Abstract
Zoothamnium is a speciose genus, most species of which have incomplete morphological data based on modern criteria. In the present study, the morphology of three species of Zoothamnium, i.e., Z. apoarbuscula n. sp., Z. apohentscheli n. sp., and Z. alternans, collected in Qingdao, China, was revealed using living observation and silver staining. In addition, the SSU rDNA of each species was sequenced for phylogenetic analyses. Zoothamnium apoarbuscula n. sp. is characterized by its umbellate colony which is up to 900 μm high, dichotomously branched stalk, differentiation of zooids, and infundibular polykinety 3 comprising three equal-length ciliary rows. Zoothamnium apohentscheli n. sp is characterized by its large colony up to 1700 μm high, alternately branched stalk, and infundibular polykinety 3 comprising three equal-length ciliary rows. A population of Z. alternans is described in detail. Phylogenetic analyses based on SSU rDNA sequence data revealed that species with an alternately branched stalk cluster together in gene trees and probably represent an independent lineage within the genus Zoothamnium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subclass Peritrichia Stein, 1859, is one of the largest groups of ciliates with over 1000 nominal species. It has a global distribution and its members occupy a wide range of marine, freshwater and terrestrial habitats (Entz 1884; Foissner et al. 1992, 2010; Hu et al. 2019; Kahl 1935; Kent 1880–1882; Lynn 2008; Penard 1922; Wilbert and Song 2005; Zhou et al. 2019). Peritrichia are divided into two orders, the Sessilida Kahl, 1933 and the Mobilida Kahl, 1933. Among the sessilids, some are solitary (e.g., Vorticella, Scyphidia); whereas, others are colonial (e.g., Epistylis, Opercularia, Zoothamnium).
Members of the genus Zoothamnium are colonial and have a continuous spasmoneme that runs throughout the entire colony (although sometimes detached from stalk base) causing the stalk to contract in a “zig-zag” fashion (Corliss 1979; de St. Vincent 1824; Lynn 2008). Nearly, 140 nominal species are included in this genus but most are poorly characterized due to their lack of information based on modern criteria, i.e., silverline system, infraciliature, and molecular data (Kahl 1933, 1935; Nenninger 1948; Sommer 1951; Stiller 1971). A combination of morphological similarity and inaccurate or incomplete descriptions renders it difficult to separate and identify species of Zoothamnium (Ji et al. 2006, 2011; Kahl 1933, 1935; Lu et al. 2020; Precht 1935; Sommer 1951; Stiller 1953a, b; Stiller and Stevčić 1967). Furthermore, new species of Zoothamnium are continuously reported suggesting that there is a large, undiscovered diversity (Ji et al. 2015; Lu et al. 2020; Schuster and Bright 2016; Shen et al. 2017; Sun et al. 2015; Wang and Ji 2019).
In the present study, we describe three marine Zoothamnium species collected from a marine fish aquarium in Qingdao, China, based on the observations of both living and silver-stained specimens. After comparison with known congeners, two were identified as new species, i.e., Z. apoarbuscula n. sp. and Z. apohentscheli n. sp. Another species was identified as Zoothamnium alternans Claparède & Lachmann, 1858. In addition, we analyze the molecular phylogeny of these three species based on SSU rDNA sequence data.
Results
Class: Oligohymenophorea de Puytorac et al., 1974.
Subclass: Peritrichia Stein, 1859.
Order: Sessilida Kahl, 1933.
Genus: Zoothamnium Bory de St. Vincent, 1824.
Zoothamnium apoarbuscula n. sp. (Fig. 2; Table 1)
Diagnosis Colony inverted dome-like in outline with dichotomously branched accessory branches all of which radiate from apical end of main stalk. With micro- and macrozooids; microzooids inverted bell-shaped, 25–50 × 20–30 μm in vivo; macrozooids globular, located on lower portions of accessory branches. Peristomial lip single layered, strongly everted. Peristomial disc conspicuously elevated. One contractile vacuole apically located on dorsal wall of infundibulum. Macronucleus C-shaped, transversely oriented. Polykinety 3 (P3) consists of three equal-length rows, terminates above polykinety 1 (P1). Marine habitat.
Type locality Marine fish aquarium (300 × 50 × 70 cm) in Laboratory of Protozoology (N36°03′44″; E120°19′52″), Ocean University of China, Qingdao, China. The water temperature was 28 °C and the salinity was 30 ‰.
Deposition of slides One protargol slide (registration number: WT-20190819-01-01) containing the holotype specimen and a silver nitrate slide (registration number: WT-20190819-01-03) containing paratype specimens were deposited in the Laboratory of Protozoology, Ocean University of China (OUC), Qingdao, China. One protargol slide (registration number: NHMUK 2020.4.23.1) containing paratype specimens was deposited in the Natural History Museum, London, UK.
Etymology The species-group name apoarbuscula is a composite of the Greek prefix “apo-” (away from) and the species-group name arbuscula reflecting its similarity to the well-known species Zoothamnium arbuscula Ehrenberg, 1838 in terms of its colony shape.
Description Colony with micro- and macrozooids, often densely covered with detritus. Microzooids about 25–50 × 20–30 μm in vivo, inverted bell-shaped (Fig. 2a, b, g–n). Peristomial lip about 30–35 μm in diameter, single layered and strongly everted (Fig. 2a, b, h–n). Peristomial disc conspicuously elevated in fully extended zooids (Fig. 2a, b, h, i). Macrozooids globular, located on lower portions of accessory branches (Fig. 2d, g, q). Pellicular striations transverse, easily recognizable above 1000 × magnification. Oral cilia about 13 μm in length.
Cytoplasm colorless, usually full of yellow or gray food granules. Single contractile vacuole located at the center of peristomial disc, slightly above peristomial lip and at dorsal wall of infundibulum (Fig. 2a, b, j, m). Macronucleus C-shaped, transversely oriented (Fig. 2a–c, r, s). Micronucleus not observed.
Colony up to 900 μm tall, mostly contains fewer than 50 zooids, with accessory branches that radiate from apical end of main stalk. Accessory branches equal length and dichotomously branched: all branches extend upwards collectively forming an inverted dome-like outline (Fig. 2d, g). Spasmoneme sturdy, comprising bundles of fibrils (stalk myonemes) within a transparent sheath and with a reticulate surface, detached from stalk base (Fig. 2o, p).
