Abstract
The process of selecting a good quality embryo to improve the pregnancy outcomes is very important. The aim of our study was to elaborate the embryo selection process in a single vitrified-warmed blastocyst transfer (VBT) cycle by analyzing pre-vitrified and post-warmed blastocyst morphological factors to improve pregnancy outcomes. In this retrospective cohort study, we performed 329 single VBT cycles. The pre-vitrified and post-warmed morphological factors of all blastocysts were analyzed. Logistic regression analysis was conducted to select the independent morphological factor associated with ongoing pregnancy. The expansion of blastocoel (mid blastocoel; aOR 2.27, 95% CI.0.80–6.42, p = 0.12, expanded blastocoel; aOR 3.15, 95% CI.1.18–8.44, p = 0.02) in a pre-vitrified blastocyst and the grade of inner cell mass (ICM) (grade B; aOR 0.47, 95% CI.0.27–0.83, p = 0.01, grade C; aOR 0.22, 95% CI 0.09–0.56 p < 0.01) in post-warmed blastocysts significantly predicted the ongoing pregnancy. After fertilization, the embryo developed as a blastocyst on day 5 (day 5) showed a higher ongoing pregnancy than that on day 6 (day 6) (aOR 0.50, 95% CI.0.26–0.94, p = 0.03). The results suggest that while selecting a vitrified-warmed blastocyst in a single VBT cycle, the day 5 vitrified blastocyst should be considered, and a higher expansion grade in the pre-vitrified blastocyst should be selected. Our study has shown that post-warmed ICM grade tends to be a predictive indicator for the selection of the best blastocyst and allows for successful pregnancy, with ongoing pregnancy in a single blastocyst transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of the culture system and the cryopreservation of an embryo make it possible to increase the number of freeze-all strategies [1, 2]. Cryopreservation allows an embryo to escape the adverse effects of controlled ovarian hyperstimulation, such as decreased endometrial receptivity [3]. In addition, it has become a treatment option for women who are at risk of ovarian hyperstimulation syndrome [2].
Recently, IVF clinics have been conducting a single embryo transfer to reduce the prevalence of multiple pregnancies that can cause adverse neonatal and obstetric outcomes [4,5,6,7]. In an elective single embryo transfer, blastocyst transfer has been introduced to achieve a higher pregnancy outcome [8]. For a single blastocyst transfer, embryo quality is traditionally evaluated based on morphological characteristics. The blastocyst grading system introduced by Gardner has been validated and is the most widely accepted morphological method used to select blastocysts [9].
Many studies have reported a correlation between the morphological factors of blastocysts and pregnancy outcomes. Numerous studies have attempted to identify the most important morphological factor and accurate criteria for the quality of embryos. However, there still exists controversy on this issue, and follow-up studies on the resultant important morphological factors have reported conflicting outcomes [3, 10,11,12,13,14,15,16,17,18]. Three scenarios may explain the lack of correlation in the studies. First, the decision of blastocyst grading is subjective and prone to disagreement among embryologists. Second, many studies have been conducted using various embryo cohorts. Third, various cryopreservation methods have been used for embryo preservation in each study.
To cryopreserve blastocysts, vitrification is widely used, and currently, it is the recommended technique [19, 20]. Safe and efficient vitrification allows a broad range of applications in clinical adaptation to patients [16, 21,22,23]. However, the vitrification process requires the exposure of cells to high levels of cryoprotectants, increased osmolality, and extreme changes in temperature, all of which can have significant effects on embryo physiology [24, 25]. During the vitrified-warmed process, the blastocyst undergoes morphological changes such as artificial shrinkage, dehydration, and rehydration with cryoprotectant. These artificial morphological changes can lead to cell damage. Therefore, after the vitrified-warmed process, it is important to reevaluate the morphological factor of an embryo, which is associated with pregnancy outcome.
Consequently, the aim of this study was to enhance the embryo selection process by analyzing pre-vitrified and post-warmed blastocyst morphological factors to improve pregnancy outcomes.
Materials and Methods
Patients and Ethical Approval
This single-center, retrospective, cohort analysis included infertile patients undergoing an autologous IVF cycle. Patients who had single vitrified-warmed blastocyst transfer from March 2017 to December 2018 were identified in an electronic medical record database and included in the study. We precluded women who underwent an in vitro maturation protocol, pre-implantation genetic testing, and oocyte donor cycle in a controlled ovarian hyperstimulation. We also excluded women with uterine anomalies or the endometrial thickness (EMT) < 7 mm. The ethics committee of the Institutional Review Board of CHA Gangnam Medical Center (IRB approval number: GCI 19-29) approved the study.
