Abstract
The primexine formation and plasma membrane undulation are the crucial steps of pollen wall formation in many angiosperms. However, the molecular mechanism underlining these processes is largely unknown. In Arabidopsis, NEW ENHANCER OF ROOT DWARFISM1 (NERD1), a transmembrane protein, was reported to play pleiotropic roles in plant development including male fertility control; while, how NERD1 disruption impacts male reproduction is yet unclear. Here, we revealed that the male sterility of nerd1 mutants is attributed to defects in early steps of pollen wall formation. We found that nerd1-2 is void of primexine formation and microspore plasma membrane undulation, defective in callose deposition. Consequently, sporopollenin precursors are unable to deposit and assemble on the microspore surface, but instead accumulated in the anther locule and tapetal cells, and ultimately leading to microspore abortion. NERD1 is localized in the Golgi and is expressed in both vegetative and reproductive organs, with the highest expression in reproductive tissues, including the tapetum, male meiocytes, tetrads and mature pollen grains. Our results suggest that NERD1 is required for the primexine deposition and microspore plasma membrane undulation, thus essential for sporopollenin assembly and pollen exine formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In angiosperms, generation of the male gametophyte (pollen) is critical for successful sexual reproduction. To adapt to the harsh environmental conditions and facilitate pollination, pollen grains have developed a highly organized and protective cell wall which contains an outer exine layer consisting of nexine and sexine, and an inner intine layer (Ariizumi and Toriyama 2011; Piffanelli et al. 1998). The exine is mainly composed of sporopollenin; while the intine consists mainly of cellulose and pectin (Ariizumi and Toriyama 2011). Increasing evidence suggests that both the innermost anther wall, the tapetum, and microspores contribute to exine development; while, the formation of intine is largely controlled by microspores (Shi et al. 2015).
Cytological observations show that the formation of pollen exine starts with the appearance of a cellulosic primexine (Ariizumi and Toriyama 2011). During meiosis, male meiocytes are surrounded by a temporary callose wall. At the tetrad stage, primexine is developed between the plasma membrane of microspores and the callose wall, and the plasma membrane exhibits distinctive undulations that are common to various species. In Arabidopsis, sporopollenin precursors initially deposit on the top of the undulations and assemble in a species-specific pattern. The deposition and assembly of sporopollenin continues till about the second mitotic division of male gametophytes (Ariizumi and Toriyama 2011; Shi et al. 2015). Several lines of evidence indicate that the primexine provides information for initial sporopollenin deposition sites (Ariizumi and Toriyama 2011). Disruption of genes involved in primexine deposition disturbs or abolishes pollen exine formation and patterning (Xu et al. 2016).
Several transmembrane proteins have been reported to be essential for primexine formation in Arabidopsis. DEFECTIVE IN EXINE FORMATION1 (DEX1) and its homolog in rice, OsDEX1 encode a novel membrane-associated protein that contains several potential calcium-binding domains (Paxson-Sowders et al. 1997, 2001; Yu et al. 2016). The dex1 mutant has defective pollen wall pattern due to significantly reduced primexine deposition and lacking plasma membrane undulation (Paxson-Sowders et al. 1997, 2001). Mutants of NO EXINE FORMATION1 (NEF1), encoding a predicted plastid integral membrane protein, fail to form pollen exine due to the coarsely developed primexine and disrupted sporopollenin deposition onto the microspore plasma membrane (Ariizumi et al. 2004). NO PRIMEXINE AND PLASMA MEMBRANE UNDULATION (NPU) encodes a membrane protein, and its mutation led to significantly impaired early stage pollen wall formation, causing defective callose synthesis, and no primexine deposition and plasma membrane undulation (Chang et al. 2012). Meanwhile, genes involved in carbohydrate and lipid metabolism or transport have also been reported to be important for the primexine formation. RUPTURED POLLEN GRAIN1 (RPG1)/(AtSWEET8) and RPG2 belong to the MtN3/saliva family and function as sugar efflux transporters (Chen et al. 2010). The rpg1 mutant exhibits much less primexine deposition and abnormal plasma membrane undulation. rpg1 is partially male sterile; while, rpg1rpg2 is almost completely male sterile, suggesting that RGP2 may be also involved in the primexine formation (Guan et al. 2008; Sun et al. 2013). KAONASHI4 (KNS4)/UNEVEN PATTERN OF EXINE1 (UPEX1) encodes a type-II arabinogalactan β-(1,3)-galactosyltransferase. Mutation of KNS4 significantly diminishes arabinogalactan-protein (AGP) and changes location of AGPs as well as pectins, leading to defective primexine matrix (Li et al. 2017; Suzuki et al. 2017). Recently, ACYL-COA SYNTHETASE5 (ACOS5), one of the key enzymes responsible for sporopollenin biosynthesis, has been identified to be also critical for pollen wall formation by affecting primexine formation and sporopollenin assembly (Xie et al. 2017). The primexine formation is tightly regulated at the transcription level and by plant hormones. Hackly Microspore (HKM)/MALE STERILITY1 (MS1), a PHD transcription factor (Ariizumi et al. 2005; Ito and Shinozaki 2002; Wilson et al. 2001) and EXINE FORMATION DEFECT (EFD), a nuclear-localized de novo DNA methyltransferase (Hu et al. 2014) have been reported to be key regulators of the primexine formation and exine patterning. TRANSIENT DEFECTIVE EXINE1 (TDE1)/DE-ETIOLATED2 (DET2) is a key enzyme in the brassinosteroid (BR) biosynthesis. det2 is defective in primexine deposition and probaculae formation, which can be recovered by exogenous application of brassinosteroids (Ariizumi et al. 2008).
