Abstract
The aim of this review is to summarize the current literature regarding a link between endometriosis and adverse pregnancy outcomes. We also present an overview of common pathogenic mechanisms between endometriosis and obstetric complications. A computerized literature search was performed to identify relevant studies. The search covered the period between January 2008 and October 2018. Emerging evidence has revealed that endometriosis increased the risk of preterm birth, miscarriage, preterm premature rupture of membranes, placenta previa, preeclampsia, pregnancy-induced hypertension (PIH), gestational diabetes, gestational cholestasis, small for gestational age (SGA) babies, antepartum hemorrhage, postpartum hemorrhage, placental abruption, retained placenta, malpresentation, labor dystocia, cesarean delivery, stillbirth, neonatal death, and congenital malformations of the uterus, but the data are based on limited information. However, some studies have found that endometriosis did not affect pregnancy outcomes. Previous studies are heterogenous and the existing data are controversial. Limited evidence from a few studies also indicated that surgical excision of endometriosis may not reduce the risk of adverse pregnancy outcomes. Endometriosis and obstetric complications may share common pathophysiologic mechanisms, in which abnormal activation of inflammation, structural and functional alterations in the junctional zone, and perturbed uterine peristalsis may play important roles. In this review, we outlined evidence that women with endometriosis have a high risk of obstetric complications. We describe the common crucial features between endometriosis and obstetric complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is one of the common gynecologic diseases in women of reproductive age [1]. It is defined as the presence of endometrial tissue outside the uterine cavity. The complex etiology is still unclear and it is based on at least two main theories: retrograde menstruation with peritoneal seeding and the coelomic or Müllerian duct metaplasia theory [2]. Menstrual cyclicity, inflammation, aberrant hormone signaling, genetic profile, epigenetic modifications, prostaglandin metabolism, immunological factors, or the in utero environment factors may play a possible role in the pathogenesis of endometriosis [3]. It causes pelvic pain, dysmenorrhea, and infertility, which affect the quality of life in women [1]. It has been reported that many comorbidities may arise during the course of endometriosis, including chronic diseases (irritable bowel syndrome, migraine headache, pelvic inflammatory diseases, infertility, obesity, chronic liver disease, rheumatoid arthritis, chronic renal disease, diabetes mellitus, cardiovascular diseases, hypertension, hyperlipidemia, and obstetric complications) and malignancies (ovarian cancer, breast cancer, endometrial cancer, and colorectal cancer) [4, 5]. Among these comorbidities, recent epidemiological studies focused on an association between endometriosis and obstetric complications [6,7,8]. Endometriosis promotes adverse pregnancy outcomes, but underlying mechanisms are poorly understood. The aim of this review is to summarize the current literature regarding a link between endometriosis and adverse pregnancy outcomes and describe the possible mechanisms underlying this complex relationship.
Methods
Literature Search
A computerized literature search was conducted to identify relevant studies reported in the English language. We collected a comprehensive literature search from PubMed and Embase database between January 2008 and October 2018, combining the keywords “endometriosis,” “obstetric complications,” “adverse pregnancy outcomes,” “adenomyosis,” and “Inflammation.” A variety of combinations of these terms were used, depending on which database was searched. Furthermore, the references of each article were searched to identify potentially relevant studies. Publications of original studies, review papers, and multi-/single-center studies in human participants were included, while those documenting opinions, points of view, or anecdotes were excluded. Thirty-four articles were eligible, including retrospective and systematic review studies.
Results
Study Characteristics
A selection flow chart for the search strategy is presented in Fig. 1. Based on the search strategy, a total of 226 articles were initially identified on endometriosis and obstetric complications. After reading titles and abstracts, 176 articles were excluded because they were irrelevant and 50 potential articles were reviewed for full text. After reading the full texts, 8 articles were excluded to duplicate (n = 3) or lack of the relevant date (n = 5). Finally, according to the study inclusion/exclusion criteria, a total of 42 publications were used for the final analysis.