Ovarian Stimulation and Oocyte Collection
In this study, controlled ovarian stimulation was performed using the GnRH agonist (leuprorelin acetate 0.5 mg daily) or antagonist protocols (Orgalutran; MSD, Cetrotide; Merck Serono Ltd., Ganireve; LG Chem.). Gonadotropin (Pergoveris, Gonal-F; Merck Serono Ltd., Follitrope; LG Chem., Puregon; MSD) dose was adjusted according to the patient’s anti-Müllerian hormone (AMH), antral follicular count, and previous response to stimulation. Transvaginal ultrasound and serum estradiol levels were used to monitor follicular response to ovarian stimulation. Final oocyte maturation was triggered using hCG (Ovidrel; Merck Serono Ltd) when more than 2 follicles were over 18 mm. Ultrasound-guided oocyte retrieval was performed for 34–36 h after the hCG injection.
Embryo Culture
Standard conventional insemination or intracytoplasmic sperm injection (ICSI) was performed for fertilization. Using the COOK embryo culture medium (COOK, Queensland, Australia), embryos were cultured in an incubator (HERA cell 240, Thermo Fisher Scientific, MA, USA) at 5% O2, 6% CO2, and 37 °C. Oosafe 4-well dish (SparMED, Ryttermarken, Denmark) and oil-drop culture (Ovoil, Vitrolife AB, Sweden) were used. After fertilization, on day 5, the best quality blastocyst based on Gardner’s score was transferred to a fresh embryo transfer. Surplus blastocysts were graded and vitrified. The slow-developing embryos, such as morula or early blastocyst stage on day 5, were left to culture for one more day. The remaining embryos were re-assessed on day 6 and then cryopreserved. The freeze-all embryo strategy was performed in patients who suffered from OHSS, elevated progesterone, or high estradiol levels; we followed the same procedure for cryopreservation. All procedures were conducted using an electronic witnessing system (Witness®, RI Ltd., UK) to record the exact times of blastocyst vitrification and warming procedures.
Artificial Shrinkage and Assist Hatching
The shrinkage technique has been previously described in a study by Mukaida et al. [26], and this procedure was conducted 20–30 min prior vitrification. We used a holding pipet to stabilize the blastocyst. The ICM was located at the 6 or 12 o’clock direction, and the injection micro-pipet was penetrated through the trophectoderm cell in the blastocoel cavity. After the artificial shrinkage of the blastocoels, a hole in the zona pellucida was introduced using a laser system (ZILOS-tk Laser Zona pellucid Drill System; Hamilton Thorn Bioscience, Inc., Beverly, MA, USA) with a single laser shot (200 μs).
Vitrification and Warming of Blastocysts
All other chemicals used in this study were purchased from Sigma Chemical Co. (Saint Louis, Mo, USA) unless otherwise indicated; the plastic was purchased from Oosafe 4 Well Dish (SparMED, Ryttermarken, Denmark).
The vitrification and warming procedure used in this study have been described in detail in [27]. Briefly, the basic solution (BS) was HEPES buffer (SAGE), and 20% human serum albumin (HSA) was used. The equilibration solution (V1) was supplemented with 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO). The vitrification solution (V2) was supplemented with 15% of EG, 15% of DMSO, and 0.5 M of sucrose. The blastocyst was plunged into V1 solution for 2.5 min. Next, it was transferred to V2 solution. After 20 s of exposure in the vitrification solution, blastocysts were loaded onto a gold EM-grid (IGG 400, Pelco International. Westchester, PA, USA) and directly plunged into slush LN2 (Vit-Master Slush LN2, IMT, Ness Ziona, Israel). The blastocysts were kept in LN2 tanks for a variable storage time. A 4-step warming process was performed for the warming procedure, and gold EM grids were immediately submerged into a sucrose solution of 0.5 M. After 2.5 min, the embryos were sequentially transferred into warming solutions containing 0.25 M, 0.125 M, and 0 M of sucrose at 2.5 min intervals at 37 °C. Approximately 2–3 h after warming, embryo survival and the degrees of re-expansion were examined.