NERD1, a transmembrane protein, was identified to play an important role in influencing primary root growth, root hair expansion, and hypocotyl elongation (Cole et al. 2018), as well as the ovule number and plant fertility (Yuan and Kessler 2019). Nevertheless, how the NERD1 regulate male gametophyte development remains unclear. In this study, we revealed that pollen abortion in nerd1 mutants is caused by the absence of primexine formation and plasma membrane undulation, as well as defective callose deposition and exine patterning, suggesting that NERD1 plays an important role in early pollen wall formation.
Results
Microsporogenesis in nerd1 is disrupted after meiosis
To understand the role of NERD1 in male reproduction, we characterized the developmental defects that occurred in the anther and pollen grains of nerd1 mutants. A previously reported allele of nerd1 (SALK_018060C, named nerd1-2), which contains a T-DNA insertion in the seventh exon of the open reading frame of At3g51050 (Fig. S1A), was used for further phenotypic analysis. We also used the CRISPR/Cas9 system to create two additional nerd1 alleles that contain mutations within the third exon of NERD1: nerd1-5 containing a 1-bp insertion and nerd1-6 containing a 32-bp deletion, both leading to frame shift and premature translational termination (Fig. S1A).
Consistent with the previous reports, we observed a shorter primary root in the nerd1-2 mutant (Fig. S2B, C). Additionally, nerd1-2 exhibited slightly dwarf plant stature with reduced rosette leaf size (Figs. 1, S2A), delayed bolting for about 10 days (Fig. S2D, E) and much fewer flowers (Fig. S2F, G) when compared with the wild type. Most prominently, nerd1-2, nerd1-5 and nerd1-6 were all completely male sterile (Fig. 1A). No pollen grains were found on the surfaces of anthers and stigmas in all three alleles (Fig. 1B). Alexander’s staining showed that pollen grains of all three nerd1 mutant alleles lost the viability at the mature stage and were completely degraded (Fig. 1C). SEM examination also confirmed the result of pollen viability analysis, revealing no pollen grains but only debris inside the anther locules of the three nerd1 mutant alleles (Fig. 1E–G). These results indicated that NERD1 affects male fertility, mainly in the process of pollen development.
Characterization of the nerd1 mutants. A Comparison of vegetative and reproductive development between wild type, nerd1-2, nerd1-5 and nerd1-6 (from left to right). B Comparison between wild-type and mutant dissected flowers at anthesis (from left to right). Note that there are no mature pollen grains on the mutant anthers and stigmas. C Alexander staining shows viable pollen in the wild-type anther and no viable pollen is detected in nerd1-2, nerd1-5 and nerd1-6 mutant anthers (from left to right). D–G SEM observation of mature pollen grains and dehiscent anthers of wild type and mutant at stage 12. Wild-type pollen grain with a regular reticulate exine pattern (D), while the nerd1-2 (E), nerd1-5 (F), and nerd1-6 (G) anthers showing degenerated microspore debris in the anther locule. A anther, De debris, Ov ovary, Sg stigma. Scale bars: A 5 cm; B 1 mm; C 100 µm; D–G 10 µm
To further reveal the function of NERD1 in male reproduction, semi-thin sections were performed to compare the process of anther and pollen development between wild type and nerd1-2, using previously defined classification of anther developmental stages (Sanders et al. 1999). At the tetrad stage (stage 7), both wild-type and nerd1-2 microspore mother cells completed meiosis and formed tetrads (Fig. 2A, F). At the early uninucleate stage (stage 8), the young microspores were normally released from the tetrads in wild-type and nerd1-2 anthers (Fig. 2B, G). At the late free microspore stage (stage 9), microspores of wild type became vacuolated. By contrast, the development of nerd1-2 microspores was arrested, with many lightly stained particles accumulating around the microspores (Fig. 2C, H). At the stages 10 and 11, wild-type microspores experienced mitotic division to form bicellular pollen, which proceeded into mature pollen grains (Fig. 2D, E). On the contrary, nerd1-2 microspores became degenerated, resulting in the presence of cell debris in the anther locule (Fig. 2I, J). These observations indicated that loss of function of NERD1 results in developmental arrest and degeneration of microspores after the early uninucleate microspore stage.