The Relationship Between Endometriosis and Obstetric Complications
Emerging evidence has revealed the possible risk of obstetric complications in endometriosis. The human studies focused on patients who had surgically proven endometriosis/adenomyosis [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Furthermore, to assess the impact of the coexistence of endometriosis/adenomyosis and pregnancy on obstetric complications, this review included patients who did not receive surgical treatment and whose diagnosis was based on imaging techniques [6,7,8, 18, 22,23,24,25,26,27,28,29,30]. In order not to be overlooked, we also selected review articles to evaluate the association between and risk of adverse pregnancy outcomes [31,32,33,34,35,36,37,38,39,40,41,42]. Peer-reviewed retrospective studies (n = 19; [9, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]), national cohort (n = 3; 10, 27, 30), systematic review and meta-analysis (n = 6; [31,32,33,34, 38, 41]), and literature review (n = 6; [35,36,37,38,39,40, 42]) reporting the link between endometriosis/adenomyosis and obstetric complications were eligible for inclusion. In the systematic review and meta-analysis articles, the sample size was from 1,496,715 to 3,280,488 pregnant women, with 95% confidence level for estimating. The sample size of retrospective studies ranged from 49 to 469 patients with endometriosis. The quality of studies varied and limited by relatively small sample sizes.
Characteristics of included studies are shown in Table 1. Endometriosis significantly increased the incidence of preterm birth [6,7,8,9,10,11,12, 24,25,26,27, 31,32,33,34,35,36], miscarriage [13, 34], preterm premature rupture of membranes [8, 10, 32], placenta previa [7, 9, 11, 14, 32, 34, 35], preeclampsia [7, 10, 27, 32, 36], pregnancy-induced hypertension (PIH) [6, 7, 31, 32], gestational diabetes [8, 32], gestational cholestasis [32, 37], small for gestational age (SGA) babies [6,7,8, 10, 31,32,33,34, 38], antepartal hemorrhage [7, 9, 26, 32, 36], postpartum hemorrhage [15], placental abruption [10], retained placenta [10], malpresentation [32], labor dystocia [32], cesarean delivery [7, 9, 27, 32, 34, 37], stillbirth [14, 32], neonatal death [10, 32], and congenital malformations of the uterus [10]. For example, in the systematic review and meta-analysis article, endometriosis was associated with obstetric complications, in the order from highest odds ratio (OR) to lowest: gestational cholestasis (OR = 4.9), placenta previa (OR = 3.3), preterm premature rupture of membranes (OR = 2.3), cesarean section (OR = 1.9), neonatal death (OR = 1.8), malpresentation (OR = 1.7), a preterm birth (OR = 1.7), antepartum hemorrhage (OR = 1.7), labor dystocia (OR = 1.5), stillbirth (OR = 1.3), gestational diabetes (OR = 1.3), SGA (OR = 1.3), gestational hypertension and/or preeclampsia (OR = 1.2), and preeclampsia (OR = 1.2) [32]. In 12 review articles on the risk of obstetric complications in endometriosis, women with endometriosis were more likely to have preterm birth, SGA, gestational hypertension and/or preeclampsia, gestational cholestasis, placenta previa, and hemorrhage: relative rates of these obstetric complications doubled or more in patients with endometriosis. In the original article, women with endometriosis had higher OR: placenta previa (OR 3.9), retained placenta (OR 3.1), preterm birth before 28 weeks (OR 3.1), hemorrhage in pregnancy (OR 2.3), placental abruption (OR 2.0), neonatal death (OR 1.8), severe preeclampsia (OR 1.7), premature rupture of membranes (OR 1.7), SGA (OR 1.5), and congenital malformations (OR 1.3) compared with women without endometriosis [10]. Among a total of 22 original articles, the top 5 obstetric complications consisted of preterm birth, SGA, placenta previa, preeclampsia, and hemorrhage. Taken together, to date, evidence shows that both the coexistence of endometriosis and pregnancy and endometriosis before pregnancy may increase the obstetrical risks.
Obstetric complications associated with endometriosis have been extensively studied but it is still controversial, possibly due to potential biases resulting from heterogeneity of previous studies. Some retrospectively published studies appear to contradict this result [16, 17]. Furthermore, limited evidence from a few studies indicated that surgical excision of endometriosis may not reduce the risk of adverse pregnancy outcomes [10, 15]. Li et al. analyzed the data from 98 primiparous women who had been diagnosed with endometriosis by previous laparoscopic surgery from 2011 to 2013 in China [15]. Pregnancy outcomes were compared between these women and 300 women without endometriosis. Women with endometriosis have a higher risk of pregnancy-related complications such as postpartum hemorrhage in the endometriosis group than in the control group. They suggested that surgery failed to improve the risk of obstetric complications [15]. Berlac et al. extracted the data from the Danish National Cohort Study for 19,331 deliveries from 1997 to 2014 [10]. Sub-analyses were performed to evaluate the risk of obstetric outcomes for women with endometriosis who underwent gynecological surgery before pregnancy. They concluded that surgery significantly increased the risk of several obstetric complications [10].