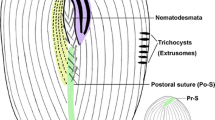
Oral ciliature of basic type for sessilid peritrichs. Haplokinety and polykinety make approximately one circuit around the peristome before entering the infundibulum where they make a further circuit (Fig. 2f, r, s). Three infundibular polykineties (P1–P3) each composed of three rows of kinetosomes (Fig. 2f). Rows of P1 are nearly equal in length. P2 and P3 are nearly equal in length, about half length of P1 (Fig. 2f, r, s, u, v). P2 ends adstomally at convergence of P1 and P3 (Fig. 2f, r, s, u). Row 1 of P2 converges abstomally with P1 (Fig. 2f, v). Row 3 of P2 is abstomally detached from rows 1 and 2 (Fig. 2f, v). P3 consists of three equal-length rows of kinetosomes, and ends above P1 adstomally (Fig. 2f, r, s, u). Two epistomial membranes (EM 1 and EM2): EM 1 located at the entrance of infundibulum (Fig. 2f, t); EM 2 located in front of distal ends of haplokinety and polykinety (Fig. 2f, s, t). Germinal kinety lies parallel to haplokinety in the upper half of infundibulum (Fig. 2f). Trochal band consists of dikinetids, located about two-thirds down length of zooid (Fig. 2r, s).
Silverline system consists of closely spaced transverse silverlines (Fig. 2e, w).
Zoothamnium apohentscheli n. sp. (Figs. 3, 4; Table 1)
Diagnosis Colony up to 1700 μm high. Stalk alternately branched. Zooids inverted bell-shaped, usually 40–65 × 25–40 μm in vivo. Peristomial lip single layered and slightly everted. Peristomial disc moderately elevated. Single contractile vacuole at same level as peristomial lip, dorsally located. Macronucleus C-shaped, transversely oriented. P3 consists of three equal-length rows of kinetosomes, and ends above P1 adstomally. Number of transverse silverlines is 63–79 from peristome to trochal band, 29–31 from trochal band to scopula. Marine habitat.
Type locality Marine fish aquarium (300 × 50 × 70 cm) in Laboratory of Protozoology (N36°03′44″; E120°19′52″), Ocean University of China, Qingdao, China. The water temperature was 20 °C and the salinity was 30 ‰.
Deposition of slides One protargol slide (registration number: WT-20181224-01-01) containing the holotype specimen was deposited in the Laboratory of Protozoology, OUC. Another protargol slide (registration number: NHMUK 2020.4.23.2) containing paratype specimens was deposited in the Natural History Museum, London, UK.
Etymology The species-group name apohentscheli is a composite of the Greek prefix “apo-” (away from) and the species-group name hentscheli, reflecting the superficial similarity of this species to Z. hentscheli Kahl, 1935.
Description Zooids about 40–65 × 25–40 μm in vivo and inverted bell-shaped (Figs. 3a–e, 4b–d, g–i). Peristomial lip about 35–40 μm in diameter, single layered, moderately thickened and everted (Figs. 3a–e, 4c, d, g–i). Peristomial disc convex, clearly elevated above peristomial lip in fully extended zooids (Figs. 3a–e, 4b–d, g–i). Pellicular striations conspicuously recognizable at 1000 × magnification, numbering 63–79 from peristome to trochal band, 29–31 from trochal band to scopula (Figs. 3e, 4j). Oral cilia about 13 μm in length.
Cytoplasm colorless, usually containing several yellow or colorless food granules. Contractile vacuole situated at the dorsal wall of infundibulum, at same level as peristomial lip (Figs. 3a, b, 4d, g, i). Macronucleus C-shaped, transversely oriented (Figs. 3a–e, 4o). Micronucleus not observed.
Colony up to 1700 μm tall, usually with over 50 zooids. Stalk alternately branched, branches progressively narrowed and shortened from primary stalk to terminal branches (Figs. 3f, 4a). Stalk sheath colorless, with inconspicuous longitudinal striations (Fig. 4e, f).
Oral ciliature of usual type for sessilid peritrichs. Haplokinety and polykinety make approximately 1.5 circuits around peristome and a further turn within infundibulum (Figs. 3g, 4k, l). Three infundibular polykineties (P1–P3) each composed of three rows of kinetosomes (Figs. 3g, 4k, l, n). Three rows of P1 nearly equal length. P2 ends adstomally at convergence of P1 and P3 (Figs. 3g, 4k, l, n). Abstomal ends of rows 1 and 2 in P2 converge with P1 and diverge from row 3 (Figs. 3g, 4k, l). Two epistomial membranes (EM 1 and EM2): EM 1 located at entrance of infundibulum (Figs. 3g, 4k, m); EM 2 close to distal ends of haplokinety and polykinety (Figs. 3g, 4m). Germinal kinety lies parallel to haplokinety in upper half of infundibulum (Figs. 3g, 4l). Trochal band consists of dikinetids, located about two-thirds down length of zooid (Figs. 3e, 4j, k, l).
Zoothamnium alternans Claparède & Lachmann, 1858 (Fig. 5; Table 1)
1858 Zoothamnium alternans—Claparède & Lachmann, Mém. Inst. natn. Génev. 5 (year 1857): 103–104, Pl. II, Figs. 1, 2, 3 and 4 (original description).