Transverse section analysis of developing anther in wild-type (A–E) and nerd1-2 (F–J). A, F Anther at tetrad stage. B, G early uninucleate microspore stage. C, H late uninucleate microspore stage. Degenerated microspores clearly seen within the locule of nerd1-2. D, I vacuolated pollen stage. E, J bicellular pollen stage. Complete degeneration of nerd1-2 microspores in anther locule. E epidermis, En endothecium, Msp microspores, PG pollen grains, Rm remnants of cell, T tapetum, Tds tetrads. Scale bars: 20 µm
No exine formation occurs in nerd1-2
To further elucidate the cause of pollen abortion in nerd1-2 plants, TEM observations were carried out using wild-type and nerd1-2 microspores from the tetrad to tricellular pollen stage (Figs. 3, S3). At the tetrad stage (stage 7), wild-type tetrads were enclosed by a thick layer of callose wall and a thin layer of primexine. The incipient baculae (probaculae) was seen to develop on the peaks of undulating plasma membrane (Figs. 3A, S3A). In contrast, neither primexine formation nor microspore membrane undulation was observed in nerd1-2 (Figs. 3B, S3B). At the uninucleate microspore stage (stage 8), sporopollenin accumulated to form baculae with similar size that was regularly deposited on wild-type microspores (Figs. 3C, S3C). However, in nerd1-2 microspores, sporopollenin accumulated into a few crescent-shaped particles which were randomly distributed surrounding the microspores (Figs. 3D, S3D). At the bicellular pollen stage (stage 11), the baculae and tectum became larger and thicker to form complete exine in the wild type (Figs. 3E, S3E). However, plasma membrane of the nerd1-2 microspores became disintegrated, and the anther locule filled with a lot of irregular aggregated sporopollenin particles (Figs. 3F, S3F). At the tricellular pollen stage (stage 12), pollen wall formation in the wild-type pollen was completed. Tryphine (also called pollen coat) filled in cavities of the exine, the underneath nexine and intine became thicker (Figs. 3G, S3G). While in nerd1-2, the microspores were completely degraded, only debris and sporopollenin aggregates were seen inside the anther locules (Fig. S3H). TEM observations showed that pollen abortion in nerd1-2 was the consequence of absence of primexine formation and plasma membrane undulation.
Ultrastructural analysis of pollen wall development in wild-type (A, C, E and G) and nerd1-2 (B, D and F) plants. A, B tetrad stage. Arrowheads in A show the microspore membrane undulation whereas no primexine deposition and microspore membrane undulation observed in nerd1-2 (B). C, D uninucleate microspore stage. No baculae and tectum formation were observed in nerd1-2 microspores, only sparsely globular sporopollenin observed deposited on the microspore membrane (D). E, F bicellular pollen stage. nerd1-2 microspore become degenerated and only globular sporopollenin particles spotted surrounding the degenerated microspore in the anther locule (F). G tricellular pollen stage. Ba baculae, Cw callose wall, deb debris, Dmsp defective microspore, In intine, Msp microspore, Ne nexine, Pb probaculae, Pe primexine, Pm plasma membrane, Sp sporopollenin, Tc tectum, Ty tryphine, UPM undulated plasma membrane. Scale bars: 0.5 µm in (A–G)
It has been reported that xylan and pectin are components of the primexine in Arabidopsis (Li et al. 2017; Suzuki et al. 2017). To further confirm the absence of the primexine in nerd1-2, we used specific antibodies for xylan (LM10) and de-esterified pectin (CCRC-M38) to visualize the differences in wild-type and mutant microspores. Confocal laser scanning micrographs showed that the LM10 and CCRC-M38 strongly labeled the peripheries of wild-type microspores in late tetrad stage (Fig. 4A, C). In contrast, no signals were observed in nerd1-2 tetrads (Fig. 4E, G). In wild-type microspores at early unicellular microspore stage, the LM10 and CCRC-M38 signals were distributed at the base of incipient sporopollenin which counterstained with Auramine O (Fig. 4I–L). However, in nerd1-2, microspore did not show any detectable xylan and pectin signals, while surrounding with numerous crescent-shaped sporopollenin granules verified by Auromine O staining (Fig. 4M–P). These results demonstrate that nerd1-2 microspores were unable to develop the primexine.
Confocal images showing the distribution of LM10- and CCRC-M38-recognized xylan and pectin, respectively, and Auramine O-stained exine in microspores at late tetrad stage and early unicellular stage. A–D (wild type) and E–H (nerd1-2): anthers at late tetrad stage. I–L (wild type) and M-P (nerd1-2): anthers at early unicellular stage. Images of red, blue and green channels represent the signals of LM10 (A, E, I and M), CCRC-M38 (C, G, K, O) and Auramine O (B, F, J and N), respectively, and the merges images are shown (D, H, L and P). Scale bar: 2 μm
Abnormal accumulation of sporopollenin in the locule wall of nerd1-2
TEM observations revealed no difference in the tapetal layer between wild type and nerd1-2 at meiotic stages (Fig. 5A, E). The tapetum in Arabidopsis has specialized storage organelles, i.e., endoplasmic reticulum (ER)-derived tapetosomes and plastid-derived elaioplasts, which synthesize and store pollen coat materials, such as flavonoids, neutral lipids, and steryl esters (Hsieh and Huang 2007). At early uninucleate microspore stage, elaioplasts containing multiple electron-translucent plastoglobules were present throughout the wild-type tapetal cytoplasm, and clustered at the cell periphery (Fig. 5B). In nerd1-2, although the appearance of the elaioplasts was very similar, numerous electron-dense granule aggregations that were not observed in the wild-type anther appeared in the tapetum facing locule wall between the middle layer and the tapetum (Fig. 5F; indicated by red arrows). These deposits looked similar to sporopollenin aggregates that failed to attach properly to the microspore plasma membrane in nerd1-2 (Fig. 3M, N). Sporopollenin-like aggregates continued to accumulate in the locule wall at later stages (Fig. 5G, H; indicated by the red arrows). These observations suggest that mutations in NERD1 interfere with the secretion of pollen wall components from the tapetum into microspore cell wall.