Blastocyst Survival and Re-expansion Assessment
Vitrified-warmed blastocyst survival assessment was performed, and embryos were defined as fully surviving if more than 50% of intact cells were observed under the microscope. Viable cells were defined if the cell membrane shows a clear margin and homogeneous content. Dead cells were defined if the cell membrane appeared dark and granular. The expansion was graded into three categories: absent (if less than 10% of the original volume was recovered), partial (if approximately 10–59% of expansion was achieved), and full (if approximately 60–100% of expansion was achieved).
Embryo Quality Evaluation and Grading
The methods used to evaluate embryo quality and grading were parallel to the method described by Kim et al. [28]. The quality of warmed blastocysts was assessed 16 to 24-h post-culture on an inverted microscope by two independent embryologists. Blastocyst morphology was assessed according to the degree of blastocoel expansion, ICM, and trophectoderm (TE) morphology (summarized in Appendices 1 and 2) [29]. The quality of blastocysts was divided into four groups according to their morphological grades: excellent (E; expanded AA, hatching AA, hatched AA), good (G; early AA, mid AA, expanded AB, BA, hatching AB, BA, hatched AB, BA), average (A; early AB, BA, Mid AB, AC, BA, BB, expanded AC, BB, CA, hatching AC, BB, CA, CB, BC, hatched AC, BB, BC, CB, CA), and poor (P; early AC, BB, BC, CA, CB, CC; mid BC, CA, CB, CC, expanded BC, CB, CC; hatched BC, CB, CC).
Transfer of Warmed Blastocysts
The vitrified-warmed blastocyst was transferred to natural cycles or hormonal replacement cycles for endometrial preparation. The start day of progesterone and the day of ovulation were considered day zero. The luteal phase support was provided either through progesterone vaginal suppositories or intramuscular injections starting 6 days before the embryo transfer (ET). Blastocyst transfer was performed under ultrasound guidance using an embryo transfer catheter on day 5 after ovulation or progesterone supplementation.
Pregnancy Test and Clinical Outcomes
The primary outcome was an ongoing pregnancy that was defined as a viable pregnancy beyond 12 weeks of gestation. The other outcomes were clinical pregnancy and early pregnancy loss. Clinical pregnancy was defined as a pregnancy identified by visualizing a gestational sac with a fetal heartbeat. Early pregnancy loss was defined as a pregnancy loss between clinical pregnancy and ongoing pregnancy.
Statistical Analysis
All data were analyzed using IBM SPSS Statistics software version 22.0. Continuous variables, such as maternal age, body mass index, infertility duration, previous IVF cycle, and basal hormone, were analyzed by conducting Student’s t test. Categorical variables such as etiology of infertility and endometrial preparation method were analyzed by conducting Pearson’s χ2- tests. To predict pregnancy outcomes, we analyzed pre-vitrified and post-warmed morphological factors using logistic regression. The confounders used in multivariate logistic regression analysis are as follows. Variables are maternal age at oocyte retrieval (< 30, 31–34, ≥ 35 years old), infertility duration (< 2, ≥ 2 years), previous IVF cycles (< 2, ≥ 2), endometrial thickness in (VBT) (< 10, ≥ 10 mm), cryosurvival rate(< 100, ≥ 100%), and day of vitrification (day 5, day6). The clinically important factors that are thought to affect pregnancy outcomes were determined through discussion. For multivariate logistic regression analysis, the continuous variables converted into categorical variables and blastocyst grade (expansion of blastocoel, ICM, and TE grade) were categorized. Multivariate logistic regression analysis was performed in a backward stepwise selection using likelihood ratio, and evaluation of model fit was performed using omnibus tests of model coefficients and Hosmer and Lemeshow goodness of fit tests. In addition, this analysis is performed using a two-separated model with pre-vitrified blastocyst grade shown in Table 1 and the post-warmed blastocyst grade shown in Table 2 as a variable. A p value of less than 0.05 was considered statistically significant.
Results
For 273 patients enrolled in the study, 329 blastocysts were evaluated in a single VBT cycle. The overall implantation rate was 48.0% (158/329), the clinical pregnancy rate was 41.9% (138/329), and the ongoing pregnancy rate was 39.8% (131/329). The patients’ characteristics of the transferred blastocysts are summarized in Table 3. In the ongoing pregnancy group, maternal age at oocyte retrieval (32.9 ± 3.1, 34.6 ± 4.3, p < 0.01) and VBT (33.4 ± 3.2, 35.1 ± 4.3, p < 0.01) were less than those in the non-ongoing pregnancy group. Infertility duration (3.0 ± 1.7, 3.5 ± 1.9, p < 0.01) was shorter, and the number of previous cycles (1.4 ± 0.7, 1.6 ± 1.2, p = 0.03) was lower in the ongoing pregnancy group. There was no significant difference between groups in terms of the remaining characteristics.
