Abstract
Endometriosis is an estrogen-dependent gynecological disorder that affects 10% of reproductive-aged women and causes pelvic pain and infertility. Bone marrow–derived stem cells (BMDCs) are known to engraft endometriosis in association with lesion growth; however, they do not undergo significant clonal expansion. The indirect effects of BMDCs on endometriosis growth and cell proliferation are not well characterized. Here, we demonstrate that BMDCs’ co-culture increased endometrial stromal cell proliferation. In vitro studies using endometrial cells showed that BMDCs increased cell proliferation and activation of CDK1 in both an endometriosis cell line and primary endometrial stromal cells from women with endometriosis, however not in normal endometrial cells. In vivo studies using a mouse model of endometriosis showed increased CDK1+ expression associated with engrafted GFP + BMDCs. These results suggest that endometrial cell proliferation is induced by stem cell–derived trophic factors leading to the growth of endometriotic lesions. Targeting the specific signaling molecules secreted by BMDC may lead to novel therapeutic strategies for controlling cell proliferation in endometriosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a common, chronic inflammatory disorder in women of reproductive age. This disease has an unknown pathogenesis and is characterized by pain, infertility, and ectopic pelvic lesions resembling endometrium [1, 2]. Current medical therapies treat endometriosis by altering sex steroid hormones. However, side effects that develop during these treatments may be cumbersome and threaten patient compliance.
Bone marrow–derived cells (BMDCs) migrate to multiple organs and differentiate into tissue-specific cells. BMDCs are involved in the pathogenesis of endometriosis and contribute to the development of this disease [3]. They participate in epithelial and stroma regeneration in endometrial tissue [4] and endometriotic lesions [5, 6]; they are also likely the principal source of extra-pelvic endometriosis [3, 6]. However clonal expansion and cell replacement alone do not account for the extensive effects of these stem cells on the engrafted tissues [7]. The effect of BMDCs’ engraftment on the endogenous resident endometrial cells is still poorly characterized.
The peritoneal microenvironment is significantly altered in women with endometriosis. Endometrial cells refluxed into the peritoneal cavity secrete chemokines [8], creating a feed-forward loop [9] that stimulates the infiltration of BMDCs including immune cells [10] and bone marrow–derived stem cells. Both endometriotic and immune cells [11,12,13] produce pro-inflammatory cytokines and prostaglandins [14, 15], and they suppress anti-inflammatory interleukins [16], creating an inflammatory imbalance. The ability of this altered microenvironment to support endometriotic cells is dependent on kinase signaling pathways [17]. In this study, we focused mainly on three signaling pathways involving nuclear factor (NF)kB, mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin (mTOR) as well as factors related to the cell cycle. We carried out co-culture of endometrial stromal cells and BMSCs to examine the differential expression of molecules involved in these kinase signaling pathways to delineate the effects of BMDCs on endometrial cells.
Materials and Methods
Sample Collection
Institutional Review Board (IRB) approval was obtained from Yale School of Medicine (New Haven, Connecticut) allowing the use of human samples (Human Investigations Committee protocol, #1004006657). Recruited subjects were women who underwent surgery for benign disease in the reproductive endocrinology practice of the Yale School of Medicine. Written informed consent was obtained from subjects admitted to the hospital prior to undergoing laparoscopy or laparotomy for suspected benign indications such as pelvic masses, pelvic pain, infertility, or endometriosis. Endometrial biopsies in the secretory phase were obtained from women with moderate-to-severe endometriosis (stages III and IV). Inclusion criteria included women who were aged 18–49 years, had regular menstrual cycles, and used no hormonal therapy for at least 3 months preceding surgery. Exclusion criteria included post-menopausal status, previous hormone use within 3 months of surgery, hyperplasia, polyps, malignancy, autoimmune disease, or use of anti-inflammatory medications. Twenty subjects were included in each of the endometriosis and control study groups. The endometriosis group was surgically diagnosed and had histologically verified endometriosis, and the control group was visually verified to be free of endometriosis during the surgery. The phase of the menstrual cycle was determined based on the subjects’ menstrual history and confirmed by histologic evaluation.
