Abstract
To study how biofilm-forming rhizobacteria isolated from mines and dumpsites improved the phytoremediation efficacy of B. juncea in metal-contaminated soil. Out of 91 isolates, six were chosen for research based on their tolerance to metals, and their efficient PGPR properties, and subjected to the design of a consortium. A compatibility study revealed no antagonistic interaction between rhizobacterial-consortiums. The results of the biofilm formation and FEG-SEM studies revealed that a consortium-BC8 formed a strong biofilm on the root surface of B. juncea seedlings. Based on results obtained with the phytoextraction efficiency of B. juncea in consortium-BC8 (SMHMZ46 and SMHMP23), they were identified as Klebsiella variicola and Pseudomonas otitidis, respectively, and submitted to NCBI GenBank with accession numbers MZ145092 and OK560623. This rhizobacteria is the first to be reported as assisting Ni and Pb phytoremediation by employing B. juncea. Soil inoculation with consortium-BC8 increased the amount of soluble Ni and Pb by 13.25-fold and 10.69-fold, respectively, when compared to the control. These consortiums-BC8 significantly increased vegetative growth and metal accumulation in root and shoot with a translocation-factor of 1.58 for Ni and soil to root with a bioconcentration-factor of 1.3 for Pb in B. juncea grown in individual soil contamination with 96.05 mg/kg NiCl2 and 89.63 mg/kg Pb(NO3)2, which are significantly higher than other consortium treatments and the non-inoculated control. B. juncea amendments with a biofilm-forming consortium-BC8 having TF, BCF, and BAC > 1 for Ni, whereas BCF > 1, TF, and BAC < 1 for Pb, are appropriate for green remediation of Ni and phytostabilization of Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive metal pollution of soil has become a severe concern in recent decades as a result of expanding industry and population, excessive input of NPK (nitrogen, phosphorus, and potassium) fertilizers, weedicides, pesticides, and land irrigation with polluted water sources (Prajapati et al. 2022). Heavy metal contamination in agricultural soils is extremely crucial since heavy metals are readily absorbed into the food chain via fruits, vegetables, and edible plant components (Kaur et al. 2018). Increased levels of potentially toxic heavy metals (HMs) in soils are universally acknowledged as one of the principal drivers of environmental degradation worldwide, posing a threat to both the environment and human health (Ekoa Bessa et al. 2021). To reduce the detrimental effects of HMs bioaccumulation and biomagnification in biological systems while also protecting the environment, careful remediation approaches for HMs-contaminated soils are urgently needed (Wang et al. 2021). Numerous physical, chemical, and/or biological therapies are now available to clean up contaminated environments (Sharma 2020). Physical and chemical approaches, on the other hand, are constrained by high costs and manpower requirements (Zainab et al. 2020).
“The term "green remediation" refers to a gentle in situ remediation technique that tries to address the environmental effects of remedial actions at every stage of the process, increasing the net environmental benefit of a clean-up. One of the green remediations is phytoremediation, a plant-based approach that uses plants to extract and remove heavy metal pollutants or lower their bioavailability in soil (Jeyasundar et al. 2021; Wang et al. 2021). Bioenergy generation is appropriate for plants like Brassica juncea because of their efficiency in creating biomass (Ying et al. 2021). Plants are considered a low-cost technique for eliminating HMs with relatively minor environmental management issues (Amin et al. 2018). Furthermore, plant growth-promoting rhizobacteria assist phytoremediation of HMs-contaminated soils, which is being investigated as a potential green solution for soil management”. In this study, plant growth-promoting rhizobacteria from the Zawar mines in Udaipur, Rajasthan, India, and the Pirana landfill dumpsite in Ahmedabad, Gujarat, India, as well as the bioenergy crop B. juncea, were collectively used to meet the goals of "green remediation” of Ni and Pb.
Heavy metal bioavailability, plant resistance to stress caused by HMs, and plant productivity could all be factors limiting the effectiveness of phytoremediation (Antoniadis et al. 2021). The remediation potential of biofilm formed plant growth-promoting rhizobacteria-assisted phytoremediation has recently attracted more attention due to multiple effective mechanisms. These mechanisms primarily increase plant biomass, root surface area, and health (Rochlani et al. 2022; Shaikh et al. 2022), metal bioavailability, and the makeup of the microbial community, as well as metal translocation within plants via microbe-metal-plant interactions. Extracellular polymeric substances (EPS), proteins, and DNA form highly organized cell communities called biofilms that attach to both biological and non-biological surfaces (Altaf & Ahmad 2017). Rhizobacterial biofilms adhering to plant roots help maintain colonization sites and act as a storage space for nutrients in the rhizosphere in the form of root exudates, which reduces the availability of nutrients to phytopathogens (Altaf & Ahmad 2017; Jeyasundar et al. 2021; Prajapati et al. 2022; Rochlani et al. 2022). It has been suggested that biofilm-forming PGPR has several advantages for plants, including facilitating resource acquisition, regulating phytohormone levels, and lowering biotic and abiotic stress factors (Jeyasundar et al. 2021; Prajapati et al. 2022). In addition, biofilm-forming microorganisms can interact with metals, nutrients, and toxins (Altaf & Ahmad 2017; Sullivan & Gadd 2019), and their extraordinary adaptability and metabolic activities show why they are so beneficial for remediation (Sharma 2020; Shaikh et al. 2022). Recent research (Zhang et al. 2020; Jeyasundar et al. 2021) concentrated on the interactions between a single strain and a single host plant, but in the field, microbial populations are always complex mixtures. Microbial coexistence and communication may result in interspecies interactions, which, depending on certain environmental factors, may result in unexpected functional and metabolic capabilities (Jeyasundar et al. 2021). Bacteria, actinomycetes, and fungi working together in harmony is another effective microbial biotechnological approach for a productive phytoremediation procedure (Rajkumar et al. 2012).