The characteristics of the previous COH cycles are summarized in Appendix 3. There were no significant statistical differences in previous COH cycle variables.
The clinical results of the VBT cycles are summarized in Table 4. In the ongoing pregnancy group, the cryosurvival rate of the post-warmed embryo (98.5 ± 8.6%, 95.4 ± 14.5%, p = 0.02) and the proportion of day 5 vitrified blastocysts were significantly higher (87.8%, 71.7%, p < 0.01). The pre-vitrified and post-warmed qualities of blastocysts were significantly associated with the ongoing pregnancy (p < 0.01). Therefore, we conducted a logistic regression analysis to identify the independent association between a morphological factor and an ongoing pregnancy.
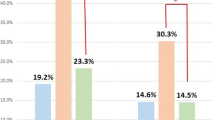
Univariate logistic regression analysis was conducted. Maternal age at oocyte retrieval, infertility duration, day of vitrification, and cryosurvival rate were significantly associated with the ongoing pregnancy. According to the univariate logistic regression analysis, the ongoing pregnancy was higher in younger patients (31–34; OR 0.66, 95% CI.0.37–1.23, p = 0.19, ≥ 35; OR 0.31, 95% CI.0.16–0.61, p = 0.01). The shorter infertility duration (OR 0.50, 95% CI 0.25–0.97, p = 0.04) and higher cryosurvival rate (OR 3.37, 95% CI 1.12–10.14, p = 0.03) were associated with higher ongoing pregnancy. In case of days of vitrification, day 5 vitrified blastocyst showed higher ongoing pregnancy (OR 0.35, 95% CI 0.19–0.65, p < 0.01). The number of previous IVF cycles (OR 0.69, 95% CI 0.42–1.14, p = 0.15) and EMT in VBT (OR 1.26, 95% CI 0.81–1.97, p = 0.31) were not affected by ongoing pregnancy. In univariate logistic regression analysis to predict morphology of the embryo (Table 1), a more expanded blastocyst shows higher ongoing pregnancy (mid blastocoel; OR 1.96, 95% CI. 0.72–5.33, p = 0.19, expanded blastocoel; OR 2.73, 95% CI.1.06–7.04, p = 0.04) than an early blastocyst. The ICM grades B (OR 0.50, 95% CI 0.26–0.99, p = 0.05) and C (OR 0.16, 95% CI 0.06–0.45, p < 0.01) showed lower ongoing pregnancy than ICM grade A. The TE grades B (OR 0.51, 95% CI 0.26–1.01, p = 0.05) and C (OR 0.29, 95% CI 0.13–0.65, p < 0.01) showed lower ongoing pregnancy than TE grade A.
In multivariate logistic regression analysis to predict associated morphological factor of the pre-vitrified blastocyst (Table 1), the omnibus tests of model coefficients are significant for the model estimated as χ2 (p < 0.05). In addition, the model was a fit in the Hosmer–Lemeshow goodness of fit test (χ2=2.33, p > 0.05). The ongoing pregnancy was higher for younger maternal age at oocyte retrieval (31–34; aOR 0.73, 95% CI.0.38–1.40, p = 0.35, ≥ 35; aOR 0.37, 95% CI.0.18–0.74, p < 0.01) and shorter infertility duration (aOR 0.41, 95% CI.0.20–0.86, p = 0.02). The day 6 vitrified blastocyst showed lower ongoing pregnancy (aOR 0.38, 95% CI.0.20–0.72, p < 0.01). The expansion degree of blastocoel was significantly correlated with the ongoing pregnancy. The ongoing pregnancy in a more expanded blastocyst was significantly higher (mid blastocoel; aOR 2.27, 95% CI.0.80–6.42, p = 0.12, expanded blastocoel; aOR 3.15, 95% CI.1.18–8.44, p = 0.02) compared with early blastocysts.
