Abstract
To present a new day 4 (D4) embryo grading system to assess embryos in frozen–thawed embryo transfer (FET) cycles. A new grading system (grades A–E) was developed from the 2011 ESHRE Istanbul Consensus for D4 embryos in FET cycles. Embryos with complete compaction were classified as grade A; those with partial compaction were assigned as grade B; and those without compaction were classified as grades C, D, and E according to their different blastomere number ratio (BNR; number of embryo blastomeres on D4/number of embryo blastomeres on D3, D4/D3). Embryos with a BNR of ≥ 1.5 were defined as grade C, those with a BNR of ≥ 1.2 and < 1.5 were defined as grade D, and those with a BNR of ≥ 1.0 and < 1.2 were defined as grade E. Using this proposed grading model, 5460 embryos with known implantation data were retrospectively analyzed after D4 transfer in FET cycles. The transferred embryos exhibited a similar declining trend in implantation and live birth rates from the top grade A to the lowest grade E. The in vitro fertilization group showed increased implantation rates of grade B and E embryos compared with the intracytoplasmic sperm injection group (grade B: 41.99%, 34.63%, χ2 = 5.84, p < 0.05 and grade E: 18.98%, 14.08, χ2 = 75.62, p < 0.01). Receiver-operating characteristic analysis revealed that our proposed model predicted the implantation outcomes and live birth rates of all embryos (area under the curve = 0.65; 95% confidence interval [CI],0.63–0.66; p < 0.01 and AUC = 0.73; 95%CI, 0.65–0.84; p < 0.001, respectively). This study demonstrates that the new grading system provided by us can be a useful tool for assisting embryo selection via changes in embryo morphology. D4 embryo transfer provides a simple and applicable method for FET cycles in daily practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There have been significant advances in the development of the in vitro fertilization–embryo transfer (IVF–ET) technology since the first IVF baby was born in the 1970s. Although there have been continuous improvements in the technology of embryo culture in vitro, there is still a lack of objective as well as accurate methods for judging embryo quality, which is an important factor affecting the outcome of clinical pregnancy [1, 2]. To date, no consensus exists on the classification of the quality of day 4 (D4) embryos in frozen embryo transfer (FET) cycles. Because embryos are usually frozen on D3, we have routinely cultured embryos overnight for transfer on D4 in FET cycles. However, it is extremely challenging to select the most viable and potent embryos for D4 ET so as to reduce the number of transplanted embryos while improving the ability of embryo implantation. This study aimed to present a new grading system (GS) for D4 embryos conceived via IVF and intracytoplasmic sperm injection (ICSI) for predicting the implantation potential of embryos in FET cycles.
Materials and Methods
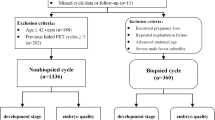
This single-center retrospective cohort study included the medical records of patients with infertility who were undergoing IVF–ICSI cycles and subsequent FET from January 2015 to September 2019.The Ethics Research Committee of the First Hospital of Lanzhou University approved this study.
Study Group
This study included patients receiving ET on D4 in FET cycles. Day 3 embryos were thawed and cultured overnight. Then, 1–2 embryos were selectively transferred. Thereafter, we assessed whether all embryos were successfully implanted or not. We excluded cycles with partial implantation.
In total, 3338 FET cycles were included for final analysis. There were 1216 cycles of single-ET and 2122 cycles of two-ET on D4. Based on embryo compaction and different blastomere number ratio (BNR; number of embryo blastomeres on D4/number of embryo blastomeres on D3, D4/D3), we calculated the implantation rates and live birth rates of the different graded D4 embryos.
Ovarian Stimulation
Patients underwent controlled ovarian stimulation in long or short protocols with gonadotropin-releasing hormone agonists (Decapeptyl®, Ferring, Germany) and recombinant follicle-stimulating hormone (FSH) (Puregon®, Organon, the Netherlands). The dose of FSH was adjusted according to ovarian response, which was evaluated via ultrasound and assessment of estrogen and progestational hormone levels. Oocyte maturation was induced using 5000–10,000 units of human chorionic gonadotropin (Pregnyl®, Saint-Prex, Switzerland). Ultrasound-guided follicular aspiration was performed at 36 h after ovulation induction.