The current study was designed to:
-
(1)
Screen selected metal-tolerant rhizobacteria for the production of plant growth-promoting substances and biofilm formation.
-
(2)
Determine if toxic metal-tolerant biofilm-forming rhizobacterial consortiums can boost heavy metal availability and translocation in the root and shoot of B. juncea in order to improve phytoremediation, thereby making it a suitable option for the amelioration of metal-contaminated sites (Table 1).
Materials and methods
Metal-tolerant rhizobacteria isolation and selection
In our previous work, we isolated 91 metal-tolerant rhizobacteria: 51 from Zawar mines in Udaipur, Rajasthan, India, and 40 from the Pirana dumpsite in Ahmedabad, Gujarat, India. Six rhizobacteria (SMHMZ2, SMHMZ4, SMHMZ46, SMHMP4, SMHMP23, and SMHMP38) were chosen for further research due to their high tolerance to heavy metals such as Pb, Ni, and Cd (Sharma et al. 2020, 2021, 2022a) and were subjected to screening for plant growth-promoting traits and biofilm formation.
Qualitative and quantitative analysis of the plant growth-promoting characteristics of rhizobacteria in the presence of heavy metals (Ni and Pb)
Solubilization of phosphate
In Pikovskaya's medium, the ability of isolates to solubilize insoluble phosphates was investigated (Pikovskaya 1948). Each rhizobacterium was spot inoculated on Pikovskaya's agar plates having 100 µg/ml of Ni and Pb. For 72 h, the plates were incubated at 28 °C. Isolates that can dissolve insoluble phosphates will develop yellow haloes around colonies. The P solubilization index (PSI) was determined using the formula shown below.
Quantitative phosphate solubilization was performed in a 100 ml liquid Pikovskaya’s medium in 250 ml flasks for 21 days. The concentration of the soluble phosphate was determined every 7 days by the stannous chloride method and the change in pH as per the method mentioned by Shah and Saraf 2019.
Siderophore production
The CAS agar method was used to do qualitative siderophore detection (Schwyn and Neilands, 1987). The CAS agar medium was additionally supplemented with 1 mM concentrations of Pb and Ni. The cultures were inoculated one at a time onto the CAS agar plate and incubated at 37 °C ± 2 °C for 72 h. The results were interpreted based on the presence of orange-yellow haloes around the colonies. A quantitative determination of siderophore synthesis in liquid succinic acid medium supplemented with 100 µg/ml metals (Ni and Pb) was performed after 48 h of incubation to investigate the effect of metal on PGPR siderophore production (Saraf et al. 2017; Wang et al. 2022). The following tests were used to determine the kind of siderophore produced by rhizobacterial isolates:
-
1.
Csaky’s assay (Csaky, 1948) for hydroxamate-type siderophore
-
2.
Arnow’s assay (Arnow 1937) for catecholate type of siderophore
Ammonia and hydrogen cyanide (HCN) production
All six isolates were evaluated for ammonia production under the influence of metals in 30 ml peptone water containing (gm per liter) peptone 10 g, NaCl 5 g, yeast extract 5 g, a pH of 7.6, and 100 µg/ml metals (Ni and Pb). Incubated for 48 h at 37 °C ± 2 °C. The supernatant was collected after centrifugation every 7, 10, and 13 days of incubation, and 0.5 mL of Nessler's reagent was added. The appearance of brown indicates the presence of ammonia. The ammonia concentration was spectrophotometrically calculated using a standard curve of ammonium sulfate concentrations ranging from 1 to 100 µg/ml at 450 nm (Goswami et al. 2013).
Castric (1975) described methods for screening bacterial isolates for hydrogen cyanide (HCN) synthesis. HCN production was indicated by the development of a light brown to dark brown shade of filter paper.
Design the rhizobacterial consortium and their compatibility testing
Consortium Design: Two factorial design approaches, i.e., two habitats (mines and landfill areas), were used to design rhizobacterial consortium. Three rhizobacteria SMHMZ2, SMHMZ4, and SMHMZ46, isolated from the Zawar mines area, Udaipur, Rajasthan, and another three, SMHMP4, SMHMP23, and SMHMP38, isolated from the Pirana landfill site in Ahmedabad, Gujarat, India, showed good metal tolerance against Ni and Pb and PGPR properties, so they were used to prepare microbial consortiums. A total of 09 rhizobacterial consortium combinations (BC1-BC9) were prepared. Coding of various Rhizobacteria Consortium combinations as per below-BC.
Isolates from Pirana landfill site | ||||
|---|---|---|---|---|
SMHMP4 | SMHMP23 | SMHMP38 | ||
Isolates from Zawar Mines | SMHMZ2 | BC1 | BC2 | BC3 |
SMHMZ4 | BC4 | BC5 | BC6 | |
SMHMZ46 | BC7 | BC8 | BC9 | |
Under aseptic circumstances, equal amounts of overnight-grown cultures of the different PGPRs (~ 107 cfu/ml) were mixed together to generate bacterial consortia combinations BC1– BC9, which were then employed for host treatment.