Cell Culture
The immortalized endometrial stromal cell line (HESC) and endometriosis stromal cell line were a gift from Prof. Gil Mor at Yale School of Medicine, New Haven, CT, USA. Eutopic endometrial tissues were processed and stromal cells cultured using a protocol previously described by Ryan et al. [18] with minor modifications [19]. Briefly, endometriotic or endometrial tissue was minced finely and digested in digestion medium containing collagenase B (1 mg/ml, Roche Diagnostics, Indianapolis, IN, USA) and deoxyribonuclease I (0.1 mg/ ml, Sigma-Aldrich, St. Louis, MO, USA) in DMEM medium, at 37 °C for 30 min, and tissue was pipetted gently to disperse the cells every 10 min. Stromal cells were filtrated through a 40-μm cell strainer and cultured in DMEM/F-12 containing 10% fetal bovine serum and 1% penicillin-streptomycin. The fibroblast-like appearance of endometriosis-derived stromal cells in culture under phase contrast microscopy appeared identical to that of endometrial stromal cells. Primary eutopic endometrial stromal or endometriosis derived cells were used at 2 to 3 passages.
BMDCs were obtained from the American Type Culture Collection (ATCC, cat. # PCS-500-012, Manassas, VA, USA). Co-culture between BMDCs and endometrial stromal cells was carried out using 4-μm pore size polycarbonate membrane (Millipore, Burlington, MA, USA). Endometrial stromal cells were seeded into a 6-well plate at a concentration of 1 × 105 cells per well. BMDCs were plated into the trans-well insert at a concentration of 1 × 104 cells per insert and the insert was placed into the 6-well plate. Each well contained 3 ml growth media (ATCC CRL-11731) with a final concentration of 15% fetal bovine serum. Total RNA and protein were collected from the cell cultures grown for 24, 48, and 72 h. Total RNA was used for the analysis of gene expression by qRT-PCR while protein was analyzed by western blot.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from endometriotic lesions by TRIzol reagent (Life Technologies), followed by purification using Qiagen cleaning kit (Qiagen, Valencia, CA, USA) to prepare cDNA with 50 ng RNA in a 20 μl reaction mixture by iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative real-time PCR was performed using SYBR Green (Bio-Rad) and optimized in the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). Twenty-six genes which were significantly differentially expressed between normal and endometriosis cells were selected from in the initial screen of more than 150 genes. qRT-PCR was used to quantify gene expression using specific primers for these 26 genes (Table 1). The specificity of the amplified transcript (39 cycles) and absence of primer-dimers were confirmed by a melting curve analysis. Gene expression was normalized to the expression of β-actin for each sample. Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method, also known as the 2-ΔΔCT method [20]. All experiments were carried out three times and each experiment was performed in duplicate. Nuclease-free water was used as a negative control replacing the cDNA template.
Western Blotting
Protein was extracted from the cells with RIPA lysis buffer containing protease inhibitors (Bio-Rad Laboratories, Hercules, CA, USA). Total protein concentration was determined by BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein (25 μg) from lysates were separated on 4–20% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad) and transferred on to a polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked in 5% non-fat milk at room temperature for 1 h and incubated overnight at 4 °C with anti-CDK1(1:500) and anti-GAPDH (1:1000) primary antibodies purchased from Abcam (Cambridge, MA, USA) and Cell Signaling Technology, (Beverly, MA, USA) , respectively. On the following day, membranes were washed three times each for 5 min in 1% TBST, followed by incubation with a goat anti-rabbit IgG conjugated to horseradish peroxidase secondary antibody (Abcam) in 5% BSA. The protein bands on the membrane were visualized using enhanced chemiluminescence solutions A and B mix for 3 min (PerkinElmer, Inc., Waltham, MA). The density of the bands was assessed by the ImageJ software, and values were normalized to the densitometric values of GAPDH. Western blots were run twice with duplicate samples.