Regarding the morphological factors of the post-warmed blastocyst (Table 2), in univariate logistic regression, the grades of ICM and TE exhibit a significant association with ongoing pregnancy. The ICM grades B (OR 0.41, 95% CI 0.24–0.71, p < 0.01) and C (OR 0.15, 95% CI 0.06–0.36, p < 0.01) showed lower ongoing pregnancy than ICM grade A. The TE grades B (OR 0.45; 95% CI 0.26–0.81; p < 0.01) and C (OR 0.22, 95% CI 0.11–0.44, p < 0.01) showed a significantly lower ongoing pregnancy than TE grade A.
Multivariate logistic regression analysis is performed to evaluate the independent effect of morphological factors while adjusting for variables in Table 2. The omnibus tests of model coefficients were significant for the model estimated as χ2 (p < 0.05). In addition, the model was a fit in the Hosmer–Lemeshow goodness of fit test (χ2 = 4.51, p > 0.05).
The infertility duration, day of vitrification, and ICM morphology were still statistically significant predictors of ongoing pregnancy. Shorter infertility duration was associated with higher ongoing pregnancy (aOR 0.41, 95% CI.0.20–0.88, p = 0.02). The day 6 vitrified blastocyst embryo exhibited significantly lower ongoing pregnancy than day 5 vitrified blastocyst (aOR 0.50, 95% CI 0.26–0.94, p = 0.03). The ongoing pregnancy was significantly higher for ICM grade A than for grades B (aOR 0.47, 95% CI 0.27–0.83, p = 0.01) and C (aOR 0.22, 95% CI 0.09–0.56, p < 0.01).
Discussion
Because cryopreservation of the human embryo is an essential part of the routine procedure, it is important to establish an embryo selection criteria based on the developmental competence of embryos. The aim of this study was to improve pregnancy outcomes by enhancing the embryo selection process using pre-vitrified and post-warmed embryo morphological factors in a single vitrified-warmed blastocyst transfer cycle. Our results indicate that day 5 vitrified blastocysts exhibit better pregnancy outcomes. In case of the morphological factor, the degree of expansion in pre-vitrified blastocysts and ICM grade in post-warmed blastocysts were associated with ongoing pregnancy outcomes.
The ICM cells eventually develop into a fetus; hence, the ICM grade may serve as a predictive marker for ongoing pregnancy. The importance of ICM grade for clinical outcomes has been previously documented [13, 14, 16, 17, 30], which is consistent with the correlation between morphology and implantation of euploid human blastocysts [13]. The larger size and slightly oval shape of ICM are significantly associated with the blastocyst implantation potential [14]. The ICM is positively associated with the clinical ongoing pregnancy rate and early pregnancy loss rate [16, 30]. These studies evaluated blastocyst grade in a fresh single blastocyst transfer cycle. Our study strongly supports the hypothesis that the post-warmed ICM grade is correlated with ongoing pregnancy in single VBT cycles. Because low-quality embryos were also included in our study, our results confirm these observations and support that ICM grade C had a lower ongoing pregnancy rate than the ICM grade of A.
In contrast, previous studies have suggested that TE is statistically related to clinical outcomes [15, 18, 31,32,33]. Hill et al. showed that trophectoderm morphology, but not ICM and blastocoel expansion, was related to clinical outcomes [31]. However, this study supports this notion when only high-quality embryos are vitrified. Low-quality embryos were not included in the embryo cohort. Another study focused on the number of cells in the TE biopsy. However, the number of TE cells was evaluated in the ICM section and did not represent the whole blastocyst [32].
Meanwhile, several studies suggesting the predictive value of blastocoel expansion have reported that the blastocoel expansion degree significantly affects pregnancy outcomes in the FET cycle [15, 23, 34]. These studies excluded low-grade blastocysts. Goto et al. included a small proportion of blastocysts with grade C for ICM and TE, and did not consider the effect of each blastocyst morphological factor [23]. Our study has many advantages because it retains a significant number of blastocysts with an expansion of blastocysts, including early to hatch with ICM and TE grades A, B, and C. The degree of pre-vitrified blastocoel expansion may be important because an increased number of cell divisions increase the quality of cell junction. With more blastocoel expanding, the blastocyst has a larger number of smaller blastomeres. Therefore, smaller cells have a larger surface area to volume ratio. This enables cryoprotectants to permeate faster [35]. This might allow the embryo to tolerate vitrification and osmotic stress. This mechanism is probably the influencing factor that associates the grade of expansion of the pre-vitrified blastocyst with ongoing pregnancy.