Morphology of Zoothamnium apoarbuscula n. sp. in vivo (a, b, d, g–p), after protargol staining (c, f, q–v) and after “wet” silver nitrate staining (e, w). a, b, h–k, m, n Different individuals, showing the variation of zooid shape, arrows mark the contractile vacuole. (c) Showing the variation of macronucleus shape. d, g Mature colony, arrows mark the macrozooid. e, w Detail of silverline system and pellicular pores. f Oral ciliature. l Upper portion of a relatively immature colony. o Part of primary stalk, showing the reticulate surface of spasmoneme. p Posterior portion of stalk, arrow marks the end of spasmoneme. q A stained colony, arrows mark macrozooids. r, s Two protargol-stained zooids showing ciliature, arrow in r marks P3, arrowhead in r marks trochal band, arrow in s marks the end of P3, arrowhead in s marks EM2. t Part of oral ciliature, arrow marks EM1, arrowhead marks EM2. u, v Infundibular polykineties, arrow in u marks P3, arrow in v marks P2. EM1, 2 epistomial membrane 1, 2, G germinal kinety, H haplokinety, Po polykinety, P1–3 infundibular polykineties 1–3. Scale bars: 25 μm in (a, b); 400 μm in (d); 300 μm in (g); 20 μm in (h–k, m, n); 15 μm in (r, s)
Zoothamnium apohentscheli n. sp. in vivo (a–f) and after protargol staining (g). a–d Different individuals, showing the variation of zooid shape. e Schematic of a zooid, showing pellicular striations and position of the trochal band. f A mature colony. g Oral ciliature. EM1, 2 epistomial membrane 1, 2, G germinal kinety, H haplokinety, Po polykinety, P1–3 infundibular polykineties 1–3, TB trochal band. Scale bars: 30 μm in (a–e); 500 μm in (f)
Photomicrographs of Zoothamnium apohentscheli n. sp. in vivo (a–j), after protargol staining (k–n) and after DAPI staining (o). a A mature colony. b–d, g–i Different zooids, showing the variation of zooid shape, arrows mark the contractile vacuole. (e, f) Parts of stalk, arrows mark the spasmoneme. j Pellicular striations, arrow marks the trochal band. k, l Two protargol-stained zooids, showing ciliature, arrow in k marks polykinety 3, arrowhead in k marks epistomial membrane 1, double arrowhead in k marks the trochal band, arrow in l marks the germinal kinety, arrowhead in l marks the haplokinety. m Part of oral ciliature, arrow marks epistomial membrane 2, arrowhead marks epistomial membrane 1. n Infundibular polykineties. o DAPI-stained zooid, showing the macronucleus. P1–3 infundibular polykineties 1–3. Scale bars: 500 μm in (a); 25 μm in (c, d, g–i); 20 μm in (k, l)
1930 Zoothamnium alternans Claparède & Lachmann, 1858—Fauré-Fremiet, Biol. Bull., 58: 28–51, Figs. 1–15 (growth and differentiation).
1933 Zoothamnium alternans Claparède & Lachmann, 1858—Kahl, Tierwelt N.- und Ostsee 23 (Teil II. c3): 132, Fig. 23 (2, 2a).
1935 Zoothamnium alternans Claparède & Lachmann, 1858—Kahl, Tierwelt Dtl., 30: 748, Fig. 139 (9, 10).
1938 Zoothamnium alternans Claparède & Lachmann, 1858—Summers, Biol. Bull., 74: 117–129, Fig. 1 (development of colony).
1938 Zoothamnium alternans Claparède & Lachmann, 1858—Summers, Biol. Bull., 74: 130–159 (regulative development).
2001 Zoothamnium chlamydis n. sp.—Hu & Song, Acta Protozool., 40: 215–220, Figs. 1–20 (synonym).
2006 Zoothamnium alternans Claparède & Lachmann, 1858–Ji et al., Acta Protozool., 45: 28–32, Figs. 1a–i, 2a–h (redescription and revision).
2009 Zoothamnium alternans Claparède & Lachmann, 1858–Song, Warren & Hu, Free-living ciliates in the Bohai and Yellow Seas, China., 260–261, Figs. 8.1E–G, 8.2C, D (redescription).
Since the original record of Zoothamnium alternans from the North Sea, Germany, this species has been reported several times, including two based on silver-stained specimens (Claparède and Lachmann 1858; Fauré-Fremiet 1930; Hu and Song 2001; Ji et al. 2006, 2009; Kahl 1933, 1935; Summers 1938a, b). The descriptions of different populations are necessary in order to better understand its intraspecific variation. A detailed redescription and an improved diagnosis are supplied here based on the present population.
Improved diagnosis Colony feather-like in appearance, stalk alternately branched. With micro- and macrozooids; microzooids inverted bell-shaped, about 35–60 × 15–32 μm in vivo; macrozooids about 55–120 × 30–60 μm in vivo, located only on primary stalk. Peristomial lip single layered and distinctly everted. Peristomial disc moderately elevated. Contractile vacuole dorsally located, at the same level as peristomial lip. Macronucleus J-shaped. P3 consists of three approximately equal-length rows, ends adstomally above P1. Number of transverse silverlines is about 27–55 from peristome to trochal band, 19–30 from trochal band to scopula. Marine habitat.
Voucher slides Two protargol slides (registration numbers: LBR-20180413-01, LBR-20180413-02), and one “wet” silver nitrate slide (registration number: LBR-20180413-01-03), with voucher specimens were deposited in the Laboratory of Protozoology, Ocean University of China, Qingdao, China.
Description based on the present population Colony with micro- and macrozooids. Microzooids usually inverted bell-shaped, 30–45 × 15–30 μm in vivo (Fig. 5a, c, d, j–o). Peristomial lip about 30–40 μm in diameter, single layered and distinctly everted (Fig. 5a, c, d, j–o). Peristomial disc moderately elevated above peristomial lip in fully extended zooids (Fig. 5a, c, d, j–o). Macrozooids about 55–65 × 30–40 μm in vivo (Fig. 5g–i, q), located only on primary stalk. Pellicular striations conspicuous above 1000 × magnification. Oral cilia about 15 μm in length. In some colonies, zooids at the top of primary stalk are larger than microzooids (Fig. 5i).
Morphology of Zoothamnium alternans in vivo (a–d, h–q), after protargol staining (f, r–v), after “wet” silver nitrate staining (e, w), after DAPI staining (x) and a mature colony of Zoothamnium alternans (g). a, c, d, j–p Showing the variation of zooid shape and macronucleus shape, arrows in a, l and p mark the contractile vacuole. b A relatively larger zooid at the top of primary stalk. e Silverline system. f Oral ciliature. g Mature colony of Z. alterans, arrows mark macrozooids (from Grell, 1968). h A mature colony the top of which is damaged: arrows mark macrozooids. i An immature colony, arrows mark macrozooids, arrowhead marks the end of the spasmoneme, double arrowhead marks the zooids at the top of primary stalk. q A macrozooid. r, s Two protargol-stained zooids showing ciliature, arrow in r marks P3, arrowhead in r marks the germinal kinety, double arrowhead in r marks the the trochal band, arrow in s marks the haplokinety, arrowhead in s marks EM1. t–v Infundibular polykineties, arrow in t marks P1, arrowhead in t marks P2, arrow in u marks P3, asterisk in v marks the adstomal end of P3. w Silverline system and pellicular pores. x DAPI-stained zooid, showing the macronucleus. EM1, 2 epistomial membrane 1, 2, G germinal kinety, H haplokinety, Po polykinety, P1–3 infundibular polykineties 1–3, Ma macronucleus. Scale bars: 20 μm in (a, c, d, k, m, n, q); 30 μm in (b); 600 μm in (g); 500 μm in (h, i); 15 μm in (r, s)
Cytoplasm colorless, includes several gray or colorless granules. Single contractile vacuole dorsally located at the same level as peristomial lip (Fig. 5a, l, p). Macronucleus J-shaped (Fig. 5a, c, d, r, s, x). Micronucleus not observed.