Endometriotic Lesions of Mice
C57BL/6J wild-type and ubiquitin-GFP mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and The Jackson Laboratory (Bar Harbor, ME, USA), respectively. All animal experiments were conducted in accordance under an approved protocol from the Yale University Institutional Animal Care and Use Committee (IACUC). Briefly, wild-type mice (6 weeks) received 125 mg/kg of 5-fluorouracil (5-FU) by i.p. on 6 and 1 days (day 6 and 1) before bone marrow transplantation (BMT, day 0). In addition, stem cell factor (SCF, 50 mg/kg) was injected twice before BMT, as previously described [21], and fresh BM cell transplantation was performed as described previously [5].
Endometriosis in BM transplanted mice (N = 12) was surgically induced under aseptic conditions and anesthesia using a modified method previously described [6, 22]. Surgery was performed 30 days following BMT. Uterine horns were removed from wild-type female donor mice at diestrus (low estrogen stage), opened longitudinally, cut into fragments of 3-mm size, and transplanted onto the peritoneal wall of recipient mice. Three fragments were systematically transplanted and sutured to the peritoneal wall of each mouse. Control mice were subjected to sham surgery, where, in place of uterine tissue, peritoneal tissue from the ventral midline was used.
Tissue from endometriotic lesions was fixed in 4% paraformaldehyde and embedded in paraffin. Five-micrometer tissue sections were mounted on slides, steamed in sodium citrate at pH 6 for 10 min for antigen retrieval, and blocked using 10% donkey serum (Vector Laboratories, Burlingame, CA, USA). Sections on slides were incubated at 4 °C overnight with primary antibodies, rabbit anti-Cdk1 (catalog #PA5–82086; 1:200; Invitrogen, CA, USA) and goat anti-GFP (catalog #ab6673; 1:400, Abcam, Cambridge, MA, USA). Rabbit anti-CDK1 specifically targets human, mouse, and rat species. Secondary antibodies used were Alexa Fluor 568-conjugated donkey anti-goat (catalog #A11057, Invitrogen) and Alexa Fluor 488-conjugated donkey anti-rabbit (catalog #A21206, Invitrogen). Sections were mounted under coverslips using Vectashield fluorescent mounting media with 46-diamidino-2-phenylindole (DAPI) (catalog #H-1200; Vector Laboratories.). Visualization of the slides was performed using a laser scanning confocal microscope (LSM 710; Zeiss) and the ZEN software (Carl Zeiss).
Statistical Analysis
Data were analyzed using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). An unpaired Student’s t test for percentage of labeled cells (PLC) and RT-PCR data were used to determine statistical significance. Data are expressed as means ± standard error mean (SEM).
Results
BMDCs Increase CDK1 Expression in Endometriosis Cells
In order to understand the effect of BMSCs on endometriosis gene expression, we determined the expression of several genes of interest in immortalized human endometrial cells (HESC) as well as an endometriosis stromal cell line with and without BMDC co-culture. Gene expression was determined in these cells after 24 h (day 1), 72 h (day 3), and 144 h (day 6). As shown in Table 1, we screened 26 genes related to the NFkB, MAPK, and mTOR signaling pathways, the cell cycle, and apoptosis. Among the 26 genes, a single one, CDK1, was significantly downregulated in endometrial cells while upregulated in endometriosis cells. As shown in Fig. 1A, CDK1 mRNA levels significantly decreased on days 3 and 6 in HESC cells co-cultured with BMDCs compared with the mRNA levels in HESC cells alone. In contrast, we found the opposite in endometriosis stromal cells when co-cultured with BMSCs. In endometriosis cells that were co-cultured with BMDCs, CDK1 mRNA levels were reduced on days 1 and 3 but interestingly increased significantly on day 6 compared with the mRNA levels in endometriosis cells alone (Fig. 1A). Further, we observed that Ki67 (cell proliferation marker) mRNA levels were increased significantly on day 6 in endometriosis cells co-cultured with BMDCs compared with control endometrial cells (HESCs) as shown in Fig. 1B.