Ultrastructure of the anther wall layers in wild type (A–D) and nerd1-2 (E–H). A, E Tetrad stage. B and F, Early uninucleate microspore stage. Electron-dense granules (red arrows) stack along the locule wall in nerd1-2 (F). C, G Late uninucleate microspore stage. Well-developed elaioplasts with numerous electron-lucent plastoglobules and clusters of electron-dense tapetosomes were observed in tapetal cell of the wild type (C), electron-dense granules (red arrows) which stack in the locule wall were increased in nerd1-2 (G). D, H Bicellular pollen stage. In the wild-type tapetal cell, there were substantial tapetosomes, lipid bodies and elaioplasts which are full with lipidic components within the plastoglobuli (D); however, the electron-dense granules (red arrows) are still sticking on the locule wall in nerd1-2 (H). EI elaioplast, L lipid body, LW locule wall, ML middle layer, T tapetum, Te tapetosome. Scale bars: 1 µm
NERD1 is highly expressed in male reproductive organs
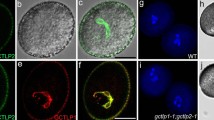
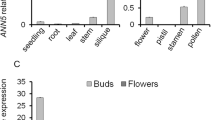
In silico expression analysis using public database showed that NERD1 is ubiquitously expressed in both vegetative and reproductive tissues (Fig. S4). To validate these data, spatial and temporal expression of NERD1 was analyzed by qRT-PCR, which detected NERD1 expression in vegetative tissues including roots, stems, and leaves, with the highest expression in flowers (Fig. 6A). GUS staining of transgenic plants expressing the β-glucuronidase marker protein (GUS) driven by the NERD1 promoter (NERD1pro:GUS) showed that the GUS signal could be detected in young seedlings, flowers, and particularly in late-stage anthers and pollen grains (Fig. 6B, C, E, F). Sections of stained floral buds exhibited the GUS signals mainly in the tapetum, microspores at vacuolated stage, and mature pollen grains (Fig. 6D, G).
Expression of NERD1 during plant development. A qRT-PCR analysis of the NERD1 transcript. Error bars indicate SD. Each reaction represents three biological repeats. B–G GUS expression in various tissues of NERD1pro:GUS transgenic lines. B Young seedling. C Inflorescences. D Semi-thin section of anthers at stage 9. E An anther at mature pollen stage. F Mature pollen grains released from (E). G Semi-thin section of anthers at stage 12. Scale bars: 1 mm in (B), 2 mm in (C), 50 µm in (D) and (G), 20 µm in (F), 100 µm in (E)
To further elucidate the localization of NERD1 in the anther, a translational fusion of the full-length genomic DNA of NERD1 and eGFP under the control of the native NERD1 promoter was created (Fig. 7A). Transgenic plants expressing NERD1pro::NERD1-eGFP fully restored the fertility of nerd1 (Fig. 7B, C). Consistent with the previous reports (Cole et al. 2018; Yuan and Kessler 2019), transgenic plants expressing NERD1pro::NERD1-eGFP showed fluorescence signal accumulating in punctate compartments in root cells, indicating that it is a Golgi-localized protein (Fig. S5). In the anther of transgenic plants expressing NERD1pro::NERD1-eGFP, the GFP fluorescence was first detected in the male meiocytes and tapetum at the meiosis stage (stage 6; Fig. 7D), and became stronger in tapetum and tetrads at tetrad stage (stage 7; Fig. 7E), and then reached the highest level in both tapetum and anther locules at early free microspore stage (stage 8; Fig. 7F); the fluorescence gradually decreased at late free microspore stage (stage 9, Fig. 7G). The fluorescence in the tapetum and anther locules disappeared at stage 10 together with the degeneration of the tapetum layer, while the signal in the pollen wall could still be detected (Fig. 7H). These gene expression and protein localization data supported the role of NERD1 in early anther and pollen development.