Colony up to 1200 μm tall. Stalk alternately branched forming a feather-like outline. Lengths of accessory branches increase progressively from two ends towards middle portion of colony (Fig. 5g–i). Spasmoneme sturdy, comprising bundles of fibrils (stalk myonemes) within a transparent sheath, with sparsely distributed mitochondria (Fig. 5h, i).
Oral ciliature of usual type for sessilid peritrichs. Haplokinety and polykinety make approximately 1.5 circuits around peristome before entering infundibulum (Fig. 5f, r, s). All three infundibular polykineties (P1–P3) three-rowed (Fig. 5f, v). Three rows of P1 nearly equal length (Fig. 5f, t). Adstomal end of P2 ends at convergence of P1 and P3 (Fig. 5f, v). Abstomal end of row 1 in P2 converges with P1 (Fig. 5f, t, v). Abstomal end of row 3 in P2 detached from rows 1 and 2 (Fig. 5f, t, v). Three rows of P3 equal length and end adstomally above P1. In abstomal half part of P3, there is a gap between rows 1 and 2 (Fig. 5f, r, u). Two epistomial membranes (EM 1 and EM2): EM 1 located at entrance of infundibulum (Fig. 5f, s); EM 2 located near distal ends of haplokinety and polykinety (Fig. 5f). Germinal kinety lies parallel to haplokinety in upper half of infundibulum (Fig. 5f, r). Trochal band consists of dikinetids, located two-thirds down length of zooid (Fig. 5e, r, s).
Silverline system consists of densely transverse silverlines, numbering 33–35 from peristome to trochal band (n = 2), 23–25 from trochal band to scopula (n = 2) (Fig. 5e, q, w).
Molecular data and phylogenetic trees (Fig. 6)
The newly obtained SSU rDNA sequences of the three Zoothamnium species are deposited in the GenBank database with length (bp), GC content and accession numbers as follows: Z. apoarbuscula n. sp.—1686, 43.12%, MT031923; Z. apohentscheli n. sp.—1686, 43.18%, MT031924; Z. alternans—1684, 41.75%, MT031922.
Maximum likelihood tree inferred from SSU rDNA sequences, revealing the phylogenetic positions of Zoothamnium apoarbuscula n. sp., Z. apohentscheli n. sp. and Z. alternans. Numbers near nodes denote maximum likelihood (ML) bootstrap value and Bayesian inference (BI) posterior probability, respectively. Species identity of sequences (marked with asterisks) called “Zoothamnium plumula” (KY675162 and DQ662854) and “Zoothamnium alternans” (DQ662855) could be from misidentified materials and need to be confirmed. The scale bar indicates two substitutions per 100 nucleotide positions
Phylogenetic trees based on SSU rDNA sequence data using Bayesian inference (BI) and maximum likelihood (ML) analyses had almost identical topologies, therefore only the ML tree is shown here. Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. group into one of the three clades (clade I) of the family Zoothamniidae. Within clade I, Zoothamnium apoarbuscula n. sp. groups with Z. pararbuscula, Z. intermedium, Z. arbuscula, Z. plumula, Z. wangi, and Z. apohentscheli n. sp. with low support (53% ML, 0.51BI). This is sister to the other main group within clade I which comprises Z. hentscheli and Z. parahentscheli. Zoothamnium alternans groups within clade III of the family Zoothamniidae with moderate support (77% ML, 1.00 BI).
Discussion
Zoothamnium apoarbuscula n. sp.
Branching pattern of colony
We found numerous colonies of Zoothamnium apoarbuscula n. sp., most of which had fewer than 30 zooids. The largest colony we observed had about 45 zooids. All colonies had an umbellate shape, dichotomously branched stalk and macrozooids on lower portions of accessory branches. By contrast, its morphologically most similar species, Z. arbuscula and Z. pararbuscula Ji et al. 2005, typically form very large colonies with hundreds of zooids. Therefore, mature colonies of Z. apoarbuscula n. sp. may not have been observed. Based on these observations, colonies of Zoothamnium apoarbuscula n. sp. most likely branch in a dichotomous pattern.
Comparison with Zoothamnium pararbuscula
Zoothamnium apoarbuscula n. sp. is characterized by its umbellate colony shape, differentiated zooids and marine habitat. These features distinguish it from most other congeners except Z. pararbuscula from which it can be separated by: (1) the relatively smaller size of microzooids (25–50 × 20–30 μm vs. 36–62 × 32–38 μm); (2) the uneven diameter of the primary stalk which is conspicuously narrowed in the rear region above the base (vs. even diameter); (3) the reticulated surface of the spasmoneme (vs. smooth surface with granules) in the primary stalk; and (4) polykinety 2 about the same length as polykinety 3 (vs. about twice as long as polykinety 3) (Ji et al. 2005a). The separation of these two species is further supported by the molecular data (Fig. 6).
Zoothamnium apohentscheli n. sp.
Comparison with congeners in similar morphology (Fig. 7a–h; Table 2)
Zoothamnium apohentscheli n. sp. is mainly characterized by the alternately branched stalk. Considering its stalk branching pattern and single-layered peristomial lip, six congeners should be compared with Z. apohentscheli n. sp., namely Z. hentscheli, Z. commune Kahl, 1933, Z. sinense Song, 1991, Z. wangi Ji et al., 2005, Z. xuianum Sun et al., 2005, and Z. parahentscheli Sun et al., 2009.