Embryo Culture
The choice of the fertilization method depended on the infertility of the substantial male factor. Fertilization status was evaluated on D1 (18 h after insemination) for the appearance of two polar bodies and two pronuclei. Embryos were then cultured in Vitrolife G-I plus cleavage medium (Vitrolife, Goteborg, Sweden) in a CO2 incubator (37 °C, 6% CO2, and 5% O2). Embryonic development was assessed on D3 by grading using the Gardner method [3]. All embryos were cultured to the D3 stage and vitrified using the VitriFreeze™ Media Kit (Kitazato, Japan) after thawing with the VitriThaw™ Media Kit (Kitazato). All samples were stored in liquid nitrogen.
D4 Embryo Grading
A new GS modified from the 2011 ESHRE Istanbul Consensus for D4 embryos in FET cycles was established [4]. Typically, the phenomenon of compaction occurs on D4 of human embryonic development. Embryos with complete compaction were assigned as grade A and those with partial compaction were classified as grade B. Using our GS, we classified the embryos without compaction as grades C, D, and E based on their different BNRs (D4/D3). Embryos with a BNR of ≥ 1.5 were defined as grade C, those with a BNR of ≥ 1.2 and < 1.5 were defined as grade D, and those with a BNR of ≥ 1.0 and < 1.2 were defined as grade E (Table 1).
Frozen Cycle Endometrial Preparation
Artificial Hormone Replacement
The cycle started on D3, where in the patients were orally administered 2–6 mg of estradiol (Estrace; Allergan Pharmaceuticals) for endometrial preparation. Endometrial ultrasound was conducted to assess the lining of the endometrium for its readiness for the ET procedure. Endometrial thickness was evaluated weekly until it was ≥ 7 mm thick. Based on the proposed day of thawing and ET, a progesterone suppository (200 mg, three times daily, tid) was vaginally administered to women to initiate luteal support. The embryos were thawed on D3 of progesterone administration and transferred after overnight culture.
Natural Cycles
After spontaneous menstruation, endometrial thickness and follicular development were regularly monitored using serial ultrasound procedures. Progesterone and luteinizing hormone (LH) levels were observed until the LH peak appeared, corresponding to the day before oocyte ovulation (i.e., when the LH level exceeded 180% of the baseline). On D2, progesterone suppositories (200 mg tid) started to show their effects. The embryos were thawed on D3 of progesterone administration and transferred after overnight culture.
ET and Confirmation of Implantation
Based on the patient’s age and anamneses, 1–2 embryos were thawed and cultured overnight for transplantation during the natural cycle or hormone replacement FET cycle. All ET procedures were operated under the guidance of ultrasound using standardized techniques. Clinical pregnancy was confirmed by the detection of a fetal heartbeat under ultrasound at 7 weeks of pregnancy.
Statistical Analysis
Data were analyzed using the IBM SPSS v.22 (IBM Corporation). Continuous variables were expressed as mean values with SD and were analyzed using Student’s t test. The chi-square test was performed to compare the categorical variables. The area under the receiver-operating characteristic (ROC) curve (AUC) was used to test the predictive ability of ROC on the outcome of embryo implantation. A p value of < 0.05 was considered statistically significant. Correlation analysis was based on Pearson’s and Spearman’s correlation coefficients and was considered statistically significant at p < 0.01.
Results
Demographic Data and Cycle Characteristics
Our study included 2182 controlled ovarian hyperstimulation cycles (1314 cycles in the IVF group and 868 cycles in the ICSI group). There were no significant differences in the baseline characteristics and demographics, including female age, anti-Müllerian hormone level, serum estradiol and progesterone levels on trigger day, and number of metaphase II oocytes retrieved, between the IVF and ICSI groups (Table 2).