Distribution of NERD1-GFP in the anther. A Schematic diagrams of the NERD1-GFP construct contains the NERD1 genomic DNA and fused with eGFP. B siliques of the wild-type, nerd1-2 and NERD1pro::NERD1-eGFP transgenic lines. C KI-I2 staining of the anther of wild-type, nerd1-2 and NERD1pro::NERD1-eGFP transgenic lines. D–H Confocal images of different stage anthers in NERD1pro::NERD1-eGFP transgenic lines. I–M Confocal images of the control anther. The green channel shows the GFP signal, and the magenta channel shows the chlorophyll autofluorescence. Images were taken from the anther at stage 6 (D, I), stage 7 (E–J), stage 8 (F–K), stage 9 (G–L), stage 10 (H–M). L locule, M microspore, Mc Meiotic cell, T tapetum, Tds tetrads. Scale bars: 100 µm in (C), 50 µm in (D–M)
Mutation in NERD1 alters expression patterns of several genes associated with primexine formation or callose formation
Because the primexine formation and plasma membrane undulation were disrupted and no exine was formed in nerd1-2, qRT-PCR was used to examine the expression of several genes that are known to be associated with callose deposition, primexine and exine formation. The expression levels of NEF1, NPU, CALS5, RPG1, DEX1 and EFD were significantly down-regulated in nerd1-2 anthers to different extents (Fig. 8). CALS5 is vital for callose wall formation (Dong et al. 2005; Nishikawa et al. 2005) and the decrease in CALS5 expression was consistent with the observation that the callose wall surrounding the microspores in the nerd1-2 was thinner than that in wild type (Fig. S6). In addition, the expression of CALS5 was also largely down-regulated in several other primexine formation defective mutants, including rpg1, npu, dex1 and efd (Chang et al. 2012; Hu et al. 2014; Ma et al. 2013; Sun et al. 2013). These results demonstrated that mutation of NERD1 had remarkable effects on the expressions of several genes in the gene network of early pollen wall formation.
Expression of several primexine synthesis and callose wall deposition-related genes in the wild type and nerd1-2. qPCR analysis of NERD1, NEF1, NPU, CALS5, RPG1, DEX1 and EFD transcript level in the inflorescences of wild-type and nerd1-2 plants. Error bars indicate SD; each reaction represents three biological repeats. *P < 0.05; **P < 0.01 (Student’s t test)
Discussion
NERD1 is essential for initial sporopollenin deposition during microsporogenesis
Recently, Yuan et al. indicate that nerd mutants, including nerd1-2 and nerd1-4 show male reproductive defects to different extents (Yuan and Kessler 2019). They observed microspore abortion at tetrad stage in nerd1-2 and suggested that NERD1 may function in early stages of anther and pollen development. However, how NERD1 controls male fertility is yet unknown. In this study, our findings revealed previously undescribed roles of NERD1 in the anther and pollen development.
Although NERD1 affects various aspects of plant growth and development, such as shorter roots, smaller rosette leaves (Fig. S2A–C), delayed flowering (Fig. S2D and E) and decreased flower number (Fig. S2F and G), the most prominent phenotype of nerd1 knockout mutants is complete male sterility. Cytological analysis revealed that male sterility of nerd1 is mainly caused by the defects in pollen wall formation (Fig. 2C, H). TEM analysis further revealed that the primexine formation and microspore plasma membrane undulation are disrupted in nerd1 at tetrad stage, which causes the failure of sporopollenin deposition and assembly on the surface of microspores, and eventually leading to microspore abortion and complete male sterility (Figs. 3, 5 and S3). Abnormal aggregates of sporopollenin precursors were found randomly accumulated in the anther locule, suggesting that the biosynthesis of sporopollenin appears to be not affected in the mutant. These phenotypes are also observed in mutants that exhibit reduced or completely loss of primexine deposition, including dex1, nef1, tde1, rpg1, efd, and npu (Ariizumi et al. 2004; Chang et al. 2012; Guan et al. 2008; Hu et al. 2014; Li et al. 2017; Paxson-Sowders et al. 2001; Suzuki et al. 2017; Xie et al. 2017). It is widely believed that the primary function of the primexine in pollen wall formation is to provide a scaffold on the developing microspore for sporopollenin deposition, polymerization, and patterning (Ariizumi and Toriyama 2011; Heslop-Harrison 1968; Quilichini et al. 2015). Our results provide further evidence for the important role of the primexine in the initiation of exine pattern formation.
The predicted roles of NERD1
Primexine is presumed to be largely composed of cellulose components including neutral and acidic polysaccharide material, which was evidenced by chemical staining (Heslop-Harrison 1968). It has been reported that xylan and pectin are also components of primexine wall and could potentially be cross-linked with each other (Li et al. 2017; Suzuki et al. 2017). In nerd1-2, no xylan and pectin were detected on the surface of microspores at the primexine formation stage, suggesting that NERD1 could be involved in the transportation or assembly of these components (Fig. 4).
NERD1 was recently reported to be involved in plant exocytosis to regulate root development, although no evidence shows its direct interaction with exocyst (Cole et al. 2018). The localization of NERD1 primarily in the Golgi stacks supports its function in plant exocytosis (Cole et al. 2018; Yuan and Kessler 2019). An intriguing hypothesis raised by these authors is that NERD1 may directly affects the secretion of cell wall matrix, such as polysaccharide, glycoproteins or proteoglycans via exocyst-mediated trafficking to influence cell wall growth in root system (Cole et al. 2018). Therefore, it is likely that NERD1 is also involved in the secretory pathway of primexine components during anther and pollen development (Fig. 4).