Morphologically similar congeners (a–h) of Zoothamnium apohentscheli n. sp., illustrations from historical reports (i, j) and morphologically similar congeners (k–r) of Zoothamnium alternans. a Z. kentii (from Grenfell 1884). b Z. hentscheli (from Hentschel 1916). c Z. commune (from Kahl 1933). d Z. commune (from Ji et al. 2006). e Z. sinense (from Ji et al. 2006). f Z. wangi (from Ji et al. 2011, copyrighted). g Z. xuianum (from Sun et al. 2005, copyrighted). h Z. parahentscheli (from Ji et al. 2015). i Z. alternans (from Claparède and Lachmann 1858). j Z. alternans (from Ji et al. 2009). k Z. alternans sensu Greeff (1870). l Z. alternans sensu Kent (1880–1882). m Z. niveum (from Bauer-Nebelsick et al. 1996). n Z. perejaslawzewae (from Perejaslawzewa 1886). o Schematic of Z. perejaslawzewae (from Kahl 1933). p Z. plumula (from Perejaslawzewa 1886). q Z. plumula (from Ji et al. 2011, copyrighted). r Z. ignavum (from Schuster and Bright 2016). Scale bars: 400 μm for colony; 40 μm for zooid
Zoothamnium hentscheli, which is the most morphologically similar species to Z. apohentscheli n. sp., was originally described by Hentschel (1916) as “Zoothamnium sp. a.” and was renamed as Z. hentscheli by Kahl (1935). Foissner et al. (1992) synonymized Z. hentscheli with Z. kentii Grenfell, 1884 based on both species having a characteristic coat of detritus. However, the stalk of Z. hentscheli is usually alternately branched which is conspicuously different with the regularly dichotomously branched stalk of Z. kentii (Grenfell 1884; Hentschel 1916; Kahl 1935) (Fig. 7a, b). Furthermore, the mature colony of Z. hentscheli is shorter and has fewer zooids than that of Z. kentii, i.e., up to 1200 μm high with up to 78 zooids in Z. hentscheli vs. up to 2300 μm high with 80–90 zooids in Z. kentii (Grenfell 1884; Hentschel 1916; Kahl 1935). Thus, we consider these to be separate species. This is consistent with Ji et al. (2006, 2009) who did not accept the synonymy of Z. hentscheli and Z. kentii.
Like Z. hentscheli, Z. apohentscheli n. sp. is typically covered with detritus. However, the latter differs from Z. hentscheli by its relatively smaller zooid (40–65 μm vs. 63–84 μm in length) and marine (vs. freshwater) habitat (Hentschel 1916) (Fig. 7b).
Zoothamnium commune also has a marine habitat, but it differs from Z. apohentscheli n. sp. by its larger zooid size (55–104 × 48–56 μm vs. 40–65 × 25–40 μm), the size of its terminal zooids which are usually larger than (vs. the same size as) zooids on lateral branches, and having more pellicular striations (38–43 vs. 29–31) between the trochal band and the scopula (Ji et al. 2006; Kahl 1933, 1935) (Fig. 7c, d).
Zoothamnium sinense can be separated from Z. apohentscheli n. sp. by its smaller colony size (ca. 400 μm vs. up to 1700 μm high), relatively smaller zooid size (36–48 μm vs. 40–65 μm in length) and the abstomal half of the innermost row of P3 being separated from (vs. parallel with and adjacent to) the other two rows (Ji et al. 2006; Song 1991) (Fig. 7e).
Zoothamnium wangi can be easily separated from Z. apohentscheli n. sp. by its larger zooid size (65–90 × 45–55 μm vs. 40–65 × 25–40 μm), more pellicular striations (38–50 vs. 29–31) between trochal band and scopula, and the two-rowed (vs. three-rowed) P3 (Ji et al. 2005b, 2011) (Fig. 7f).
Zoothamnium xuianum can be separated from Z. apohentscheli n. sp. by its smaller colony size (up to 800 μm vs. up to 1700 μm), relatively stiff (vs. flexible) accessory branches, sparsely (vs. densely) distributed zooids on the accessory branches, fewer pellicular striations (12–17 vs. 29–31) between the trochal band and scopula and its brackish water (vs. marine) habitat (Ji et al. 2009; Sun et al. 2005) (Fig. 7g).
Zoothamnium parahentscheli differs from Z. apohentscheli n. sp. by its remarkably stronger primary stalk (20–28 μm vs. 14–19 μm in dia.) and its shorter accessory branches, i.e., mostly 50–200 μm long vs. mostly over 300 μm (Ji et al. 2009, 2015) (Fig. 7h).
Zoothamnium alternans Claparède & Lachmann, 1858
Identification
Apart from the relatively smaller size of its zooids, which could be interpreted as a population-dependent difference, our population closely matches Z. alternans in the alternately branched stalk, the presence of both micro- and macrozooids, location of macrozooids on the main stalk, the J-shaped macronucleus, and the oral ciliature (especially the gap between rows1 and 2 of P3) (Claparède and Lachmann 1858; Ji et al. 2006, 2009; Kahl 1935) (Figs. 5g, 7i). Thus, we identify our population to Z. alternans.
Comparison with congeners in similar morphology (Figs. 5g, 7k–r; Table 3)
Superficially, Z. niveum Ehrenberg, 1838, Z. plumula Kahl, 1933, Z. perejaslawzewae Kahl, 1933, and Z. ignavum Schuster & Bright, 2016 resemble Z. alternans in terms of the feather-shaped colony. However, Z. niveum can be clearly separated from Z. alternans by its considerably larger colony (up to 1 cm high vs. up to 1.2 mm high) (Bauer-Nebelsick et al. 1996) (Fig. 7m). Zoothamnium plumula can be easily separated from Z. alternans by the absence (vs. presence) of macrozooids and the C-shaped (vs. J-shaped) macronucleus (Ji et al. 2011; Kahl 1933, 1935; Perejaslawzewa 1886; Song et al. 2002) (Fig. 7n, o). Zoothamnium perejaslawzewae can be separated from Z. alternans by its coupled (vs. individual, alternately attached) zooids on the accessory branches, the absence (vs. presence) of macrozooids and the uniformly decreasing (vs. increasing and then decreasing) length of the accessory branches with the height of the colony (Kahl 1933, 1935; Perejaslawzewa 1886) (Fig. 7p, q). Zoothamnium ignavum differs from Z. alternans by its clustered (vs. dispersed along primary stalk) macrozooids and S-shaped (vs. J-shaped) macronucleus (Schuster and Bright 2016) (Fig. 7r).