A BMDCs increased CDK1 expression in an endometriosis cell line. CDK1 mRNA levels were significantly increased in endometriosis cells on day 6 compared with day 1 and 3 and also compared with the control endometrial stromal cells (HESC). In controls, CDK1 levels were reduced significantly on days 3 and 6 compared with day 1. Each bar represents the mean ± SEM for data from three individual experiments and each experiment was performed in duplicate. Asterisk denotes statistical significance (p < 0.05) between cells without and with BMDCs. B BMDCs increased Ki67 expression in an endometriosis cell line. Ki67 mRNA levels were significantly increased in endometriosis cells on day 6 compared with day 1 and 3 and also compared with control endometrial stromal cells (HESC). Each bar represents the mean ± SEM for data from three individual experiments and each experiment was performed in duplicate. Asterisk denotes statistical significance (p < 0.05) between cells without and with BMDCs
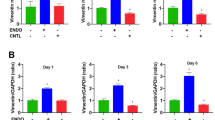
Effect of BMDCs on CDK1 Expression in Primary Endometrial Stromal Cells
We confirmed the changes in CDK1 expression observed in cell lines, in eutopic endometrial primary stromal cells that were cultured from subjects with and without endometriosis. Primary stromal cells from control subjects showed a similar pattern of CDK1 mRNA levels as observed in HESC controls on day 1, day 3, and day 6 when co-cultured with BMSCs (Fig. 2A). BMDCs have a similar effect on CDK1 protein levels as shown in Fig. 2C. CDK1 mRNA and protein levels are not significant on day 1 in primary cells in accordance with the mRNA and protein levels in HESC cells on day 1 (Fig. 2A and C). Primary stromal cells from endometriosis subjects had significantly upregulated CDK1 mRNA expression as well as protein levels on all other days (3 and 6) when co-cultured with BMSCs, as shown in Fig. 2B and D respectively.
BMDCs increased CDK1 expression in primary stromal cells from endometriosis. After co-culture with BMDCs, CDK1 mRNA levels were significantly decreased in primary stromal cells from normal subjects (A) while increased in stromal cells from subjects with endometriosis (B). C and D show the CDK1 protein levels in normal subjects and subjects with endometriosis, respectively. Quantification of protein bands were measured by densitometry and normalized to respective GAPDH. Bar graphs represent the average values of three individual experiments performed in duplicate. Asterisk denotes statistical significance (p < 0.05) between cells without and with BMDCs
BMDCs Increased Eutopic Stromal Cell Proliferation
In order to determine the effect of BMDCs on cell proliferation in eutopic endometrial primary cells, we co-cultured primary cells from eutopic endometrial tissue obtained from subjects with and without endometriosis with BMSCs. Cell proliferation was determined by quantifying the number of cells using bromophenol blue staining for live cells. As shown in Fig. 3, when primary cells from control subjects were co-cultured with BMDCs, the number of cells was significantly decreased compared with the primary cells alone, while the number of primary cells from endometriosis subjects was significantly increased by co-culture with BMDCs.
Increased cell proliferation in endometriosis induced by BMDCs: Representative microscopic images showing cell growth on days 1, 3, and 6 from primary stromal cell cultures from normal subjects (controls) and subjects with endometriosis (endometriosis). Primary stromal cells from normal endometrium co-cultured with BMSCs showed decreased and cell count significantly on all days, Stromal cells from endometriosis subjects demonstrated increased numbers of cells on all days. Each bar represents the mean ± SEM for data from three individual experiments and each experiment was performed in duplicate. Asterisk denotes statistical significance (p < 0.05) primary stromal cells vs those co-cultured with BMSCs
BMDCs Increase Cdk1 Expression in Endometriotic Lesions In Vivo
To characterize BMDCs’ engraftment in endometriosis in vivo, we used a 5-FU-based submyeloablation mouse bone marrow transplantation model according to our published protocol [21]. Sections from endometriotic lesions from endometriosis mice and uterus from sham mice that received GFP expressing BM cell transplantation were stained with anti-GFP (green) and anti-Cdk1 (red). Gross morphology of the endometriotic lesions induced in our mouse model is shown in supplemental figure 1. The stained endometriosis sections were visualized for green and red labeled cells under confocal microscopy. Immunofluorescence results showed that the number of Cdk1 expressing stromal cells (red) nearby engrafted GFP-positive cells (green) were increased (Fig. 4A) and significantly higher (p < 0.05) as shown in Fig. 4B in endometriotic lesions from mice with endometriosis compared with the sham group mice.