Moreover, not only a laparoscopically confirmed endometriosis but also suspicion of endometriosis, dysmenorrhea, level of cramps with period, and pain at ovulation are the risk factors that predispose to preterm birth [24]. Among primiparous singleton pregnancies, women with endometriosis had an increased risk of adverse obstetric outcomes: SGA fetuses, gestational diabetes, preterm premature rupture of membranes, and preterm birth [8, 10]. Furthermore, the meta-analyses of pooled data showed that women with endometriosis who conceived by assisted reproductive technology (ART) are significantly associated with a higher risk of preterm birth, postpartum hemorrhage, placenta previa, and SGA than those who conceived naturally [6, 11, 15, 18, 31, 38]. On the other hand, some studies reported that ART does not seem to entail an increased risk of obstetrical complications. They concluded that women with endometriosis were at an increased risk of preterm birth, preeclampsia, and cesarean section, irrespective of use of ART [17, 25, 27, 38].
Three distinct clinical subtypes of endometriosis have been identified: ovarian endometrioma, peritoneal endometriosis, and deep infiltrating endometriosis (DIE). In general, there were no statistically significant differences in the obstetrical risks among the subgroups (DIE, ovarian endometrioma, and peritoneal endometriosis) analyzed [19]. Patients with peritoneal endometriosis with superficial lesions are at an increased risk of miscarriage [21]. Similar to the case of ovarian endometrioma, women with DIE at pregnancy had an increased risk of obstetrical outcomes, such as placenta previa, preterm birth, placental abruption, miscarriage, intrauterine growth restriction, hypertensive disorders, and cesarean delivery compared with the general population [19, 28,29,30]. DIE is associated with higher rates of placenta previa; for other obstetrical outcomes, results are controversial [39]. Women with gynecological surgery for DIE before pregnancy carried an elevated risk of not only placenta previa but also gestational hypertension and intrauterine growth restriction during pregnancy [19, 20].
Adenomyosis constitutes an established nosological entity. According to Kishi et al., adenomyosis consists of at least 2 different subtypes regarding their substructures: subtype I adenomyosis (intrinsic) and subtype II adenomyosis (extrinsic) [43]. Subtype I is defined as a deep endometrial invasion into the myometrium and occurs in the uterine inner layer without affecting the outer structures, whereas subtype II is defined as a direct infiltration into the outer shell of the uterus from pelvic endometriosis, without affecting the inner structures [43]. Depending on the subtype of adenomyosis, subtype I was characterized as premenopausal adenomyosis of the parous woman, and subtype II was recognized as adenomyosis in association with endometriosis of the infertile woman [40]. To exclude the influence of endometriosis, clinical parameters of the subtype I adenomyosis were analyzed in order to identify risk factors [41]. Pregnancy with subtype I adenomyosis was a risk factor for preterm birth [19, 22, 23, 30, 33, 41], premature rupture of membranes [19, 22, 41], low birth weight [30], SGA neonates [23, 30, 33, 41], hypertensive disorders [23, 41], malpresentation [41], uterine rupture [19], intrauterine infection [23], postpartum hemorrhage [19], ectopic pregnancy [19], and miscarriage [23, 41]. Several studies provided important results that adenomyosis itself may be associated with an increased risk of obstetric complications or adverse pregnancy outcomes. Since the study populations were small and confounders could not be totally excluded, evidence is insufficient to draw conclusions.
Finally, spontaneous hemoperitoneum in pregnancy (SHiP) is a very rare, but life-threatening condition and occurs predominantly in the second and third trimesters of pregnancy, particularly relevant to women with endometriosis with in vitro fertilization [42, 44, 45]. SHiP occurs as a result of spontaneous bleeding from decidualized endometriosis implants.