We agree with Ji et al. (2006) who concluded that Z. alternans sensu Greeff (1870) and Z. alternans sensu Kent (1880–1882) are misidentifications. These two forms are also easily separated from Z. alternans, i.e, Z. alternans sensu Greeff (1870) has conspicuously larger zooids on accessory branches which is not the case in Z. alternans (Fig. 7k), and Z. alternans sensu Kent (1880–1882) has several slender, elongated zooids on some of its accessory branches (vs. absent in Z. alternans), and lacks macrozooids (vs. present in Z. alternans) (Fig. 7l).
Wang and Nie (1932) reported Z. alternans found in Xiamen, China. However, the presence of macrozooids, which is a key character of Z. alternans, was not reported in their population. Thus, the Xiamen population needs to be reinvestigated to confirm its identity. Shen and Gu (2016) reported a population which they identified as Z. alternans but which needs to be reinvestigated because of the following characters which do not match with Z. alternans: 1) the maximum width of zooid nearly equal with (vs. consciously narrow than) the width of peristomial lip; and 2) the obliquely oriented C-shaped (vs. J-shaped) macronucleus.
Phylogenetic analyses
In the SSU rDNA tree, species of Zoothamnium grouped into three clades. The genus Zoothamnium is non-monophyletic which is consistent with the previous studies (Li et al. 2008; Zhuang et al. 2018). Both Z. apoarbuscula n. sp. and Z. apohentscheli n. sp. were grouped in clade I. We failed to find morphological support for this clade. Zoothamnium apoarbuscula n. sp., Z. pararbuscula and Z. arbuscula do not group together in a single clade although they share similar morphologies. Zoothamnium apohentscheli n. sp. is most closely related with Z. wangi and Z. plumula (?, KT675162) with strong support (99% ML, 1.00 BI). Each of them has an alternately branched stalk. Our population of Z. alternans clusters with Z. alternans (?, DQ662855), Z. pelagicum, Z. plumula (?, DQ662854, deposited in GenBank database erroneously as Z. pluma), Z. ignavum and Z. niveum which collectively formed clade III. This grouping is supported by a shared morphological character, i.e., their alternately branched stalk forming a feather-shaped colony, which clearly differentiates them from other species of Zoothamnium.
It is noteworthy that the SSU rDNA sequence of Z. alternans (?, DQ662855) differs from the sequence of our population by six base pairs, and no morphological information or voucher specimens are available for sequence DQ662855. Thus, the species identity of sequence DQ662855 needs to be confirmed. In addition, there is a marked difference between the two SSU rDNA sequences (DQ662854 and KT675162) of Z. plumula (?) and neither morphological information nor voucher specimens are available for these two sequences. Thus, the species identities of these two sequences also need to be confirmed.
Materials and methods
Sample collection and morphological methods
Samples were collected from a marine aquarium (300 × 50 × 70 cm; Fig. 1c) in the Laboratory of Protozoology, Ocean University of China, using glass microscope slides as artificial substrates. Briefly, the slides were fixed onto a frame that was immersed in the tank for about 7–10 days to allow colonization of peritrichs to occur (Small 1973).
Zoothamnium apoarbuscula n. sp. was collected on August 19, 2019 (water temperature 28 °C, salinity 30‰). Zoothamnium apohentscheli n. sp. was collected on December 24, 2018 (water temperature 20 °C, salinity 30‰). Zoothamnium alternans was collected on April 13, 2018 (water temperature 25 °C, salinity 30‰).
Colonies were removed from the slide using acupuncture needles and collected using glass micropipettes. Live specimens were investigated using differential interference contrast microscopy at magnifications of 40 × to 1000 × . The infraciliature was revealed by protargol staining according to Ji and Wang (2018). The silverline system was demonstrated using the “wet” silver nitrate method (Song and Wilbert 1995). Measurements and counts were performed under 400–1000 × magnifications. Drawings of live specimens were based on living observations, freehand sketches and photomicrographs. Drawings of stained specimens were made with a drawing device. Terminology is according to Foissner et al. (1992) and Warren (1986).
DNA extraction, PCR amplification, and sequencing
Genomic DNA was extracted from cleaned cells using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The small subunit (SSU) rDNA sequence was amplified by PCR according to Lian et al. (2019), using the primers: 18SF (5ʹ-AAC CTG GTT GAT CCT GCC AGT-3ʹ) (Medlin et al. 1988) and 5.8SR (5ʹ-CTG ATA TGC TTA AGT TGA GCG G-3ʹ) (Gao et al. 2012). To minimize the possibility of errors, Q5® Hot Start High-Fidelity DNA Polymerase (New England BioLabs, USA) was used in PCR amplification. The fragments were sequenced bidirectionally by the Tsingke Biological Technology Company (Qingdao, China).
Phylogenetic analyses
Besides the three new sequences in present work, another 59 sequences of peritrichs used in the present phylogenetic analyses were acquired from GenBank. Four species of Hymenostomatia (Glaucoma chattoni X56533; Ichthyophthirius multifiliis U17354; Tetrahymena corlissi U17356; Tetrahymena pyriformis EF070254) were selected as the outgroup taxa. Sequences were aligned by the GUIDANCE2 algorithm online using the default parameters (Landan and Graur 2008; Sela et al. 2015). The resulting alignment was manually refined using the program BioEdit 7.0 (Hall 1999). The length of the final alignment was 1728 positions.
Maximum likelihood (ML) bootstrapping analysis was carried out with 1000 replicates, using RAxML-HPC2 v.8.2.10 on XSEDE (Stamatakis 2014) on CIPRES Science Gateway (http://www.phylo.org), with the GTR + gamma model. Bayesian inference (BI) analysis was carried out using MrBayes v.3.2.6 on XSEDE (Ronquist et al. 2012) with the best fit model GTR + I + G selected by the Akaike Information Criterion in MrModeltest 2.2 (Nylander 2004). Markov chain Monte Carlo simulations were run for 6,000,000 generations, and trees were sampled every 100 generations with a burn-in of 6000 trees (10%). Tree topologies were manually formatted with MEGA 7.0 (Kumar et al. 2016). Systematic classification is based on Lynn (2008) and Gao et al. (2016).