In nerd1-2, abnormal sporopollenin aggregates distributed not only in the anther locule but also in the locule wall (Fig. 5), which is another indication of unsuccessful sporopollenin deposition and assembly onto the microspore surface. This phenomenon also occurs in some of the primexine defective mutants, including dex1, nef1, rpg1 and rpg1rpg2, indicating that they may share similar mechanisms to control primexine formation. Notably, these genes all encode transmembrane proteins and most of them are highly expressed in the tapetum, microsporocytes and microspores. RPG1 and RPG2 encode membrane-localized sugar transporters functioning in glucose transport (Chen et al. 2010; Guan et al. 2008; Sun et al. 2013). Glucose transported by RPG1/2 may be used to synthesize the cellulose components in the primexine. NERD1 might be also involved in the trafficking of the glucose or other carbon skeletons required for the primexine formation.
Furthermore, in silico co-expression analysis (Fig. S7) indicates that NERD1 may function together with several early pollen wall formation-associated proteins, such as NEF1 and two Secretory (SEC) proteins, AtSEC23A (AT4G01810) and AtSEC23B (AT1G05520). AtSEC23/SEC24 and AtSEC13/31 are coat subunits of the coat protein complex II (COPII)-coated vesicle. COPII vesicles mediate the transport of newly synthesized lipids and proteins from endoplasmic reticulum (ER) to the Golgi apparatus in the early secretory pathway. SEC components have been reported to be involved in several aspects of male reproduction in Arabidopsis. Mutation in both AtSEC24B and AtSEC24C leads to gametophyte development defects (Tanaka et al. 2013). AtSEC31B is required for pollen wall development, probably by regulating the early secretory pathway of tapetal cells (Zhao et al. 2016). A very recent report indicates that AtSEC23A and AtSEC23D play pivotal roles in pollen wall development and exine patterning (Aboulela et al. 2018). Interestingly, atsec23ad displays similar pollen wall developmental defects to that observed in nerd1. Although disruption of both AtSEC23A/D seems not affecting primexine formation, sporopollenin precursors could not deposit on the microspore surface, but accumulate into large aggregates in the atsec23ad anther locule. In addition, sporopollenin aggregates deposit in the anther locule wall. These phenotypes suggest that AtSEC23A/D may participate in the trafficking of important components in the primexine that are required for attaching sporopollenin precursors to the microspore plasma membrane. Based on its Golgi localization, NERD1 might be one of the cargoes that are exported by the COPII and exocyst secretory systems. Consistent with this hypothesis, NERD1 proteins are observed to be secreted into the anther locule at the tetrad stage (Fig. 7).
Another common feature of many of primexine defective mutants is a thinner callose wall around the tetrad which consistent with a substantial decrease in CalS5 expression (Chang et al. 2012; Hu et al. 2014; Ma et al. 2013; Sun et al. 2013). Several genes involved in primexine formation were also significantly down-regulated in nerd1-2. It is not clear how NERD1 affect the expression of these genes. One possibility is that changes in sugar or other primexine component trafficking inhibit the expression of these genes via a feedback signaling.
In summary, we demonstrate that NERD1 is indispensable for primexine formation, plasma membrane undulation and male fertility in Arabidopsis, which extends our understanding of early pollen wall formation in Arabidopsis.
Materials and methods
Plant material, growth conditions, and nerd1 mutant identification
Arabidopsis T-DNA insertion mutant line nerd1-2 (SALK_018060C, AT3g51050) was obtained from the Arabidopsis Biological Resource Center. Arabidopsis thaliana Columbia ecotype plants and Tobacco (Nicotiana benthamiana) plants were grown under long-day conditions (16 h of light/8 h of dark) in greenhouse at 22 °C, and 70% relative humidity under light intensity of 30,000 lumens/m2. To select homozygous lines, genomic DNA was extracted from young seedlings and PCR-based genotyping was performed using gene-specific primers LP and RP together with T-DNA vector primer LBa1 (Table S1).
Phenotypic characterization of nerd1
Whole plants were photographed by an E995 digital camera (Nikon, Japan). Flowers and dehiscent anthers were photographed with a M205A microscope (Leica, Germany). For pollen viability analysis, anthers were stained with Alexander’s solution (Alexander 1969) or iodine–potassium iodide (I2–KI) solution (Xu et al. 2017) at room temperature and then photographed with an Eclipse 80i microscope (Nikon, Japan). Semi-thin cross sections of developing anthers were performed with the Technovit embedding kits (Heraeus Kulzer, Germany) as described previously (Hong et al. 1995). For scanning electron microscopy (SEM) analysis, freshly dissected anthers were covered with 5-nm layer of gold using an EMSCD050 vacuum coater (Leica, Germany), and observed with a scanning electron microscope (Hitachi S3400N, Tokyo, Japan). For transmission electron microscopy (TEM) observation, anthers were fixed in 2.5% glutaraldehyde, post-fixed by 1% OsO4, dehydrated, embedded in Epon812 resin, and ultrathin sectioned (60–70 nm thick), before examined with a Tecnai G2 spirit biotwin Transmission Electron Microscope. For callose analysis, semi-thin sections of anthers at tetrad stage were stained with 0.05% (w/v) aniline blue in 0.1-M phosphate buffer (pH 8.0), viewed under UV illumination by the Nikon’s Eclipse Ni-E microscope, and analysis by the NIS-Elements AR software (Nikon).