Compatibility testing
These nine (09) rhizobacterial consortium combinations (BC1-BC9) were subjected to compatibility testing to see if there was any antagonistic activity between rhizobacteria. The compatibility test was devised so that every PGPR member of Group-I (Zawar mine isolates) was confronted with every PGPR member of group-II (Pirana waste site isolates). Overnight-grown broth cultures of the respective PGPRs (one from group I and one from group II) were streaked on the opposing halves of the nutrient agar plates. All co-cultivation experiments were done in triplicate. After 48 h of incubation at 37 °C ± 2 °C, the plates were examined for the existence of any zones of inhibition at the colony borders where the two cultures met (Mishra, and Sundari, 2017).
Qualitative and quantitative assays for biofilm formation
Test-tube method
In test tubes, 10 ml of NB containing 100 ppm of the metals Pb, Ni, and Cd was inoculated with a loopful of rhizobacterial isolates. The test tubes were then incubated at 28 °C for two days. Next, the test tubes were rinsed with phosphate buffered saline after the supernatant was discarded (pH 7). The stained dry glass tubes were washed with distilled water after being dyed for 15 min with 0.1% crystal violet. A visible film that lined the tube's bottom and wall indicated the presence of biofilm (Christensen et al. 1982).
Congo red agar plate method
Using Congo Red Agar (CRA) medium, it is a straightforward qualitative method to identify the production of biofilms. CRA medium was prepared with 100 µg/ml (Pb and Ni). CRA plates were inoculated with test organisms and incubated at 37 °C for 24 h. Black colonies with a dry, crystalline consistency indicated biofilm production (Freeman et al. 1989).
Microtiter plate assay method
An experiment for quantifying biofilm on 96 well microtiter plates was carried out using the methodology of Ansari and Ahmad, 2018. Selected rhizobacteria were inoculated in 10 mL of NB broth that included 100 µg/ml of Pb and Ni and 5% sucrose and incubated at 37° C for 24 h. After that, the broth was diluted to an optical density of 0.025 at 600 nm. 200 µl (4*106 cfu/ml) of the diluted cultures were added to each well of sterile 96-well flat-bottom polystyrene plates (Corning), which were then incubated for 48 h at 28° C. Following incubation, each well's contents were removed through slight tapping. To remove free-floating bacteria, the wells were rinsed four times with 0.2 mL of phosphate buffered saline (pH 7.2). The biofilm developed by rhizobacteria adherent to the wells was preserved with 2% sodium acetate and stained with 200 µl of 0.1% crystal violet solution added to each well and incubated at 28 °C for 20 min. Excess stain was removed with deionized water, and the plates were let dry. After adding 70% ethanol to each dry well, the intensity of the crystal violet staining was measured. The optical density (OD) of a stained adherent biofilm was measured at 590 nm using a micro-ELISA auto reader (Micro Lab Instruments). The interpretation of biofilm generation was done using Stepanovic et al. (2017) (Table 2).
Biofilm development on the root Surface
A separate experiment was designed to evaluate the biofilm that was developed by the BC8 consortium on the root surface of Brassica juncea. In a nutshell, 10 days after transplantation, the B. juncea seedling in the sterile gnotobiotic soil system was immersed in an overnight-grown culture of consortia; sterile broth was used as a control. To get rid of cells that were loosely adhered to the root surface, all plants were uprooted and cleaned. According to Ansari and Ahmad (2018), the root samples were fixed in 2.5% glutaraldehyde (v/v) in 0.1 M phosphate buffer (pH 7.2) for 2 h and then dehydrated through a graded ethanol series (30%–100%, v/v). The root samples were shipped to the SICART facility in Anand, Gujarat, where a scanning electron microscope (Nova NanoSEM 450) was used to observe the biofilm on the root surface.
Identification of heavy metal-tolerant rhizobacteria using the 16S rRNA gene sequence
Isolates with higher heavy metal resistance, PGP characteristics, biofilm formation, and increased heavy metal accumulation in plants were chosen for identification. The pure culture's DNA was extracted. Its purity was determined using agarose gel electrophoresis, and one band of high-molecular-weight DNA was discovered. Using 16S rRNA primers, a gene fragment was amplified by PCR. There was only one unique PCR amplicon band visible when resolved on Agarose Gel. The PCR amplicon was purified using SLS' PCR Purification Kit to get rid of contaminants (column-based purification). Using the BDT v3.1 Cycle Sequencing Kit and 16S rRNA Primers, the DNA sequencing reaction of the PCR amplicon was carried out on an ABI 3730xl Genetic Analyzer at SLS Research Pvt. Ltd., Surat, Gujarat, India (Saitou and Nei 1987). The NCBI Genbank database was searched using BLAST on the gene sequence. The following is a primer on the details.
Primer name | Sequence (5′–3′) |
|---|---|
27F | AGA GTT TGA TCM TGG CTC AG |
1492R | CGG TTA CCT TGT TAC GAC TT |
Rhizobacteria's influence on soil metal mobility
In 50-ml falcon tubes, a 1 ml aliquot (8*109 cfu/ml) of rhizobacterial consortium suspension was added to 10 gm of soil contaminated with 100 mg kg−1 of NiCl2 and Pb (NO3)2 and shaken at 200 rpm at room temperature (Treatment 2). 1 mL of sterile distilled water was applied to the control soil (Treatment 1). After 7 days, each set of falcon tubes received 10 mL of sterile, double-distilled water to extract water-soluble heavy metals (Ni and Pb). All falcon tubes were centrifuged at 8000 rpm for 10 min to remove dirt particles, and the resultant solution was filtered using Whatman filter paper No.40. An atomic absorption spectrophotometer (ELICO SL 194) was used to determine the concentrations of Ni and Pb in the filtrate (Rajkumar and Freitas 2008; Ndeddy Aka et al. 2016).