References
Bauer-Nebelsick M, Bardele CF, Ott JA (1996) Redescription of Zoothamnium niveum (Hemprich & Ehrenberg, 1831) Ehrenberg, 1838 (Oligohymenophora, Peritrichida), a ciliate with ectosymbiotic, chemoautotrophic bacteria. Eur J Protistol 32:18–30
Claparède É, Lachmann J (1858) Etudes sur les infusoires et les rhizopodes. Mém Inst Natn Génev 5(year 1857):1–260
Corliss JO (1979) The ciliated protozoa: characterization, classification and guide to the literature, 2nd edn. Pergamon Press, Oxford
de St. Vincent BJB, Vincent JB (1824) Essai d’une Classification Des Animaus Microscopiques. In: Lamouroux JVF, de St. Vincent JB, Deslongchamps E (eds) Encyclopédie Méthodique. Agasse, Paris, pp 816–817
Ehrenberg CG (1838) Die Infusionsthierchen als Volkommene Organismen. Ein Blick in das tiefere organische Leben der Natur. Verlag von Leopold Voss, Leipzig
Entz G (1884) Über Infusorien des Golfes von Neapel. Mitt Zool Stat Neapel 5:289–444
Fauré-Fremiet E (1930) Growth and differentiation of the colonies of Zoothamnium alternans (Clap. and Lachm.). Biol Bull 58:28–51
Foissner W, Berger H, Kohmann F (1992) Taxonomische und okologische Revision der Ciliaten des Saprobiensystems. Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft 5/92, pp 1–502
Foissner W, Blake N, Wolf K, Breiner HW, Stoeck T (2010) Morphological and molecular characterization of some peritrichs (Ciliophora Peritrichida) from tank bromeliads, including two new genera: Orborhabdostyla and Vorticellides. Acta Protozool 48:291–319
Gao F, Katz LA, Song WB (2012) Insights into the phylogenetic and taxonomy of philasterid ciliates (Protozoa, Ciliophora, Scuticociliatia) based on analyses of multiple molecular markers. Mol Phylogenet Evol 64:308–317
Gao F, Warren A, Zhang QQ, Gong J, Miao M, Sun P, Xu DP, Huang J, Yi ZZ, Song WB (2016) The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Sci Rep 6:24874
Greeff R (1870) Untersuchungen über den Bau und die Naturgeschlichte der Vorticellen. Arch Naturgesch 37:353–384
Grell KG (1968) Protozoologie, 2nd edn. Springer, Berlin
Grenfell JG (1884) On some new infusoria from Bristol. J Microsc Nat Sci 3:133–138
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hentschel E (1916) Biologische Untersuchungen über den tierischen und pflanzlichen Bewuchs im Hamburger Hafen. Mitteil Zool Mus Hamb 33:1–176
Hu XZ, Song WB (2001) Description of Zoothamnium chlamydis sp. n. (Protozoa: Ciliophora: Peritrichida), an ectocommensal peritrichous ciliate from cultured scallop in North China. Acta Protozool 40:215–220
Hu XZ, Lin XF, Song WB (2019) Ciliate atlas: species found in South China Sea. Science Press, Beijing
Ji DD, Wang YF (2018) An optimized protocol of protargol staining for ciliated protozoa. J Eukaryot Microbiol 65:705–708
Ji DD, Song WB, Al-Rasheid KAS, Li LF (2005a) Taxonomic characterization of two new marine peritrichous ciliates, Pseudovorticella clampi n. sp. and Zoothamnium pararbuscula n. sp. (Protozoa: Ciliophora: Peritrichida) from North China. J Eukaryot Microbiol 52:159–169
Ji DD, Song WB, Warren A (2005b) Two new marine sessile peritrichous ciliates (Protozoa, Ciliophora). Acta Zootax Sin 30:702–705
Ji DD, Song WB, Warren A (2006) Redescriptions of three marine peritrichous ciliates, Zoothamnium alternans Claparède et Lachmann, 1859, Z. sinense Song, 1991 and Z. commune Kahl, 1933 (Ciliophora: Peritrichia), from North China. Acta Protozool 45:27–39
Ji DD, Sun P, Warren A, Song WB (2009) Colonial sessilid peritrichs. In: Song WB, Warren A, Hu XZ (eds) Free-living ciliates in the Bohai and Yellow Seas, China. Science Press, Beijing, pp 217–256
Ji DD, Shin MK, Choi JK, Clamp JC, Al-Rasheid KA (2011) Redescriptions of five species of marine peritrichs, Zoothamnium plumula, Zoothamnium nii, Zoothamnium wangi, Pseudovorticella bidulphiae, and Pseudovorticella marina (Protista, Ciliophora). Zootaxa 2930:47–59
Ji DD, Kim JH, Shazib SUA, Sun P, Li LQ, Shin MK (2015) Two new species of Zoothamnium (Ciliophora, Peritrichia) from Korea, with new observations of Z. parahentscheli Sun et al., 2009. J Eukaryot Microbiol 62:505–518
Kahl A (1933) Ciliata libera et ectocommensalia. In: Grimpe G, Wagler E (eds) Die Tierwelt der Nord-und Ostsee 23. Akademische Verlagsgesellschaft Becker & Erler, Leipzig, pp 29–146
Kahl A (1935) Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 4 Peritricha und Chonotricha. Tierwelt Dtl 30:651–886
Kent WS (1880–1882) A manual of the infusoria: including a description of all known flagellate, ciliate, and tentaculiferous protozoa British and foreign, and an account of the organization and affinities of the sponges. David Bogue, London (Vol. I 1880: 1–432; Vol. II 1881: 433–720; Vol. II 1882: 721–913; Vol. III 1882: Plates)
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Landan G, Graur D (2008) Local reliability measures from sets of co-optimal multiple sequence alignments. Pac Symp Biocomput 13:15–24
Li LF, Song WB, Warren A, Shin MK, Chen ZG, Ji DD, Sun P (2008) Reconsideration of the phylogenetic positions of five peritrich genera –Vorticella, Pseudovorticella, Zoothamnopsis, Zoothamnium and Epicarchesium (Ciliophora; Peritrichia; Sessilida), based on small subunit rRNA gene sequences. J Eukaryot Microbiol 55:448–456
Lian C, Zhang TT, Al-Rasheid KA, Yu YH, Jiang JM, Huang J (2019) Morphology and SSU rDNA-based phylogeny of two Euplotes species from China: E. wuhanensis sp. n. and E. muscicola Kahl, 1932 (Ciliophora, Euplotida). Eur J Protistol 67:1–14
Lu BR, Shen Z, Zhang QQ, Hu XZ, Warren A, Song WB (2020) Morphology and molecular analyses of four epibiotic peritrichs on crustacean and polychaete hosts, including descriptions of two new species (Ciliophora, Peritrichia). Eur J Protistol 73:125670