Common Pathophysiologic Mechanisms
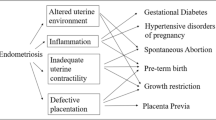
We present an overview of common pathophysiologic mechanisms between endometriosis and the top 5 obstetric complications such as preterm birth, SGA, placenta previa, preeclampsia, and hemorrhage. Two conditions may share overlapping molecular features consisting of abnormal activation of inflammatory and immune functions, (epi)genetic components, and environmental factors [46]. Inflammatory components play key roles in endometriosis. It is well established that infection and inflammation represent a risk factor in preterm birth [47]. Preeclampsia displays excessive maternal inflammation and deficient spiral artery remodeling due to an unbalanced regulation of inflammatory cytokines, immune responses, oxidative stress, maternal endothelial dysfunction, and impaired angiogenesis [48]. Excessive maternal inflammation may also contribute to several pathological conditions, including stillbirth and placental-related fetal growth restriction (FGR) [49]. These data allow us to hypothesize that endometriosis might be associated with numerous comorbidities including preterm birth, preeclampsia, FGR, and other pregnancy complications, mainly through the common pathogenic similarities regarding inflammatory response. Although traditionally viewed as a reproductive disorder, endometriosis patients are at high risk of several chronic diseases, such as not only pregnancy complications but also cardiovascular diseases, autoimmune diseases, asthma/atopic diseases, psychiatric disease, osteoporosis, and cancer [50]. Recent evidence suggests that chronic inflammation may play key roles in coronary heart disease [51], allergic disease [52], autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis [53], psychosocial disturbances or psychiatric distress [54], aromatase disruption and osteoporosis [55], and ovarian cancer, endometrial cancers, thyroid cancer, and other cancers [4, 5]. Endometriosis might be a long-term major health issue in women in later life. However, the exact mechanisms are still not fully established.
Furthermore, adenomyosis itself may be associated with an increased risk of adverse pregnancy outcomes [19, 22, 23, 30, 33, 41]. Adenomyosis represents a spectrum of lesions, ranging from an increased thickness of the junctional zone (JZ) to focal or diffuse forms. Endometriosis often coexists with adenomyosis or uterine fibroids [56]. The JZ in women with endometriosis was also expanded in comparison with controls, suggesting structural and functional abnormalities of the uterine wall in women with endometriosis [57]. The JZ is considered to play a major role in normal uterine peristalsis [58]. In women with endometriosis, the peristaltic activity of the uterus may become dysperistaltic at midcycle [57]. Thus, perturbed uterine peristalsis in women with endometriosis and adenomyosis may affect the eventual site of blastocyst implantation and thereby increase the risk of placenta previa [19, 59, 60]. Furthermore, structural and functional alterations in the inner portion of the myometrium (or the JZ) may result in failure of physiologic transformation of the spiral arteries in the uteroplacental bed and thereby affect placentation [61]. Defective placentation is frequently present in patients with placenta accreta/percreta and retained placenta [62, 63] and those with preterm premature rupture of membranes [64]. Failed deep placentation has been associated with preeclampsia, intrauterine growth restriction, preterm labor, placental abruption, and postpartum hemorrhage [61, 63].
Discussion
This article will focus on the data supporting the common epidemiology and shared pathophysiology aimed at endometriosis and obstetric complications. First, it may be possible to draw a conclusion that the coexistence of endometriosis and pregnancy is responsible for the increased risk of a variety of obstetric complications, including preterm birth, SGA, placenta previa, preeclampsia, and hemorrhage [1, 9, 11,12,13,14, 18,19,20,21,22,23]. Inclusion criteria for some studies were defined as a pathologically confirmed endometriosis [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23], while other studies reported clinical diagnosis based on ultrasonographic or MR imaging findings [6,7,8, 18, 22,23,24,25,26,27,28,29,30]. The former group is composed of a relatively small number of patients with a pathologically confirmed endometriosis, whereas the latter may include some women with misdiagnosed or undiagnosed endometriosis. Conversely, two retrospective studies described that endometriosis may not affect pregnancy outcomes [16, 17]. The data are based on limited information, because the sources of information were derived from a relatively small number of patients. They retrospectively analyzed the data from 49 to 78 patients. Therefore, limited numbers of studies may provide controversial results regarding the possible risk of obstetric complications in endometriosis.
Second, surgical excision of endometriosis may not reduce the risk of adverse pregnancy outcomes [10, 15]. Furthermore, surgical interventions for DIE before pregnancy did not prevent obstetric complications such as placenta previa, gestational hypertension, and intrauterine growth restriction during pregnancy [19, 20]. Since surgical excision of severe disease such as DIE is mandatory when women do not respond to medical therapy, incomplete or partial excision is suspected in severe cases [10, 65]. We address the possibility that previous surgical interventions may increase or not reduce the risks of obstetric complications, which may be due to minimal residual disease or disease recurrence after laparoscopic surgery. However, it is hard to draw a definite conclusion because the previous reports did not compare the same women before and after the surgical excisions.