Pot experiments
Soil sampling, metal spikes, and pot experiment
At five different places, surface soil (0–15 cm) samples were taken from an agricultural tract near the Jagatpur village, near Gota, Ahmedabad, Gujarat. The soil samples were air-dried, crushed, and sieved to a particle size of 2 mm before being bulked up to create a composite sample. NiCl2·6H2O and Pb (NO3)2 were utilized as Ni and Pb sources for spiking the soil sample, respectively. The soils were spiked using the Wuana et al. 2010 method, in which 100 ml of 1000 mg/L metal stock was added to 1000 gm (1 kg) plastic pots (6*7 inch) of air-dried parent soil (at a 10:1 solid: liquid ratio) and incubated for 4 weeks for metal stabilization in soil. The aforementioned spiking was designed to provide a target concentration of roughly 100 mg/kg (Wuana et al. 2010; Reddy and Chinthamreddy 2000). The metal concentrations in contaminated soil were determined using the acid digestion technique, followed by measurement using an atomic absorption spectrophotometer. After four weeks, the concentrations of Ni and Pb in each spike soil obtained according to the aforementioned procedures were 96.05 mg kg−1 and 89.63 mg kg−1, respectively, and these spike soils were employed in pot experiments after being sterilized in an oven at 180 °C for 2 h. The pot experiment was performed for 11 treatments, viz., Control (H2O) (control, no external metal or inoculums, only double distilled water added), Control (M) (spike with individual metal), and rhizobacterial combinations viz., BC1, BC2, BC3, BC4, BC5, BC6, BC7, BC8, BC9, and the bags were stacked in a randomized factorial design. Overall, 22 treatments with four replicates resulted in 88 black polybags for two metals (Ni and Pb).
Metals and biofilm-forming metal-tolerant rhizobacteria's effect on plant growth
For pot experiments, the seeds of B. juncea were sterilized as per the method discussed by Ndeddy Aka et al. (2016). Sterilized B. juncea seeds were seeded for 2 h in rhizobacterial cultures or sterile water (control), then allowed to dry at room temperature. In each 6-inch by 7-inch pot (6*7 inch) 1000 g of soil (ten seeds, inoculated and non-inoculated) were sown at a depth of 5 cm. The pots were trimmed eight days after germination, leaving seven seedlings per pot. A plastic tray was placed beneath the treatment pot to collect any leachate, which was then returned to the pots when the pots were watered again. The seedlings were watered daily with deionized water to keep the moisture level of the soil. The overall design of the experiment included a maximum of 11 treatments with four replicates:
-
B. juncea + non-contaminated soil (control H2O (double-distilled water))
-
B. juncea + metal-contaminated soil (separate metal: Ni and Pb)
-
B. juncea + metal-contaminated soil + rhizobacterial consortiums (BC1, BC2, BC3, BC4, BC5, BC6, BC7, BC8, and BC9) Separate pots for each rhizobacterial consortium with separate individual metal
After 30 days, the plants were harvested. Each plant's height and fresh weight were measured, and the plants were then split into roots and shoots and dried. Plant samples were properly labeled and stored in polyethylene bags for subsequent analysis.
Metal and biofilm formation by metal-tolerant rhizobacteria affect pigments, proline, and phenolic compounds in Brassica juncea
Chlorophyll pigment estimation (chlorophyll a, b and carotenoids)
0.1 gm of leaf was homogenized with 80% acetone, filtered, and the absorbance was measured at wavelengths of 663, 645, and 480 nm for chlorophyll a, b, and carotenoids, respectively (Lichtenthaler & Wellburn 1983).
Proline content
Proline levels were determined in accordance with Bates et al. (1973). L-proline was used to create the standard curve.
Total phenols
Total phenols were determined using Singleton and Rossi's method (1965). Total phenols were determined using gallic acid as a standard.
Flavonoid content
According to Zhishen et al. 1999, the flavonoid levels were measured. Quercetin was utilized as a standard for measuring flavonoid content.
Plant heavy metal measurement
For heavy metal analysis, a dried plant sample of root (0.1–0.2 gm) and shoot (1–2 gm) from each sample was precisely weighed with a balance and placed in a 100 ml Erlenmeyer flask containing 9 ml of HCL, and 3 ml of concentrate HNO3 (69 percent), and 1 ml of H2O2. The mixture was cooked on a hot plate at 100 °C in a fume hood until white fumes emitted. The mixture was allowed to cool after digestion before being diluted to 50 mL with distilled deionized water. The resultant solution was filtered twice using filter paper before the various heavy metal concentrations (Ni, and Pb) in the samples were quantified using an atomic absorption spectrophotometer (ELICO SL 194) (Ndeddy Aka et al. 2016).
Evaluating the efficacy of phytoremediation
Phytoextraction indices are a useful technique for determining the phytoextraction potential of Brassica juncea in conjunction with PGPR for the removal of Ni and Pb from polluted soil. The following are the most commonly used phytoextraction indices:
Bioconcentration factor (BCF)
The bioconcentration factor (BCF) was calculated as the metal concentration ratio in plant roots to soil (Lindsay and Norvell 1978; Amin et al. 2018):
Bioaccumulation coefficient (BAC)
The bioaccumulation coefficient (BAC) was calculated as a ratio of Pb in shoots to Pb in soil (Lindsay and Norvell 1978; Amin et al. 2018):
Translocation factor (TF)
The translocation factor (TF) was calculated as a ratio of heavy metals in plant shoots to heavy metals in plant roots (Lindsay and Norvell 1978; Amin et al. 2018):
Statistical analysis
A randomized block design was adopted in the experiment. All experiments were carried out in triplicate. The results of each treatment were reported as arithmetic means with standard error. Using IBM SPSS Statistics version 22, data were subjected to a one-way analysis of variance (ANOVA) (SPSS Inc. Chicago, USA). The DMRT (Duncan's Multiple Range Test) was also used to test the differences between means of all treatments at P ≤ 0.05 significance, while the Levene test was used to check the homogeneity of variance.