Immunofluorescence labeling
Preparation of LR White resin-embedded anther samples and immunofluorescence labeling of their sections were performed as described previously (Suzuki et al. 2017). Mouse monoclonal antibody CCRC-M38 (CarboSource Services), and rat monoclonal antibody LM10 (Plantprobes) were used as primary antibodies. Alexa Fluor405 goat anti-mouse IgG and Alexa Fluor555 goat anti-rat IgG (Thermo Fisher Scientific) were used for secondary antibodies. After washed with 0.5% Auramine O solution, samples were mounted with a 2:1 mixture of aqua-poly/mount (Polysciences) and 0.05% Auramine O solution. The specimens were observed by a CLSM (FV1000; Olympus) with Ex: 473 nm, Em: 485–545 nm for auramine O staining, Ex: 405 nm, Em: 425–460 nm for Alexa Fluor405 and Ex: 559 nm, Em: 575–675 nm for Alexa Fluor555.
Generation of nerd1-5 and nerd1-6 using CRISPR/Cas9 system
The CRISPR–Cas9 transgenic vector was constructed as described previously (Feng et al. 2013). Primers used for plasmid construction and for nerd1-5, nerd1-6 mutant genotyping are listed in Table S1. The plasmid was transformed into wild-type plants using Agrobacterium GV3101 via the floral dipping method. Transformants were selected on MS medium containing hygromycin.
Quantitative reverse transcription PCR analysis
For quantitative reverse transcription PCR (qRT-PCR) analysis, total RNA was extracted from the roots, stems, leaves and inflorescences using the Trizol kit (Thermo Fisher Scientific). First-strand cDNA was reverse transcribed from total RNA with the PrimeScript™ RT reagent Kit with gDNA eraser (Perfect Real Time; TaKaRa). Real-time RT-PCR was performed with iQ SYBR Green Supermix (Bio-rad), using the real-time PCR system (Bio-Rad C1000 CFX96). Arabidopsis Actin2 was used as the internal control. Primers used to quantify the expression of NERD1 and other regulators that participate in primexine formation are listed in Table S1.
GUS Staining
A 1622-bp DNA fragment upstream of the transcriptional start codon of NERD1 was amplified as the promoter of NERD1, using primers NERD1Pro-GUS-F and NERD1Pro-GUS-R (Table S1) from genomic DNA, and inserted into pCAMBIA1301:GUS digested with EcoR I and NcoI by infusion system (Takara Bio, Japan). The NERD1pro:GUS construct was introduced into Arabidopsis Col-0 Agrobacterium tumefaciens-mediated transformation (Clough and Bent 1998). GUS activity was determined by staining different organs of transgenic lines as described previously (Jefferson et al. 1987).
Complementation of nerd1-2 and expression analysis
To complement the male sterility phenotype of nerd1 and check the stable expression of NERD1, genomic DNA of wild-type Arabidopsis was amplified with primers NERD1-eGFP-F and NERD1-eGFP-R, which amplified a fragment that contains 1622-bp 5′ upstream region and 3673-bp genomic DNA region (without stop codon) (Table S1). The 1301-35Spro:eGFP plasmid was digested with BamH I and Bgl II, and the amplified fragment was subcloned into 1301-35Spro:eGFP by the infusion system (TAKARA; http://www.clontech.com/) to produce NERD1pro::NERD1-eGFP, which was later introduced into nerd1 heterozygotes via Agrobacterium tumefaciens mediated transformation. Anthers and young roots with NERD1pro::NERD1-eGFP were mounted in water and observed under a confocal microscope (Leica TCS SP5). GFP fluorescent signals were imaged at the excitation wavelength of 488 nm and emission wavelength of 520–580 nm. Red autofluorescence of chlorophyll was imaged at 514-nm excitation and 640–750 nm of emission wave length.
Availability of data and material
The gene sequence of NERD1 is available in the tair website (http://www.arabidopsis.org/) under accession number AT3G51050. The supporting data of the article is included within the article and its additional files.