Lynn DH (2008) The ciliated protozoa. Characterization, classification, and guide to the literature, 3rd edn. Springer, Dordrecht
Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491–499
Nenninger U (1948) Die Peritrichen der Umgebung von Erlangen mit besonderer Berücksichtigung ihrer Wirtsspezifittät. Zool Jahrb (Syst Oekol Geogr Tiere) 77:169–266
Nylander JAA (2004) MrModeltest version 2. Evolutionary Biology Centre, Uppsala University, Uppsala
Penard E (1922) Études sur les infusoires d’eau douce. Georg & Cie, Genève
Perejaslawzewa S (1886) Protozoa of the Black Sea. Schr Naturforsch Ges Odessa 10:79–114 (in Russian)
Precht H (1935) Epizoen der Kieler Bucht. Nova Acta Leopol 3:405–474
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larger B, Liu L, Suchard MA, Hurlsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Schuster L, Bright M (2016) A novel colonial ciliate Zoothamnium ignavum sp. nov. (Ciliophora, Oligohymenophorea) and its ectosymbiont Candidatus piranensis gen. nov., sp. nov. from shallow-water wood falls. PLoS ONE 11:e0162834
Sela I, Ashkenazy H, Katoh K, Pupko T (2015) GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res 43:W7–W14
Shen YF, Gu MR (2016) Fauna Sinica: Invertebrata vol. 45 Ciliophora Oligohymenophorea Peritrichida. Science Press, Beijing (in Chinese with English summary)
Shen Z, Ji DD, Yi ZZ, Al-Rasheid KAS, Lin XF (2017) Morphology and phylogenetic placement of three new Zoothamnium species (Ciliophora: Peritrichia) from coastal waters of southern China. J Eukaryot Microbiol 64:266–277
Small EB (1973) A study of ciliate protozoa from a small polluted stream in east-central Illinois. Am Zool 13:225–230
Sommer G (1951) Die peritrichen Ciliaten des Großen Plöner Sees. Arch Hydrobiol 44:349–440
Song WB (1991) Contribution to the commensal ciliates on Penaeus orientalis. II. (Ciliophora, Peritrichida). J Ocean Univ Qingdao 21:45–55 (in Chinese with English summary)
Song WB, Wilbert N (1995) Benthische Ciliaten des Süßwassers. In: Röttger R (ed) Praktikum der Protozoologie. Gustav Fischer Verlag, Stuttgart, pp 156–168
Song WB, Al-Rasheid KA, Hu XZ (2002) Notes on the poorly-known marine peritrichous ciliate, Zoothamnium plumula Kahl, 1933 (Protozoa: Ciliophora), an ectocommensal organism from cultured scallops in Qingdao, China. Acta Protozool 41:163–168
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stiller J (1953a) Die Protozoen des Pécsely-Baches in Ungarn. Ann Hist Nat Mus Nat Hung 44:47–70
Stiller J (1953b) Epizoische Peritrichen aus dem Balaton III. Hydrobiologia 5:189–211
Stiller J (1971) Szájkoszorús csillósok-peritricha. Fauna Hung 105:1–245
Stiller J, Stevčić Z (1967) Epibionten der Meeresspinne Majasquinado (Herbst) Nebst Beschreibung Drei Neuer Entokommensaller Peritrichen Arten. Thal Jugosl 3:161–172
Summers FM (1938a) Some aspects of normal development in the colonial ciliate Zoothamnium alternans. Biol Bull 74:117–129
Summers FM (1938b) Form regulation in Zoothamnium alternans. Biol Bull 74:130–154
Sun P, Ji DD, Song WB (2005) Notes on a new marine peritrichous ciliate (Ciliophora: Peritrichida), Zoothamnium xuianum n. sp., with redescription of Z. paraentzii Song, 1991 from northern China. Zootaxa 1075:41–53
Sun P, Warren A, Al-Farraj SA, Song WB (2015) Morphology of three new colonial sessile peritrich ciliates, Pseudepistylis paramphora n. sp., Zoothamnium paranii n. sp. and Z. hartwigi n. sp., with notes on Epicarchesium variabile (Ciliophora, Peritrichia). Eur J Protistol 51:186–195
Wang XY, Ji DD (2019) Morphological and molecular identification of a new ciliate, Zoothamnium palmphlatum nov. spec. (Ciliophora, Peritrichia) from north China. J Eukaryot Microbiol 66:670–679
Wang CC, Nie DS (1932) A survey of the marine Protozoa of Amoy. Cont Biol Lab Sci Soc China 8:285–385
Warren A (1986) A revision of the genus Vorticella (Ciliophora: Peritrichida). Bull Br Mus Nat Hist (Zool) 50:1–57
Wilbert N, Song WB (2005) New contributions to the marine benthic ciliates from the Antarctic area, including description of seven new species (Protozoa, Ciliophora). J Nat Hist 39:935–973
Zhou T, Wang Z, Yang H, Gu ZM (2019) Two new colonial peritrich ciliates (Ciliophora, Peritrichia, Sessilida) from China: with a note on taxonomic distinction between Epistylididae and Operculariidae. Eur J Protistol 70:17–31
Zhuang Y, Clamp JC, Yi ZZ, Ji DD (2018) Phylogeny of the families Zoothamniidae and Epistylididae (Protozoa: Ciliophora: Peritrichia) based on analyses of three rRNA-coding regions. Mol Phylogenet Evol 118:99–107
Acknowledgements
This work was supported by the Natural Science Foundation of China (project number: 31801984, 31772440, 31970486) and the Fundamental Research Funds for the Central Universities (No. 201762017).
Author information
Authors and Affiliations
Contributions
WS conceived and guided the study. TW conducted sampling and performed experiments. BL identified the species. YL analyzed the phylogeny and interpreted the results. TW and BL wrote the manuscript, and AW and ZS reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statements
We declare that all applicable international, national, and or institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Edited by Jiamei Li.
Rights and permissions
About this article
Cite this article
Wu, T., Li, Y., Lu, B. et al. Morphology, taxonomy and molecular phylogeny of three marine peritrich ciliates, including two new species: Zoothamnium apoarbuscula n. sp. and Z. apohentscheli n. sp. (Protozoa, Ciliophora, Peritrichia). Mar Life Sci Technol 2, 334–348 (2020). https://doi.org/10.1007/s42995-020-00046-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-020-00046-y