Results
Plant growth-promoting characteristic of rhizobacteria in the presence of heavy metals (Pb, and Ni)
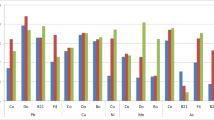
All of the rhizobacteria demonstrated plant growth-promoting traits on solid and liquid mediums containing 100 ppm metals (Fig. 1). The PSI was measured, ranging from 2.8 to 4.4. Z4, Z46, and P23 isolates had maximal phosphate solubilization in liquid medium at 7 days, with values of 75, 103, and 79 μg/ml, respectively, and the pH of the inoculated medium decreased from 7.2 to 3.0 after 21 days (Table 1). SMHMZ4, SMHMZ46, and SMHMP-23 isolates generated a bright orange-yellow halo around colonies on CAS agar plates (Fig. 1) and produced a positive reaction with the catecholate assay (Arnow's assay), which produced more siderophore, viz., 90.33, 110.67, and 85.33 µg/ml, respectively (Table 1). After 10 days of incubation, the maximum concentrations of ammonia produced by SMHMZ4, SMHMP46, and SMHMZ23 isolates were 41.44, 38.82, and 45.42 µg/ml, respectively. Four of the six isolates, SMHMZ2, SMHMZ4, SMHMP4, and SMHMP23, produced HCN (Table 1).
A schematic picture of the present work denotes, a PGPR properties of metal-tolerant rhizobacteria; b compatibility studies of rhizobacteria SMHMZ46 and SMHMP23 (BC8); c Pot experiment of biofilm-forming rhizobacteria to assist phytoremediation of Ni and Pb; d qualitative and quantitative assay for biofilm formation (A and B), biofilm development on root surface; C non-inoculated control B. juncea root surface; D consortium BC8 inoculated B. juncea root surface; e Biofilm-forming rhizobacteria assist phytoextraction strategies of phytoremediation
A compatibility study of the rhizobacterial consortium BC1–BC9 was carried out in order to determine how the individual rhizobacteria in the consortium could influence plant growth cooperatively. No zones of inhibition were observed at the points of contact between the rhizobacterial species in the culture plates (BC1–BC9). Figure 1b shows the compatibility study of the rhizobacteria SMHMZ46 and SMHP23 of consortium BC8.
Studies of biofilm formation
Out of six rhizobacteria isolates, only two, SMHMZ46 and SMHM38, showed strong biofilm formation with all screening methods. A quantitative assay for biofilm formation was performed on 96-well microtiter plates. Rhizobacterial strains SMHMZ46 and SMHMP38 showed strong biofilm development on the microtiter plates, and optical density measurements were 1.56 ± 0.21 and 1.38 ± 0.15, respectively, of crystal violet at 570 nm, while strain SMHMP23 showed weak biofilm formation. In our study, we investigated the behavior of rhizobacteria consortium BC8 on a 10-days-old B. juncea root surface for the development of biofilm. The results of root FEG-SEM analysis revealed that the consortium established biofilm on the root surface (Fig. 2).
Identification using the 16 s RNA sequence
Based on results obtained with the phytoextraction efficiency of B. juncea assist in consortium, SMHMZ46 and SMHMP23 isolates were subjected to molecular identification. BLAST was used to search for sequence homology in the partly amplified and sequenced 16S mRNA gene. The partial nucleotide sequences of bacterial strains coded by SMHMZ46 and SMHMP23 were found to be quite close to Klebsiella variicola strain 3039, and Pseudomonas otitidis P13.2, respectively, and submitted to NCBI GenBank with accession numbers MZ145092 and OK560623 (Fig. 3 a and b).
Rhizobacteria's influence on soil metal mobility
In comparison to the control, soil inoculation with the rhizobacterial consortium BC8 increased the amount of soluble Ni and Pb in soil by 13.25-fold (F = 1909.827, P < 0.0001) and 10.69-fold (F = 257.960, P < 0.0001), respectively (Fig. 4).
Effect of metal-tolerant rhizobacterial consortium on the concentration of water-soluble metals (100 mg kg−1 NiCl2 and 100 mg kg−1 of Pb (NO3)2) in soil. Treatment 1: control (metal-contaminated soils with no bacterial inoculation); Treatment 2: metal-contaminated soils + bacterial consortium. According to Duncan's Multiple Range Test (p < 0.05), similar letters in the same column are statistically non-significant. The data are means (n = 3 ± SE), with ain superscript indicating considerably higher values and later alphabets indicating significantly lower values
Effect of biofilm-forming metal-tolerant rhizobacteria on growth and accumulation of metal in Brassica juncea under Ni and Pb stress conditions
The results showed that the rhizobacterial consortium BC8 significantly (p ≤ 0.05) improved root length (1.92-fold and 1.80-fold), shoot length (1.79-fold and 1.65-fold), and the dry weight of shoot (3.08-fold and 2.87-fold) and root (2.77-fold and 1.54-fold) at 96.05 mg kg−1 of Ni and 89.63 mg kg−1 Pb-contaminated soil when compared to the same level of Ni and Pb stress without inoculation. For instance, rhizobacterial consortium BC8 significantly (p ≤ 0.05) increased Ni concentrations in the root and shoot of B. juncea by 23.95 and 95.30%, respectively. Similarly, rhizobacterial consortium BC8 enhanced Pb concentrations significantly (p ≤ 0.05) in the root and shoot by 24.21% and 85.67%, respectively (Table 2) when compared to the same level of Ni and Pb stress without inoculation.