Third, patients with endometriosis and obstetric complications may share the common pathophysiology including abnormal activation of inflammatory functions [5, 46,47,48,49, 51,52,53,54,55], structural and functional alterations in the JZ [57, 61,62,63,64], and perturbed uterine peristalsis [19, 57,58,59,60]. One of the key components characterizing endometriosis is a chronic, low level, and sterile inflammation manifested by elevated levels of local or circulatory cytokines. A variety of obstetric complications are also associated with an increased inflammation. Untimely activation of inflammatory processes is known to be associated with placental dysfunction and pregnancy complications, which can have a devastating effect on pregnancy outcomes [66]. In addition, recent evidence revealed that the common pathophysiology of endometriosis and obstetric complications may involve epigenetic changes, which are known or suspected to support cell growth and survival [67,68,69,70,71]. In another article of this journal (RSCI-19-519 revision), we discuss the underlying mechanism of epigenetic dysregulation unique to and shared between endometriosis and adverse pregnancy complications. Our study revealed some dysregulated imprinted genes common to endometriosis and obstetric complications.
Fourth, besides the pathophysiological role of inflammation, we will discuss other different contributors explaining the mechanism by which endometriosis significantly increased the risk of obstetric complications. What is the mechanism that links endometriosis and these obstetric complications? We consider the potential and multiple contributions of confounding factors in many of the reported studies, which makes the interpretation of the results difficult. Common reasons for spontaneous and indicated preterm births include preterm premature rupture of membranes, placenta previa, preeclampsia or eclampsia, hypertension, gestational diabetes, placental abruption, and fetal indications (SGA, stillbirth, and neonatal death) [72]. Placenta previa and SGA might confound results of studies evaluating the risk of malpresentation and labor dystocia. Also, the presence of a large adnexal mass including endometrioma in the third trimester of pregnancy may be associated with some complications affecting the course of pregnancy and labor via cesarean section because of the risk of dystocia or malpresentation [73]. Furthermore, it is well known that patients with uterine malformations or obstructive genital anomalies such as congenital cervical atresia may be associated with an increased incidence of pelvic endometriosis [74].
Finally, some limitations exist in this review. The reported studies have shown varying results, likely due to the differences in sample size, disease severity, definitive diagnosis, methodologic flaws, and the potential contribution of residual confounders. Because noninvasive clinical diagnosis of symptomatic endometriosis is less accurate than surgical diagnosis, the study cohort and the control cohort may include some patients with misdiagnosed endometriosis and some women with undiagnosed endometriosis, respectively.
In conclusion, endometriosis may have a negative impact on pregnancy outcomes, possibly through common pathophysiologic similarities leading to inflammation. Further investigation is needed to provide the new insight into the common epigenetic mechanisms underlying the interaction between endometriosis and obstetric complications.
References
Petraglia F, Arcuri F, de Ziegler D, Chapron C. Inflammation: a link between endometriosis and preterm birth. Fertil Steril. 2012;98:36–40.
Scutiero G, Iannone P, Bernardi G, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxidative Med Cell Longev. 2017;2017:7265238.
Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96:623–32.
Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol. 2017;209:3–7.
Teng SW, Horng HC, Ho CH, Yen MS, Chao HT. Wang PH; Taiwan Association of Gynecology Systematic Review Group. Women with endometriosis have higher comorbidities: analysis of domestic data in Taiwan. J Chin Med Assoc. 2016;79(11):577–82. https://doi.org/10.1016/j.jcma.2016.04.006.
Fernando S, Breheny S, Jaques AM, Halliday JL, Baker G, Healy D. Preterm birth, ovarian endometriomata, and assisted reproduction technologies. Fertil Steril. 2009;91:325–30.
Stephansson O, Kieler H, Granath F, Falconer H. Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod. 2009;24:2341–7.
Conti N, Cevenini G, Vannuccini S, et al. Women with endometriosis at first pregnancy have an increased risk of adverse obstetric outcome. J Matern Fetal Neonatal Med. 2015;28:1795–58.
Lin H, Leng JH, Liu JT, Lang JH. Obstetric outcomes in Chinese women with endometriosis: a retrospective cohort study. Chin Med J (Engl). 2015;128:455–8.
Berlac JF, Hartwell D, Skovlund CW, Langhoff-Roos J, Lidegaard Ø. Endometriosis increases the risk of obstetrical and neonatal complications. Acta Obstet Gynecol Scand. 2017;96:751–60.
Fujii T, Wada-Hiraike O, Nagamatsu T, et al. Assisted reproductive technology pregnancy complications are significantly associated with endometriosis severity before conception: a retrospective cohort study. Reprod Biol Endocrinol. 2016;14:73.