Implantation Rates of the Proposed Model
In total, 5460 transferred embryos were retrospectively annotated into five grades (A–E) in FET cycles, as described in Table 1. Table 3 shows the implantation rates of the different graded embryo (embryo implantation rates of IVF and ICSI), whereas Table 4 shows the implantation rates of embryos according to the age of the women. In the IVF, ICSI, nonelderly, and elderly groups, transferred embryos exhibited a similar declining trend in the implantation rates from the top grade A to the lowest grade E (p < 0.01 for all groups). The in vitro fertilization group showed increased implantation rates of grade B and E embryos compared with the intracytoplasmic sperm injection group (grade B: 41.99%, 34.63%, χ2 = 5.84, p < 0.05 and grade E: 18.98%, 14.08, χ2 = 75.62, p < 0.01).
After converting the grades (E–A) to numerical rankings (1–5), ROC analysis revealed that the proposed model predicted the implantation outcomes in a degree in the IVF group (AUC = 0.65; 95% confidence interval [CI], 0.63–0.67; p < 0.01), ICSI group (AUC = 0.64; 95%CI, 0.61–0.66; p < 0.01), nonelderly group (AUC = 0.65; 95%CI, 0.63–0.67; p < 0.01), elderly group (AUC = 0.64; 95%CI, 0.61–0.67; p < 0.01), or a combination of all embryos (AUC = 0.65; 95%CI, 0.63–0.66; p < 0.01). The sensitivity and specificity were 70.42% and 51.43% for the IVF group,70.46% and 50.18% for the ICSI group, 55.86% and 68.63% for the nonelderly group, 65.99% and 54.07% for the elderly group, and 70.44% and 50.91% for combination of all embryos, respectively. Furthermore, a positive correlation was observed between embryo grades and implantation rates (r = 0.977, p = 0.004).
Live Birth Rates of Embryos According to Different Grades
Totally, 1093 healthy fetuses were born according to the follow-up data. Table 5 shows the live birth rates of the different embryo grades. ROC analysis revealed that the proposed model efficiently predicted the live birth outcomes of all embryos (AUC = 0.73; 95%CI, 0.65–0.84; p < 0.001). The sensitivity and specificity were 84.70% and 52.06%, respectively. Embryo grades (A–E) showed a positive correlation with live birth rates (r = 0.990, p = 0.001).
Discussion
The embryo culture purpose of IVF is to select the best embryos for transfer to achieve a healthy single pregnancy. Because there is increasing global consensus to avoid multiple pregnancies after IVF/ICSI–ET, the use of selective single-ET, supported with modified embryo selection, has been promoted to maintain acceptable pregnancy rates while reducing the risk of multiple pregnancies [5,6,7].
As reported in Li et al., there is a consistent success rate between morula ET on D4 and blastocyst transfer on D5 in fresh IVF–ET cycles [8]. In addition, early blastulation was found to be an available predictor for selecting the top embryo to get a higher pregnancy rate in fresh elective single-ET cycles [9]. Usually partial compaction, with blastomeres excluded from the outset or extruded after compaction, displayed the slow morphokinetics at most stages and was associated with abnormal cleavage [10]. In the present study, we showed a useful embryonic morphological GS which was developed from a dataset of 5460 D4 embryos transferred in FET cycles. The result of each embryo after transplantation (implanted and no implanted) was obvious. The resulting GS was based on full or partial compaction (grades A or B, respectively) and BNR (D4/D3, grades C, D, or E), modified from the 2011 ESHRE Istanbul Consensus [4]. Our GS was feasible and useful for the selection of best-quality embryos on D4, which were classified from grades A to E. The embryologist only required few minutes during their routine duties to observe the embryos cultured on D4. Therefore, the GS developed by this study could be easily applied in practice.