A Representative images of Cdk1 expressing cells adjacent to BMDCs in endometriosis lesions as demonstrated by immunofluorescence. Tissue sections from uterus of sham mice and lesions from mice with endometriosis were immune-stained by anti-GFP, anti-Cdk1, and DAPI for nuclear stain. Endometriosis lesions ( right) showed more Cdk1 expressing cells (red) adjacent to engrafted BMDCs (green) comparing to tissue from sham (left) surgery treated mice. B shows that GFP positive and CDK1 protein expressing cells are significantly higher (p < 0.05) in endometriosis compared to sham mice. Scale bar: 100 μm
Discussion
In this study, we demonstrated that BMSCs increased stromal cell proliferation and upregulated CDK1 expression in endometriosis. In cell lines and primary cells obtained from eutopic endometrial tissue of subjects with endometriosis, cell proliferation and CDK1 expression were increased by co-culture with BMDCs. We further showed in vivo that engraftment of BMDCs into endometriotic lesions (ectopic) increased the number of endometrial stromal cells that express CDK1.
Endometriosis is a gynecological disorder common among reproductive-aged women characterized by the growth of endometriotic lesions in pelvic region, causing pain and infertility. Lesion growth is due to active cell proliferation and is essential to the development of endometriosis. Until now, the most widely accepted theory for pathogenesis of endometriosis is Sampson theory, in which the exfoliated menstrual endometrial cells attach to the peritoneal membrane, and subsequent cell proliferation and invasion into the underlying tissue results in endometriotic lesions [23]. Retrograde menstruation is a physiologic process that takes place almost in all menstruation cycles of reproductive-aged women. Accumulated evidence suggests that endometrial cells from both eutopic and ectopic endometrium in endometriosis exhibit excessive proliferation which is essential for the progression of endometriosis [24,25,26,27]. miRNAs, histone deacetylation, and several signaling pathways play a role in the regulation of endometrial cell proliferation and apoptosis in disease [28,29,30,31]. Our previous studies [3, 5, 6, 32] show that BMDCs are incorporated into endometriotic lesions and play a role in the development and progression of peritoneal and extra-peritoneal disease. The importance of bone marrow–derived stem cells (BMDCs) in human endometrial biology has been previously established; however, the mechanisms by which they influence endometriosis growth have not previously been characterized [3, 33,34,35].
Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine kinase and is a key mediator of cell cycle regulation [36]. Among all the CDK family members, only CDK1 is critical for cell cycle regulation and cell division [37]. CDK1 expression is upregulated in several malignancies; increased CDK1 expression or its activation has been documented in oral squamous cell carcinoma [38], ovarian cancer [39], colorectal cancer [40], and prostate cancer [41]. CDK1 participates in G2/M conversion by forming a complex with cyclin B. Silencing of CDK1 increases G2/M arrest in the cell cycle and can lead to the production of polyploid cells [42]. Tang L. et.al demonstrated that cyclin B1 play important roles in the pathogenesis of endometriosis by mediating ectopic endometrial cell proliferation [43]. Therefore, it is evident that CDK1/cyclin B1 pathway plays an important role in the development of endometriosis. Our results from both in vitro and in vivo experiments are in agreement with the above reports that CDK1 expression is upregulated in endometriosis; further, we show that in stromal cells cultured from endometriotic lesions, CDK1 is upregulated by BMDCs.