Vannuccini S, Lazzeri L, Orlandini C, Tosti C, Clifton VL, Petraglia F. Potential influence of in utero and early neonatal exposures on the later development of endometriosis. Fertil Steril. 2016;105:997–1002.
Aris A. A 12-year cohort study on adverse pregnancy outcomes in Eastern Townships of Canada: impact of endometriosis. Gynecol Endocrinol. 2014;30:34–7.
Chen I, Lalani S, Xie RH, Shen M, Singh SS, Wen SW. Association between surgically diagnosed endometriosis and adverse pregnancy outcomes. Fertil Steril. 2018;109:142–7.
Li H, Zhu HL, Chang XH, Li Y, Wang Y, Guan J, et al. Effects of previous laparoscopic surgical diagnosis of endometriosis on pregnancy outcomes. Chin Med J (Engl). 2017;130:428–33.
Mekaru K, Masamoto H, Sugiyama H, et al. Endometriosis and pregnancy outcome: are pregnancies complicated by endometriosis a high-risk group? Eur J Obstet Gynecol Reprod Biol. 2014;172:36–9.
Benaglia L, Bermejo A, Somigliana E, Scarduelli C, Ragni G, Fedele L, et al. Pregnancy outcome in women with endometriomas achieving pregnancy through IVF. Hum Reprod. 2012;27:1663–7.
Benaglia L, Candotti G, Papaleo E, Pagliardini L, Leonardi M, Reschini M, et al. Pregnancy outcome in women with endometriosis achieving pregnancy with IVF. Hum Reprod. 2016;31:2730–6.
Vercellini P, Parazzini F, Pietropaolo G, Cipriani S, Frattaruolo MP, Fedele L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: a retrospective cohort study. BJOG. 2012;119:1538–43.
Nirgianakis K, Gasparri ML, Radan AP, Villiger A, McKinnon B, Mosimann B, et al. Obstetric complications after laparoscopic excision of posterior deep infiltrating endometriosis: a case-control study. Fertil Steril. 2018;110:459–66.
Kohl Schwartz AS, Wölfler MM, Mitter V, et al. Endometriosis, especially mild disease: a risk factor for miscarriages. Fertil Steril. 2017;108:806–814.e2.
Juang CM, Chou P, Yen MS, Twu NF, Horng HC, Hsu WL. Adenomyosis and risk of preterm delivery. BJOG. 2007;114:165–9.
Tamura H, Kishi H, Kitade M, Asai-Sato M, Tanaka A, Murakami T, et al. Complications and outcomes of pregnant women with adenomyosis in Japan. Reprod Med Biol. 2017;16:330–6.
Bayram C, Osmanağaoğlu MA, Aran T, Güven S, Bozkaya H. The effect of chronic pelvic pain scoring on pre-term delivery rate. J Obstet Gynaecol. 2013;33:32–7.
Harada T, Taniguchi F, Onishi K, et al. Obstetrical complications in women with endometriosis: a cohort study in Japan. PLoS One. 2016;11:e0168476.
Brosens I, Brosens JJ, Fusi L, Al-Sabbagh M, Kuroda K, Benagiano G. Risks of adverse pregnancy outcome in endometriosis. Fertil Steril. 2012;98:30–5.
Glavind MT, Forman A, Arendt LH, Nielsen K, Henriksen TB. Endometriosis and pregnancy complications: a Danish cohort study. Fertil Steril. 2017;107:160–6.
Mannini L, Sorbi F, Noci I, Ghizzoni V, Perelli F, di Tommaso M, et al. New adverse obstetrics outcomes associated with endometriosis: a retrospective cohort study. Arch Gynecol Obstet. 2017;295:141–51.
Exacoustos C, Lauriola I, Lazzeri L, De Felice G, Zupi E. Complications during pregnancy and delivery in women with untreated rectovaginal deep infiltrating endometriosis. Fertil Steril. 2016;106:1129–1135.e1.
Yamaguchi A, Kyozuka H, Fujimori K, et al. Risk of preterm birth, low birthweight, and small-for-gestational-age infants in pregnancies with adenomyosis: a cohort study of the Japan Environment and Children’s Study. Acta Obstet Gynecol Scand. 2019;98:359–64.
Kim SG, Seo HG, Kim YS. Primiparous singleton women with endometriosis have an increased risk of preterm birth: meta-analyses. Obstet Gynecol Sci. 2017;60:283–8.
Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, Wen SW, et al. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum Reprod. 2018;33:1854–65.