Effects of metal and biofilm formation by metal-tolerant rhizobacteria on pigments, total phenolic content, flavonoid content, and proline in Brassica juncea
Under Ni and Pb stress conditions, the inoculation of rhizobacterial consortium BC8 demonstrated a significant (p ≤ 0.05) increase in chlorophyll a, chlorophyll b, and carotenoid content than non-inoculated control plants (Fig. 5a). Total phenols, flavonoids, and proline content were increased in a one-month-old Brassica juncea plant treated with Ni (167.80, 82.07, and 6.85%, respectively), and Pb (109.14, 38.68, and 4.06%, respectively) toxicity. However, supplementation with the heavy metal-tolerant rhizobacterial consortium BC8 further increased total phenols, flavonoids, and proline levels in B. juncea by 160.80% (F = 7485.089, P < 0.0001), 77.72% (F = 250.180, P < 0.0001), and 108.55% (F = 117,169.559, P < 0.0001), respectively, in Ni, 217.39% (F = 8649.235, P < 0.0001), 110.88% (F = 162.408, P < 0.0001), and 114.14% (F = 2.315E + 32, P < 0.0001), respectively, in Pb stressed conditions (Fig. 5b).
Effect of metals (Ni and Pb) and metal-tolerant rhizobacteria on (a) pigments (Chl a = Chlorophyll a, Chl b = Chlorophyll b, and Caret. = carotenoid content), (b) total phenol, flavonoid, and proline content of B. juncea under pot experiments. Note: According to Duncan's Multiple Range Test (p < 0.05), similar letters in the same column are statistically non-significant. The data are means (n = 4 ± SE), with ain superscript indicating considerably higher values, and later alphabets indicating significantly lower values
Phytoremediation potential of B. juncea
In terms of Ni, the rhizobacterial consortium BC8 significantly (p ≤ 0.05) increased the TF, BCF, and BAC of B. juncea, and the values were 1.58, 1.24, and 1.96, respectively, which were significantly (p ≤ 0.05) higher than other rhizobacterial consortium treatments and the non-inoculated control. The TF, BCF, and BAC values for Ni in the non-inoculated control were 1.01, 1.0, and 1.0, respectively. One-way ANOVA revealed that there was a statistically significant difference in mean scores between at least two group TF (F (9,30) = [150636.132], p = < 0.0001)), BCF (F (9,30) = [66048.582], p = < 0.0001)), and BAC (F (9,30) = [726212.445], p = < 0.0001)) values for Ni. However, in the case of Pb, the results revealed that B. juncea roots contained a sufficient level of Pb as compared to the non-inoculated control. For Pb, the rhizobacterial consortium BC8 considerably increases Pb concentrations in B. juncea root and shoot. Pb-BC8 inoculated treatment had TF (F (9,30) = [534.809], p = < 0.0001), BCF (F (9,30) = [37157.874], p = < 0.0001) and BAC (F (9,30) = [1095.779], p = < 0.0001) values of 0.7, 1.3, and 0.98, respectively. The TF, BCF, and BAC values for Pb in the non-inoculated control were 0.5, 1.05, and 0.53, respectively (Fig. 6).
Effect of metal (Ni and Pb) and metal-tolerant rhizobacteria consortium treatments on bioconcentration factor (BCF), bioaccumulation coefficient (BAC), and translocation factor (TF) of B. juncea for Ni and Pb. Note: According to Duncan's Multiple Range Test (p < 0.05), similar letters in the same column are statistically non-significant. The data are means (n = 4 ± SE), with ain superscript indicating considerably higher values, and later alphabets indicating significantly lower values
Discussion
Although heavy metals harm microbes, bacteria have adaptation mechanisms that let them live in environments in which heavy metals are present. These metal-tolerant bacteria can be isolated and are preferred for their ability to boost plant development and heavy metal accumulation by plants (Rajkumar and Freitas 2008; Rajkumar et al. 2012). In this investigation, six rhizobacterial isolates were selected out of 91 from the mine areas and landfill sites. Multiple plant growth-promoting rhizobacterial properties were observed in the selected metal-tolerant rhizobacterial isolates (Fig. 1), including the generation of siderophore, insoluble phosphate solubilization, hydrogen cyanide, and ammonia (Table 1). Pramanik et al. (2018), conducted a similar study in which their strain, Enterobacter spp. showed a good response to plant growth-promoting features under metal stress conditions. Wang et al. (2022) reported catecholate siderophore formation under metal stress conditions. This also demonstrates that, in addition to being iron chelators, siderophores can bind to other heavy metal ions in the medium and sequester and lower their toxicity to both microorganisms and plants (Wang et al. 2022). As a result, when these isolates are administered to heavily metal-saturated agricultural soil, they can lower metal toxicity while also improving plant growth. In order for the individual rhizobacteria in the consortium to exert cooperative effects on plant growth, a compatibility study of the rhizobacterial consortium BC1-BC9 was conducted. At the points of contact between the rhizobacterial species in the culture plates, no zones of inhibition were seen. The presence of the second rhizobacteria in the opposite half of the petri plate did not result in any visible growth inhibition in the counterpart (Fig. 1b). This shows that there are no volatile substances or spreadable toxins that might cause them to be antagonistic to one another. Thus, the experiment provided proof that the tested rhizobacterial consortium combination is growing in a mutually non-inhibitory manner, allowing further research on the consortia (Sharma et al. 2023; Mishra and Sundari 2017).