In vivo, we established a mouse model of endometriosis with BMDCs engraftment. Some CDK1+ cells were associated with GFP + BMDCs although there are areas of CDK1+ cells without BMDCs. This implies the existence of additional mechanism regulating stromal cell proliferation. As expected, BMDCs are not the sole regulators of endometriosis growth; however, we have clearly identified BMDCs and a substantial factor in inducing endometriosis cell proliferation.
The experiments in vitro and in vivo both suggested that BMDCs can upregulate CDK1 expression and proliferation of endometriosis stromal cells compared with normal endometrial stromal cells. Future studies will define the signaling molecules and the pathways by which BMDCs regulate the CDK1 expression and endometriosis cell proliferation while not stimulating the growth of normal endometrial cells. There are limitations to this study; eutopic ESCs were used in vitro studies. Primary ectopic endometrial stromal cells (ESCs) are limited in number, difficult to grow and passage, and may respond differently. Nevertheless, we have identified important distinctions in the effect of BMDCs in endometriosis. Taken together, we conclude that BMDCs increased the proliferation of primary stromal cells from endometriosis patients as demonstrated by the activation of CDK1. The effect of BMDCs on endogenous endometrial cell proliferation may explain the profound effect of BMDCs despite the relatively small number of BMDCs engrafted. This study may help in developing novel therapeutic strategies for controlling cell proliferation in endometriosis.
References
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–98.
Taylor HS, Osteen KG, Bruner-Tran KL, et al. Novel therapies targeting endometriosis. Reprod Sci. 2011;18(9):814–23.
Pluchino N, Taylor HS. Endometriosis and stem cell trafficking. Reprod Sci. 2016;23(12):1616–9.
Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–5.
Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–6.
Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology. 2014;155(4):1489–97.
Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125.
Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. J Clin Endocrinol Metab. 2001;86(10):4759–64.
Hornung D, Klingel K, Dohrn K, Kandolf R, Wallwiener D, Taylor RN. Regulated on activation, normal T-cell-expressed and -secreted mRNA expression in normal endometrium and endometriotic implants: assessment of autocrine/paracrine regulation by in situ hybridization. Am J Pathol. 2001;158(6):1949–54.
Halme J, Becker S, Hammond MG, Raj MH, Raj S. Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol. 1983;145(3):333–7.
Laird SM, Li TC, Bolton AE. The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod. 1993;8(6):793–8.
Bersinger NA, Frischknecht F, Taylor RN, Mueller MD. Basal and cytokine-stimulated production of epithelial neutrophil activating peptide-78 (ENA-78) and interleukin-8 (IL-8) by cultured human endometrial epithelial and stromal cells. Fertil Steril. 2008;89(5 Suppl):1530–6.
Bersinger NA, Gunthert AR, McKinnon B, Johann S, Mueller MD. Dose-response effect of interleukin (IL)-1beta, tumour necrosis factor (TNF)-alpha, and interferon-gamma on the in vitro production of epithelial neutrophil activating peptide-78 (ENA-78), IL-8, and IL-6 by human endometrial stromal cells. Arch Gynecol Obstet. 2011;283(6):1291–6.
Badawy SZ, Marshall L, Cuenca V. Peritoneal fluid prostaglandins in various stages of the menstrual cycle: role in infertile patients with endometriosis. Int J Fertil. 1985;30(2):48–52.
Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90(1):286–95.
Santulli P, Borghese B, Chouzenoux S, Streuli I, Borderie D, de Ziegler D, et al. Interleukin-19 and interleukin-22 serum levels are decreased in patients with ovarian endometrioma. Fertil Steril. 2013;99(1):219–26.
McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA, Mueller MD. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum Reprod Update. 2016;22(3):382–403.
Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78(3):642–9.
Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–6.
Barr A, Manning D. G proteins techniques of analysis. Boca Raton: CRC Press, Inc; 1999. p. 227–45.
Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. A murine 5-fluorouracil-based Submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology. 2016;157(10):3749–59.
Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85.
Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110 143.
Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril. 2000;74(4):760–6.