In vitrified-warmed blastocysts, the embryo undergoes morphological changes such as artificial shrinkage, dehydration, and rehydration with cryoprotectant. As the large inner water content of the blastocoels affects the diffusion of membrane-permeating cryoprotectants within the cavity, there is a high risk of ice crystal formation that may damage cellular organelles and membranes, resulting in cell lysis or induced apoptosis [36, 37]. Other studies also showed that artificial shrinkage has a beneficial effect on the process of blastocyst vitrification [26, 36]. Our study confirmed that vitrified-warmed blastocysts showed 95–98% of survival rate and 79.1% of hatching or hatched rate, regardless of performing artificial shrinkage. Thus, artificial shrinkage is an effective method in the process of blastocyst vitrification. However, long-term effects should also be considered, such as embryo functionality, pregnancy, and live birth.
In addition, it was observed in our study that day 6 vitrified blastocysts had a slow development rate. The slow developmental potential of the embryo might involve cytoplasmic immaturity of oocytes, decreased DNA integrity in blastomeres, and suboptimal culture conditions [38]. Similar findings have been reported by a few other researchers [39,40,41,42]. Therefore, day 5 vitrified blastocysts should be selected to improve clinical outcomes. This may increase the yield of embryos suitable for vitrification and improve outcomes.
To the best of our knowledge, this is the first report that evaluates both pre-vitrified and post-warmed blastocyst morphology in a single VBT cycle. We also confirmed that selecting embryos with higher ICM grade resulted in higher ongoing pregnancy. We included all types of viable blastocysts, including a low grade of blastocoel expansion with ICM grade C or TE grade C. Because only a single blastocyst was transferred, the chance of pregnancy during multiple blastocysts transfer was ruled out. We assume that our study still provides a good insight as to which blastocyst factor should be a preferred over others.
The limitations of our study are as follows. This was a retrospective study. There is a need of more studies from this perspective. We did not examine the ploidy of the embryo. The proposed scoring system is still controversial because embryo morphological factors from diverse perspectives are not considered during evaluation.
Recently, a time-lapse system has been used to monitor and analyze embryo development. It is more effective to obtain dynamic information about the blastocyst state using this morphological scoring system. Therefore, in further studies, the addition of a time-lapse system to the blastocyst grading might improve the embryo selection.
Conclusion
The key observations of our study are as follows. For single VBT cycles, selection of day 5 vitrified blastocysts is required. In addition, pre-vitrified grades of expansion need to be prioritized when warming a blastocyst. Subsequently, for post-warmed blastocysts, ICM grade can be used to select a single blastocyst that is to be transferred. Our study provides a reliable guidance to embryologists and clinicians for the optimal selection of embryos for a single VBT cycle.
References
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Freeze-all can be a superior therapy to another fresh cycle in patients with prior fresh blastocyst implantation failure. Reprod BioMed Online. 2014;29(3):286–90.
Shin JJ, Jeong Y, Nho E, Jee BC. Clinical outcomes of frozen embryo transfer cycles after freeze-all policy to prevent ovarian hyperstimulation syndrome. Obstet Gynecol Sci. 2018;61(4):497–504. https://doi.org/10.5468/ogs.2018.61.4.497.
Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. 2000;74(2):282–7.
Practice Committee of Society for Assisted Reproductive T, Practice Committee of American Society for Reproductive M. Elective single-embryo transfer. Fertil Steril. 2012;97(4):835–42.
Sullivan EA, Wang YA, Hayward I, Chambers GM, Illingworth P, McBain J, et al. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum Reprod. 2012;27(12):3609–15.
Report ECC. Prevention of twin pregnancies after IVF/ICSI by single embryo transfer*. Hum Reprod. 2001;16(4):790–800.
Johnston J, Gusmano MK, Patrizio P. Preterm births, multiples, and fertility treatment: recommendations for changes to policy and clinical practices. Fertil Steril. 2014;102(1):36–9.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69(1):84–8.
Gardner DK. In-vitro culture of human blastocyst. Towards reproductive certainty : Infertility and genetics beyond 1999. 1999;378–88.
Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31(11):1475–81.
Licciardi F, McCaffrey C, Oh C, Schmidt-Sarosi C, McCulloh DH. Birth weight is associated with inner cell mass grade of blastocysts. Fertil Steril. 2015;103(2):382–7.e2.
Roy TK, Bradley CK, Bowman MC, McArthur SJ. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101(5):1294–301.