It was reported that the formation of biofilm and exopolysaccharide produced by PGPR kept the viability of rhizobacterial cells under stress (abiotic) to protect them in the rhizosphere, significantly increase soil fertility, and enhance plant growth (Alaa 2018). In our study isolates SMHMZ46, and SMHMP38, strong biofilm formation was detected by the test tube method, Congo red agar method, and quantified by microtiter plate assay. The optical densities measured were 1.56 ± 0.21 and 1.38 ± 0.15, respectively. These strains are thought to naturally maintain a suitable niche in stressful environments with elevated metal concentrations by forming biofilms. The similar observation was obtained by Itusha et al. (2019), their Cadmium tolerant strain VITJAN13 showed strong biofilm formation on the microtiter plate, and their OD570 was 1.434 ± 0.048. Such rhizobacteria are expected to find application in plant growth-promoting rhizobacteria that assist phytoremediation (Itusha et al. 2019). The interaction of the rhizobacteria consortium BC8 with B. juncea was evaluated using FEG-SEM analysis, which revealed that the consortium BC8 established a biofilm on the root surface of Brassica juncea when compared with non-inoculated rhizobacteria-treated roots (Fig. 2). Our findings of biofilm formation on the B. juncea root surface by a Klebsiella variicola and Pseudomonas otitidis consortium. Altaf & Ahmad, (2017) reported that their heavy metal-tolerant Azotobacter vinelandii AZCH6 formed biofilm on the roots of wheat seedlings.
Based on results obtained with the phytoextraction efficiency of B. juncea in consortium BC8, SMHMZ46 and SMHMP23 isolates were identified as Klebsiella variicola and Pseudomonas otitidis and submitted to NCBI GenBank with accession numbers MZ145092 and OK560623. This is the first report on the augmentation of Klebsiella variicola and Pseudomonas otitidis in the rhizospheric region of B. juncea, showing enhanced uptake of Ni and Pb (Table 3).
Aside from limited plant biomass, another limiting element of phytoextraction is the poor availability of heavy metals in soils. The toxicity of a metal and its extraction by plants are determined by its bioavailability. Rhizosphere bacteria are well known for their role in the biogeochemical cycling of harmful heavy metals through processes involving conversions that increase metal availability for plant uptake (Ndeddy & Babalola, 2016). In the present study, the bacterial consortium BC8 significantly increased the amount of soluble Ni and Pb in the soil as compared with that without inoculation. The rise in water-soluble metal concentrations induced by rhizobacteria may be linked to the synthesis of organic acids such as indole-3-acetic acid, phosphate solubilization, and oxidation–reduction processes (Jiang et al. 2008; Rajkumar and Freitas 2008).
Optimal plant development is a critical aspect in the successful application of phytoextraction. Ni and Pb are elements in plants that have no known biological purpose. Toxic metal (Ni and Pb) poisoning significantly reduced root and shoot length when compared to uninoculated plants growing in non-contaminated soil (Halstead et al. 1969) (Table 2). Pot culture experiments revealed that rhizobacteria consortium BC8-inoculated B. juncea plants displayed a significant (p ≤ 0.05) increase in shoot length, root length, plant dry weight when compared to the same level of Ni and Pb stress without inoculation. Rhizobacteria and plants co-occur for survival, and their harmonious interaction is crucial for adapting to environments with metal stress. This interaction can therefore be studied to enhance PGPR-assisted phytoremediation. Exudates from plant roots serve as vital nutrients and energy sources for the rhizosphere's microbes, with whom they have developed complex communication networks (Shaikh et al. 2022). Some rhizobacteria that are helpful for plant growth can reduce metal phytotoxicity and promote plant growth either directly by solubilizing minerals like phosphate, nitrogen, potassium, and iron, producing phytohormones like indole-3-acetic acid, cytokinin, and gibberellic acid, and producing specific enzymes like 1-aminocyclopropane-1-carboxylate deaminase, or indirectly by starting a defense mechanism against phytopathogens (Ma et al. 2016). Previous research has shown that Ni in the soil tends to lower the quantity of phosphorus in plant species, which has a negative impact on growth (Halstead et al. 1969). This limitation can be compensated for by the inorganic phosphate-solubilizing capabilities of consortia BC8, with a corresponding decrease in medium pH. The enhanced concentration of Ni in B. juncea plants in the presence of rhizobacteria could be linked to increased Ni uptake under acidic soil conditions, which takes place as a result of phosphate solubilizer activity in soil (Rochlani et al. 2022). Several authors (Tank and Saraf 2009; Altaf & Ahmad 2017; Din et al. 2020) reported a similar observation, stating that the increase in plant growth as a result of bacterial inoculation was due to the production of siderophore, solubilization of minerals such as phosphorus, and synthesis of plant growth substances such as IAA that promote plant root elongation and shoot growth, resulting in improved mineral and nutrient uptake (Yu et al. 2017; Zainab et al. 2020). Another key feature that may influence root and plant growth indirectly is ammonia production, and ammonia synthesis by bacteria has also been found to play an important role in biocontrol or suppression of phytopathogens. HCN is another secondary metabolite produced by bacteria that may protect plants from fungal diseases (Ndeddy Aka & Babalola 2016; Sharma et al. 2022b; Rochlani et al. 2022).