Wang C, Jin A, Huang W, Tsang LL, Cai Z, Zhou X, et al. Up-regulation of Bcl-2 by CD147 through ERK activation results in abnormal cell survival in human endometriosis. J Clin Endocrinol Metab. 2015;100(7):E955–63.
Korkmaz D, Bastu E, Dural O, Yasa C, Yavuz E, Buyru F. Apoptosis through regulation of Bcl-2, Bax and Mcl-1 expressions in endometriotic cyst lesions and the endometrium of women with moderate to severe endometriosis. J Obstet Gynaecol. 2013;33(7):725–8.
Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98(5):1200–8.
Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26(4):885–97.
Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6(3):431–44.
Abe W, Nasu K, Nakada C, Kawano Y, Moriyama M, Narahara H. miR-196b targets c-myc and Bcl-2 expression, inhibits proliferation and induces apoptosis in endometriotic stromal cells. Hum Reprod. 2013;28(3):750–61.
Kawano Y, Nasu K, Hijiya N, Tsukamoto Y, Amada K, Abe W, et al. CCAAT/enhancer-binding protein alpha is epigenetically silenced by histone deacetylation in endometriosis and promotes the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2013;98(9):E1474–82.
Moridi I, Mamillapalli R, Cosar E, Ersoy GS, Taylor HS. Bone marrow stem cell chemotactic activity is induced by elevated CXCl12 in endometriosis. Reprod Sci. 2017;24(4):526–33.
Santamaria X, Mas A, Cervello I, Taylor H, Simon C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24(6):673–93.
Herraiz S, Buigues A, Diaz-Garcia C, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109(5):908–18 e902.
Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9(5):e96662.
Keaton MA. Review of “the cell cycle: principles of control” by David O. Morgan. Cell Div. 2007;2:27.
Yang Y, Xue K, Li Z, Zheng W, Dong W, Song J, et al. [Corrigendum] c-Myc regulates the CDK1/cyclin B1 dependent-G2/M cell cycle progression by histone H4 acetylation in Raji cells. Int J Mol Med. 2019;44(5):1988.
Chen X, Zhang FH, Chen QE, et al. The clinical significance of CDK1 expression in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2015;20(1):e7–12.
Wang LL, Sun KX, Wu DD, Xiu YL, Chen X, Chen S, et al. DLEU1 contributes to ovarian carcinoma tumourigenesis and development by interacting with miR-490-3p and altering CDK1 expression. J Cell Mol Med. 2017;21(11):3055–65.
Sung WW, Lin YM, Wu PR, Yen HH, Lai HW, Su TC, et al. High nuclear/cytoplasmic ratio of Cdk1 expression predicts poor prognosis in colorectal cancer patients. BMC Cancer. 2014;14:951.
Tsaur I, Makarevic J, Hudak L, et al. The cdk1-cyclin B complex is involved in everolimus triggered resistance in the PC3 prostate cancer cell line. Cancer Lett. 2011;313(1):84–90.
Zhou J, Han S, Qian W, Gu Y, Li X, Yang K. Metformin induces miR-378 to downregulate the CDK1, leading to suppression of cell proliferation in hepatocellular carcinoma. Onco Targets Ther. 2018;11:4451–9.
Tang L, Wang TT, Wu YT, Zhou CY, Huang HF. High expression levels of cyclin B1 and polo-like kinase 1 in ectopic endometrial cells associated with abnormal cell cycle regulation of endometriosis. Fertil Steril. 2009;91(4):979–87.
Funding
This work was supported by the Endometriosis Foundation of America AWD0003567.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Ethics involved in experiments carried out using cell lines approved by the Yale University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Gross morphology of endometriotic lesions: Circles (white) indicate endometriotic lesions (left). H & E staining of lesion tissue section showing glandular uterine structure (right).(DOCX 11.7 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Mamillapalli, R., Habata, S. et al. Endometriosis Cell Proliferation Induced by Bone Marrow Mesenchymal Stem Cells. Reprod. Sci. 28, 426–434 (2021). https://doi.org/10.1007/s43032-020-00294-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00294-4