Bruun MR, Arendt LH, Forman A, Ramlau-Hansen CH. Endometriosis and adenomyosis are associated with increased risk of preterm delivery and a small-for-gestational-age child: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97:1073–90.
Zullo F, Spagnolo E, Saccone G, et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil Steril. 2017;108:667–672.e5.
Vigano P, Corti L, Berlanda N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil Steril. 2015;104:802–12.
Falconer H. Pregnancy outcomes in women with endometriosis. Semin Reprod Med. 2013;31:178–82.
Ozkan S, Ceylan Y, Ozkan OV, Yildirim S. Review of a challenging clinical issue: intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2015;21:7134–41.
Pérez-López FR, Villagrasa-Boli P, Muñoz-Olarte M, Morera-Grau Á, Cruz-Andrés P, Hernandez AV, et al. Association between endometriosis and preterm birth in women with spontaneous conception or using assisted reproductive technology: a systematic review and meta-analysis of cohort studies. Reprod Sci. 2018;25:311–9.
Leone Roberti Maggiore U, Inversetti A, Schimberni M, Viganò P, Giorgione V, Candiani M. Obstetrical complications of endometriosis, particularly deep endometriosis. Fertil Steril. 2017;108:895–912.
Leyendecker G, Kunz G, Kissler S, Wildt L. Adenomyosis and reproduction. Best Pract Res Clin Obstet Gynaecol. 2006;20:523–46.
Cozzolino M, Basile F, Pontrelli G. Effects of adenomyosis on obstetric outcomes: a literature review. Minerva Ginecol. 2019;71:146–54.
Lier MCI, Brosens IA, Mijatovic V, Habiba M, Benagiano G. Decidual bleeding as a cause of spontaneous hemoperitoneum in pregnancy and risk of preterm birth. Gynecol Obstet Investig. 2017;82:313–21.
Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012;207:114.e1–7.
Lier M, Malik RF, van Waesberghe J, Maas JW, van Rumpt-van de Geest D, Coppus SF, et al. Spontaneous haemoperitoneum in pregnancy and endometriosis: a case series. BJOG. 2017;124:306–12.
Cozzolino M, Corioni S, Maggio L, Sorbi F, Guaschino S, Fambrini M. Endometriosis-related hemoperitoneum in pregnancy: a diagnosis to keep in mind. Ochsner J. 2015;15:262–4.
Jiang L, Yan Y, Liu Z, Wang Y. Inflammation and endometriosis. Front Biosci (Landmark Ed). 2016;21:941–8.
Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78. https://doi.org/10.1189/jlb.3MR0615-272RR.
Perucci LO, Corrêa MD, Dusse LM, Gomes KB, Sousa LP. Resolution of inflammation pathways in preeclampsia-a narrative review. Immunol Res. 2017;65(4):774–89. https://doi.org/10.1007/s12026-017-8921-3.
Lindner U, Tutdibi E, Binot S, Monz D, Hilgendorff A, Gortner L. Levels of cytokines in umbilical cord blood in small for gestational age preterm infants. Klin Padiatr. 2013;225(2):70–4. https://doi.org/10.1055/s-0033-1334879.
Tarín JJ, García-Pérez MA, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod Biol Endocrinol. 2015;13:31.
Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257–64. https://doi.org/10.1161/CIRCOUTCOMES.115.002224.
Caserta D, Mallozzi M, Pulcinelli FM, Mossa B, Moscarini M. Endometriosis allergic or autoimmune disease: pathogenetic aspects--a case control study. Clin Exp Obstet Gynecol. 2016;43(3):354–7.
Sundqvist J, Falconer H, Seddighzadeh M, Vodolazkaia A, Fassbender A, Kyama C, et al. Endometriosis and autoimmune disease: association of susceptibility to moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil Steril. 2011;95(1):437–40. https://doi.org/10.1016/j.fertnstert.2010.07.1060.
Pope CJ, Sharma V, Sharma S, Mazmanian D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can. 2015 Nov;37(11):1006–15.
Patel S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: a comprehensive review. J Steroid Biochem Mol Biol. 2017;168:19–25. https://doi.org/10.1016/j.jsbmb.2017.01.009.
Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res. 2019;8:F1000 Faculty Rev-283. https://doi.org/10.12688/f1000research.17242.1 eCollection 2019.
Kunz G, Beil D, Huppert P, Leyendecker G. Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum Reprod. 2000 Jan;15(1):76–82.