References
Aboulela M, Nakagawa T, Oshima A, Nishimura K, Tanaka Y (2018) The Arabidopsis COPII components, AtSEC23A and AtSEC23D, are essential for pollen wall development and exine patterning. J Exp Bot 69:1615–1633
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:437–460
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181
Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2005) The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sex Plant Reprod 18:1–7
Ariizumi T, Kawanabe T, Hatakeyama K, Sato S, Kato T, Tabata S, Toriyama K (2008) Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant Cell Physiol 49:58–67
Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, Yang ZN (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158:264–272
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cole R, Peremyslov V, Van Why S, Moussaoui I, Ketter A, Cool R, Andres Moreno M, Vejlupkova Z, Dolja V, Fowler JE (2018) A broadly-conserved NERD genetically interacts with the exocyst to affect root growth and cell expansion. J Exp Bot 69:3625–3637
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Feng ZY, Zhang BT, Ding WN, Liu XD, Yang DL, Wei PL, Cao FQ, Zhu SH, Zhang F, Mao Y, Zhu JK (2013) Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23:1229–1232
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863
Heslop-Harrison J (1968) Wall development within the microspore tetrad of Lilium longiflorum. Can J Bot 46:1185–1192
Hong SK, Aoki T, Kitano H, Satoh H, Nagato Y (1995) Phenotypic diversity of 188 rice embryo mutants. Dev Genet 16:298–310
Hsieh K, Huang AH (2007) Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19:582–596
Hu J, Wang Z, Zhang L, Sun MX (2014) The Arabidopsis Exine Formation Defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203:140–154
Ito T, Shinozaki K (2002) The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol 43:1285–1292
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Li WL, Liu Y, Douglas CJ (2017) Role of glycosyltransferases in pollen wall primexine formation and exine patterning. Plant Physiol 173:167–182
Ma L, Yang Z, Zhang S (2013) DEX1, a plasma membrane-localized protein, functions in microspore development by affecting CalS5 expression in Arabidopsis thaliana. Chin Sci Bull 58:2855–2861
Nishikawa SI, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Piffanelli P, Ross JH, Murphy D (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Quilichini TD, Grienenberger E, Douglas CJ (2015) The biosynthesis, composition and assembly of the outer pollen wall: a tough case to crack. Phytochemistry 113:170–182
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Shi J, Cui M, Yang L, Kim YJ, Zhang D (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20:741–753
Sun MX, Huang XY, Yang J, Guan YF, Yang ZN (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod 26:83–91
Suzuki T, Narciso JO, Zeng W, van de Meene A, Yasutomi M, Takemura S, Lampugnani ER, Doblin MS, Bacic A, Ishiguro S (2017) KNS4/UPEX1: a type II arabinogalactan beta-(1,3)-galactosyltransferase required for pollen exine development. Plant Physiol 173:183–205
Tanaka Y, Nishimura K, Kawamukai M, Oshima A, Nakagawa T (2013) Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238:561–575
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J 28:27–39
Xie HH, Chen L, Xu FQ, Guo WS, Wang S, Yang ZN, Zhang S (2017) ACOS5 is required for primexine formation and exine pattern formation during microsporogenesis in Arabidopsis. J Plant Biol 60:404–412
Xu T, Zhang C, Zhou Q, Yang ZN (2016) Pollen wall pattern in Arabidopsis. Sci Bull 61:832–837
Xu D, Shi J, Rautengarten C, Yang L, Qian X, Uzair M, Zhu L, Luo Q, An G, Waßmann F et al (2017) Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol 173:240–255
Yu J, Meng Z, Liang W, Kudla J, Tucker MR, Luo Z, Chen M, Xu D, Zhao G, Wang J et al (2016) A rice Ca2+ binding protein is required for tapetum function and pollen formation. Plant Physiol 172:1772–1786
Yuan J, Kessler SA (2019) A genome-wide association study reveals a novel regulator of ovule number and fertility in Arabidopsis thaliana. PLoS Genet 15:e1007934. https://doi.org/10.1371/journal.pgen.1007934
Zhao B, Shi H, Wang W, Liu X, Gao H, Wang X, Zhang Y, Yang M, Li R, Guo Y (2016) Secretory COPII protein SEC31B is required for pollen wall development. Plant Physiol 172:1625–1642
Acknowledgements
We are grateful to the Salk Genomic Analysis Laboratory for providing the T-DNA mutants seeds. The authors thank Wanwan Zhu, Ruifeng Fu and Lu Zhu for their help in TEM analysis. We thank Staffan Persson (University of Melbourne) for helpful comments and advice.
Funding
This research was supported by the National Key Research and Development Program of China (2016YFD0100903); the National Natural Science Foundation of China (U19A2031, 31670309, 31900611); China Innovative Research Team, Ministry of Education, and the Programme of Introducing Talents of Discipline to Universities (111 Project, B14016); JSPS KAKENHI Grant (JP19H05362).
Author information
Authors and Affiliations
Contributions
WQL, DBZ and DWX were involved in the design of the experiments and analyzed the data. DWX and PCM performed the experiments. IS helped for the immunolocalization experiment. DWX, DBZ, JXS and WQL wrote the manuscript. All authors have read, edited and approved publication of the present paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, D., Mondol, P.C., Ishiguro, S. et al. NERD1 is required for primexine formation and plasma membrane undulation during microsporogenesis in Arabidopsis thaliana. aBIOTECH 1, 205–218 (2020). https://doi.org/10.1007/s42994-020-00022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-020-00022-1