Several retrospective cohort studies have been conducted at different reproductive medical centers on patients who have undergone IVF with preimplantation genetic testing. Minasi et al. have reported that polyploidy and blastocyst morphology are correlated clearly [11]. Excellent- and fair-quality embryos had higher euploidy rates and a higher chance of resulting in an ongoing pregnancy [12]. There was a positive correlation between the development of D4 embryo and the time of blastocyst formation [13]. Compacted D4 embryos (grade A) may have higher chromosome aneuploidy than other graded embryos.
Compared with D3 cleavage or blastocyst ET, there are certain superiority of morula ET on D4. First, D4 embryo transplantation can eliminate the phenomenon of embryo arrest in the cleavage stage effectively [14, 15]. Although the culture conditions and laboratory techniques have been improved, only about 50% of human embryos reach the stage of blastocyst development in vitro [16]. It is well-known that human embryos enter the uterine cavity on D4 after sperm-egg fertilization. D4 ET is more natural than cleavage ET of D3 embryos to some degree. In addition, the in vitro culture time of the embryos transferred on D4 is reduced compared with that of the blastocysts. It seems clear that the time reduction decreases the sensitivity of the disruption of the epigenetic regulation [17, 18]. Another research has verified that morula ET and blastocyst transfer result in compatible pregnancy rates [19].
The cycles with partial implantation were excluded in this study. The results of embryo implantation in the studies we included were definitive and provided clear and comprehensive information for each embryo. Furthermore, our data revealed a similar decline in the rate of implantation and live birth for transferred embryos from the top grade A to the lowest grade E. The implantation rates of grade B and grade E embryos in the IVF group were obviously higher than those of embryos in the ICSI group, suggesting that the operation of ICSI fertilization has a certain impact on the potential for embryonic development. During ICSI fertilization, the position of sperm relative to meiotic spindle plays an important role in fertilization and high-quality embryo development [20]. The implantation rates of grade A and grade B embryos in the nonelderly group were 56.18% and 44.05%, respectively. Therefore, selective D4 single-ET may be attempted in the nonelderly group during FET cycles in the combination of all embryos. Moreover, based on our follow-up data, the live birth rates of the top grade A and lowest grade E embryos from FET cycles were 43.96% and 4.80%, respectively. It is well-known that many embryos with poor grades result in pregnancies, whereas some perfect embryos do not [21]. And time of morulation and trophectoderm quality could provide important predictors for pregnancy and live birth [22].
In conclusion, our new GS is a helpful tool that can assist in embryo selection via changes in embryo morphology. D4 embryo transfer provides an applicable method that can be used in daily practice. However, future research is warranted to study the transferability of this model between laboratories with independent patient populations and large-sized prospective randomized controlled studies.
References
Nasiri N, Eftekhari-Yazdi P. An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell J. 2015;16(4):392–405.
Fukui Y, Hirota Y, Matsuo M, Gebril M, Akaeda S, Hiraoka T, et al. Uterine receptivity, embryo attachment, and embryo invasion: multistep processes in embryo implantation. Reprod Med Biol. 2019;18(3):234–40.
Sanchez T, Seidler EA, Gardner DK, Needleman D, Sakkas D. Will noninvasive methods surpass invasive for assessing gametes and embryos? Fertil Steril. 2017;108(5):730–7.
Balaban B, Brison D, Calderon G, et al. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83.
Tiitinen A. Single embryo transfer: why and how to identify the embryo with the best developmental potential. Best Pract Res Clin Endocrinol Metab. 2019;33(1):77–88.
Feng G, Zhang B, Zhou H, Shu J, Gan X, Wu F, et al. Comparable clinical outcomes and live births after single vitrified-warmed and fresh blastocyst transfer. Reprod BioMed Online. 2012;25(5):466–73.
Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–9.
Li RS, Hwu YM, Lee RK, et al. Day 4 good morula embryo transfer provided compatible live birth rate with day 5 blastocyst embryo in fresh IVF/ET cycles. Taiwan J Obstet Gynecol. 2018;57(1):52–7.