Nazem TG, Sekhon L, Lee JA, Overbey J, Pan S, Duke M, et al. The correlation between morphology and implantation of euploid human blastocysts. Reprod BioMed Online. 2019;38(2):169–76.
Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76(6):1157–67.
Ahlstrom A, Westin C, Wikland M, Hardarson T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum Reprod. 2013;28(5):1199–209.
Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJG, Klein BM, et al. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod BioMed Online. 2013;27(4):353–61.
Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70.
Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26(12):3289–96.
Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90(1):186–93.
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55.
Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23(1):91–9.
Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102(1):10–8.
Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril. 2011;95(3):948–52.
Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod BioMed Online. 2004;9(6):680–91.
Dalcin L, Silva RC, Paulini F, Silva BD, Neves JP, Lucci CM. Cytoskeleton structure, pattern of mitochondrial activity and ultrastructure of frozen or vitrified sheep embryos. Cryobiology. 2013;67(2):137–45.
Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod. 2006;21(12):3246–52.
Park J-K, Go Y-E, Eum J-H, Won H-J, Lee W-S, Yoon T-K, et al. Effect on survival and developmental competence of vitrified mouse embryos using various cryoprotectants and cooling speeds. Clin Exp Reprod Med. 2010;37(4):307–19.
Kim MK, Park JK, Jeon Y, Choe S-A, Lee HJ, Kim J et al. Correlation between morphologic grading and euploidy rates of blastocysts, and clinical outcomes in in vitro fertilization preimplantation genetic screening. J Korean Med Sci. 2019;34(4).
Sakkas D, Gardner DK. Evaluation of embryo quality. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of assisted reproductive techniques: laboratory and clinical perspectives: CRC press; 2018. p. 225–42.
Subira J, Craig J, Turner K, Bevan A, Ohuma E, McVeigh E, et al. Grade of the inner cell mass, but not trophectoderm, predicts live birth in fresh blastocyst single transfers. Hum Fertil (Camb). 2016;19(4):254–61.
Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99(5):1283–9 e1.
Ebner T, Tritscher K, Mayer RB, Oppelt P, Duba HC, Maurer M, et al. Quantitative and qualitative trophectoderm grading allows for prediction of live birth and gender. J Assist Reprod Genet. 2016;33(1):49–57. https://doi.org/10.1007/s10815-015-0609-9.
Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012;98(2):361–7.
Du QY, Wang EY, Huang Y, Guo XY, Xiong YJ, Yu YP, et al. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil Steril. 2016;105(4):910–9 e1.
Tachikawa S, Otoi T, Kondo S, Machida T, Kasai M. Successful vitrification of bovine blastocysts, derived by in vitro maturation and fertilization. Mol Reprod Dev. 1993;34(3):266–71.
Vanderzwalmen P, Bertin G, Debauche C, Standaert V, Van Roosendaal E, Vandervorst M, et al. Births after vitrification at morula and blastocyst stages: effect of artificial reduction of the blastocoelic cavity before vitrification. Hum Reprod. 2002;17(3):744–51.
Poli M, Ori A, Child T, Jaroudi S, Spath K, Beck M, et al. Characterization and quantification of proteins secreted by single human embryos prior to implantation. EMBO Mol Med. 2015;7(11):1465–79.
Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod BioMed Online. 2013;27(2):140–6.
Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, et al. Live birth rate following frozen–thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33(3):390–8.
Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33(12):1553–7.
Levens ED, Whitcomb BW, Hennessy S, James AN, Yauger BJ, Larsen FW. Blastocyst development rate impacts outcome in cryopreserved blastocyst transfer cycles. Fertil Steril. 2008;90(6):2138–43. https://doi.org/10.1016/j.fertnstert.2007.10.029.
Sciorio R, Thong KJ, Pickering SJ. Single blastocyst transfer (SET) and pregnancy outcome of day 5 and day 6 human blastocysts vitrified using a closed device. Cryobiology. 2018;84:40–5.
Funding
This research was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program, 20003838) funded by the Ministry of Trade, Industry & Energy (MI, Korea).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyun Jin Kim and Jae Kyun Park are co-first authors.
Rights and permissions
About this article
Cite this article
Kim, H.J., Park, J.K., Eum, J.H. et al. Embryo Selection Based on Morphological Parameters in a Single Vitrified-Warmed Blastocyst Transfer Cycle. Reprod. Sci. 28, 1060–1068 (2021). https://doi.org/10.1007/s43032-020-00349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00349-6