Brosens JJ, Barker FG, de Souza NM. Myometrial zonal differentiation and uterine junctional zone hyperplasia in the non-pregnant uterus. Hum Reprod Update. 1998 Sep-Oct;4(5):496–502.
Leyendecker G, Kunz G, Herbertz M, Beil D, Huppert P, Mall G, et al. Uterine peristaltic activity and the development of endometriosis. Ann N Y Acad Sci. 2004;1034:338–55.
Leone Roberti Maggiore U, Ferrero S, Mangili G, Bergamini A, Inversetti A, Giorgione V, et al. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis. Hum Reprod Update. 2016;22(1):70–103. https://doi.org/10.1093/humupd/dmv045.
Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta. 2013;34(2):100–5. https://doi.org/10.1016/j.placenta.2012.11.017.
Brosens I, Derwig I, Brosens J, Fusi L, Benagiano G, Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod. 2010;25(3):569–74. https://doi.org/10.1093/humrep/dep474.
Saraswat L, Ayansina DT, Cooper KG, Bhattacharya S, Miligkos D, Horne AW, et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG. 2017;124(3):444–52. https://doi.org/10.1111/1471-0528.13920.
Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187(5):1137–42.
O’Callaghan D. Endometriosis--an update. Aust Fam Physician. 2006;35(11):864–7.
Brien ME, Baker B, Duval C, Gaudreault V, Jones RL, Girard S. Alarmins at the maternal-fetal interface: involvement of inflammation in placental dysfunction and pregnancy complications 1. Can J Physiol Pharmacol. 2019;97(3):206–12. https://doi.org/10.1139/cjpp-2018-0363.
Kobayashi H. Imprinting genes associated with endometriosis. EXCLI J. 2014;13:252–64.
Chen P, Wang DB, Liang YM. Evaluation of estrogen in endometriosis patients: regulation of GATA-3 in endometrial cells and effects on Th2 cytokines. J Obstet Gynaecol Res. 2016;42:669–77.
Cordeiro A, Neto AP, Carvalho F, Ramalho C, Dória S. Relevance of genomic imprinting in intrauterine human growth expression of CDKN1C, H19, IGF2, KCNQ1 and PHLDA2 imprinted genes. J Assist Reprod Genet. 2014;31(10):1361–8. https://doi.org/10.1007/s10815-014-0278-0.
Zadora J, Singh M, Herse F, Przybyl L, Haase N, Golic M, et al. Disturbed placental imprinting in preeclampsia leads to altered expression of DLX5, a human-specific early trophoblast marker. Circulation. 2017;136(19):1824–39. https://doi.org/10.1161/CIRCULATIONAHA.117.028110.
Burris HH, Baccarelli AA, Motta V, Byun HM, Just AC, Mercado-Garcia A, et al. Association between length of gestation and cervical DNA methylation of PTGER2 and LINE 1-HS. Epigenetics. 2014;9(8):1083–91. https://doi.org/10.4161/epi.29170.
Meis PJ, Michielutte R, Peters TJ, et al. Factors associated with preterm birth in Cardiff, Wales. II. Indicated and spontaneous preterm birth. Am J Obstet Gynecol. 1995;173:597–602.
Oprescu ND, Ionescu CA, Drăgan I, Fetecău AC, Said-Moldoveanu AL, Chirculescu R, et al. Adnexal masses in pregnancy: perinatal impact. Romanian J Morphol Embryol. 2018;59:153–8.
Boujenah J, Salakos E, Pinto M, Shore J, Sifer C, Poncelet C, et al. Endometriosis and uterine malformations: infertility may increase severity of endometriosis. Acta Obstet Gynecol Scand. 2017;96:702–6.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP16K11150, 18 K09269, and 18 K09234.
Author information
Authors and Affiliations
Contributions
NK, KO, and CY performed the literature search and collected data regarding the data supporting the common epidemiology and shared pathophysiology using the Web database. HK and CY made substantial contribution to the conception of the study. HK contributed to the study design and interpretation of included research studies. The final version of the manuscript has been read and approved by all authors.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
One-sentence summary
Endometriosis may increase the risk of obstetric complications, possibly through common pathophysiologic mechanisms such as inflammation.
Electronic Supplementary Material
ESM 1
(DTD 43 kb)
Rights and permissions
About this article
Cite this article
Kobayashi, H., Kawahara, N., Ogawa, K. et al. A Relationship Between Endometriosis and Obstetric Complications. Reprod. Sci. 27, 771–778 (2020). https://doi.org/10.1007/s43032-019-00118-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00118-0