Hsieh CE, Lee RK, Sun FJ, et al. Early blastulation (EB) of day 4 embryo is predictive of outcomes in single embryo transfer (SET) cycles. Taiwan J Obstet Gynecol. 2018;57(5):705–8.
Lagalla C, Coticchio G, Sciajno R, Tarozzi N, Zacà C, Borini A. Alternative patterns of partial embryo compaction: prevalence, morphokinetic history and possible implications. Reprod BioMed Online. 2020;40(3):347–54.
Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–54.
Barash OO, Ivani KA, Willman SP, Rosenbluth EM, Wachs DS, Hinckley MD, et al. Association between growth dynamics, morphological parameters, the chromosomal status of the blastocysts, and clinical outcomes in IVF PGS cycles with single embryo transfer. J Assist Reprod Genet. 2017;34(8):1007–16.
Viñals Gonzalez X, Odia R, Cawood S, Gaunt M, Saab W, Seshadri S, et al. Contraction behaviour reduces embryo competence in high-quality euploid blastocysts. J Assist Reprod Genet. 2018;35(8):1509–17.
Lee SH, Lee HS, Lim CK, Park YS, Yang KM, Park DW. Comparison of the clinical outcomes of day 4 and 5 embryo transfer cycles. Clin Exp Reprod Med. 2013;40(3):122–5.
Murphy BD. Under arrest: the embryo in diapause. Dev Cell. 2020;52(2):139–40.
McCollin A, Swann RL, Summers MC, Handyside AH, Ottolini CS. Abnormal cleavage and developmental arrest of human preimplantation embryos in vitro. Eur J Med Genet. 2020;63(2):103651.
Kohda T. Effects of embryonic manipulation and epigenetics. J Hum Genet. 2013;58(7):416–20.
Chronopoulou E, Harper JC. IVF culture media: past, present and future. Hum Reprod Update. 2015;21(1):39–55.
Kang SM, Lee SW, Jeong HJ, Yoon SH, Koh MW, Lim JH, et al. Clinical outcomes of elective single morula embryo transfer versus elective single blastocyst embryo transfer in IVF-ET. J Assist Reprod Genet. 2012;29(5):423–8.
Sunderam S, Boulet SL, Kawwass JF, Kissin DM. Comparing fertilization rates from intracytoplasmic sperm injection to conventional in vitro fertilization among women of advanced age with non−male factor infertility: a meta-analysis. Fertil Steril. 2020;113(2):354–63.
Xiong F, Sun Q, Li G, Yao Z, Chen P, Wan C, et al. Association between the number of top-quality blastocysts and live births after single blastocyst transfer in the first fresh or vitrified–warmed IVF/ICSI cycle. Reprod BioMed Online. 2020;40(4):530–7.
Rienzi L, Cimadomo D, Delgado A, Minasi MG, Fabozzi G, Gallego R, et al. Time of morulation and trophectoderm quality are predictors of a live birth after euploid blastocyst transfer: a multicenter study. Fertil Steril. 2019;112(6):1080–93.
Acknowledgments
The authors would like to thank Shilong Xue, Xuehong Zhang, Xiaoling Ma, and Yiqing Wang at the reproductive medicine center of the first Hospital of Lanzhou University for their ongoing support and technical assistance. And the authors thank Caixia Ma for English language editing.
Funding
This study was supported by the Scientific Research Fund of the First Hospital of Lanzhou University (No. ldyyyn2018-11), the Major Scientific and Technological Project in Gansu Province (No. 092NKDA009), Lanzhou Chengguan District Science and Technology Project (No. 2019SHFZ0026) and the Lanzhou Talent Innovation and Entrepreneurship Project (No. 2016-RC-51).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the First Hospital of Lanzhou University (LDYYLL2019-42).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, HX., Xu, XJ. & Liu, L. A New Day 4 Grading System to Assess Embryo Quality in Frozen Embryo Transfer Cycles. Reprod. Sci. 28, 1333–1338 (2021). https://doi.org/10.1007/s43032-020-00389-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00389-y