasets obtained, and was a major contributor in writing the manuscript.
JO contributed in the experimental design, data analysis, and the writing of the manuscript.
HF contributed in the experimental design, data analysis, and the writing of the manuscript.
MN performed the operations on all the patients, obtained and provided all the data regarding the patients’ medical history, and contributed in the writing of the manuscript.
All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information 1
A In ovarian samples stained following Masson’s Trichrome method, a moderate positive linear correlation was detected between the thickness of the TA layer and the age of the patients (r = 0.76, P < 0.001). This was congruent with the results obtained when the samples were stained following the PSR method. B From left to right, the picture shows an ovarian section from a young transgender donor (21 years old) and from an older transgender donor (44 years old) stained with Masson’s trichrome method. TA tunica albuginea, r Spearman coefficient, PSR Picrosirius red. Scale bars represent 200 μm (PNG 4361 kb).
Supplementary Information 2
Image composition depicting one representative ovarian section per each of the 18 patients participating in the study, organized by increasing age, from the lowest to the greatest. Scale bars represent 400 μm (PNG 7303 kb).
Rights and permissions
About this article
Cite this article
Ferré-Pujol, P., Otsuki, J., Funahashi, H. et al. The Thickness and Density of the Ovarian Tunica Albuginea Increases with Age in Transgender Patients. Reprod. Sci. 28, 1339–1346 (2021). https://doi.org/10.1007/s43032-020-00390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00390-5