Plant photosystems and photosynthetic efficiency are adversely impacted by the presence of heavy metals in soil. Total chlorophyll and carotenoid levels in non-inoculated control plants decreased as a result of Ni and Pb stress because B. juncea plants' chloroplasts and photosynthetic apparatus deteriorated and their electron transport chain might be damaged (Farid et al. 2017). On the other hand, the total chlorophyll and carotenoid contents responded favorably to the rhizobacterial consortium BC8 inoculation. The net photosynthetic rate is regulated by PGPR, which increases chlorophyll levels, carotenoid content, and confers tolerance against Ni and Pb stress while also improving nutrient uptake and hormone production (Hoque et al.2022). The current study's findings are consistent with those of Khanna et al. 2019, who found that P. aeruginosa and B. gladioli increased the total chlorophyll and carotenoid contents in tomato plants grown under cadmium stress. Phenolic compounds are the end product of secondary metabolism. They function as pigments and signal molecules and are strong non-enzymatic antioxidants that help plants tolerate heavy metal stress conditions and increase growth and productivity. By detoxifying ROS in plant cells and acting as metal chelators, flavonoids reduce the toxic effects of metals, increase biomass, and enhance photosynthesis (Sharma et al. 2023). According to numerous studies, plants produce more total phenolic compounds and flavonoids when they are under heavy metal stress (Handa et al. 2018). It is well known that proline (an osmolyte) builds up in plants under heavy metal stress. By controlling osmotic balance, stabilizing protein and membrane structures, bringing reactive oxygen species (ROS) concentrations into normal ranges, and buffering cellular redox potential under stressful conditions, proline plays a critical role in maintaining stress tolerance (Sharma et al. 2023; Kaur and Asthir, 2015). The results of the current study showed that exposure to Ni and Pb increased the accumulation of total phenolic compounds, flavonoids, and proline content. This accumulation was further increased by the inoculation of metal-tolerant rhizobacterial consortia BC8. The results of the current study are in line with those of Sharma et al. 2023, who found that the inoculation of Burkholderia gladioli and Pseudomonas aeruginosa increased the content of total chlorophyll, carotenoid, total phenol compound, flavonoid, and proline in B. juncea under Cr stress conditions. Plants were grown in metal-rich environments typically absorb metals to variable degrees in response to environmental and internal influences (Halstead et al. 1969). The increase in plant biomass and metal availability caused by inoculation with toxic metal-tolerant rhizobacterial consortia treatments resulted in a significant (p ≤ 0.05) increase in heavy metal accumulation in the root and shoot tissues of B. juncea when compared to non-inoculated controls in this study (Table 2); which is consistent with other related research (Mendoza-Hernandez et al. 2019; Jeyasundar et al. 2021), in which Brassica plants inoculated with various microbial strains exhibited increased metal concentrations compared to their respective controls. In this context, heavy metal alleviation by metal-resistant plant growth-promoting rhizobacteria is not only eco-friendly and inexpensive but also, promote plant growth by ameliorating the metal-induced stress, producing plant growth-promoting substances (Ali et al. 2021; Wang et al. 2022). Klebsiella sp. (Ahmad et al. 2016), Pseudomonas sp. (Ndeddy Aka & Babalola 2016) are a few instances of reported heavy metal-resistant PGPR that exhibited various plant growth-promoting traits.
The bioconcentration factor, bioaccumulation coefficient, and translocation factor values (Fig. 6) assist in determining plant suitability for phytoremediation (i.e., phytoextraction or phytostabilization) by describing metal accumulation and translocation behaviors in plants. Plants with BCF, BAC, and TF values > 1 are regarded as prospective phytoextractors and are appropriate for phytoextraction, whereas those with BCF and TF values < 1 are not suitable for phytoextraction (Fitz and Wenzel 2002). Plants having BCF > 1 and TF < 1 are considered potential phytostabilizers, ideal for phytostabilization (Amin et al. 2018). Pot experiments revealed that inoculated B. juncea assist with consortia BC8, translocate heavy metal underground to overground with an increase in the TF, BCF, and BAC values of Ni as compared with control. A similar finding is consistent with other observations made (Ma et al. 2015; Ndeddy Aka Babalola 2016). This is the first report that, to our knowledge, explains how a Ni-tolerant rhizobacteria consortia composed of Klebsiella variicola and Pseudomonas otitidis contributes to Ni accumulation by B. juncea while simultaneously reducing Ni phytotoxicity and promoting plant growth. By sharing the burden with the plant roots, the rhizobacteria presumably lowers the level of Ni in the soil and establishes itself during the early phases of growth. Additionally, the rhizobacteria assist the plant in acquiring enough biofilm and soluble phosphate for optimal plant growth in the presence of heavy metals when the seedlings are established in the soil (Zaidi et al. 2006).
However, in Pb-contaminated soil, these consortia greatly enhance the BCF, while the TF values were less than one. When Pb comes into contact with plant roots, it is first absorbed into the upper layers (rhizodermis, collenchymas, and parenchyma) of the root cortex, where it interacts with the active groups of polysaccharides or uronic acid, i.e., carboxyl groups, before being taken up by Ca ion channels (Sharma and Dubey 2005). Although absorbed by roots, Pb is rarely transferred higher up to aerial plant parts (Kushwaha et al. 2018), which explains why root Pb was higher in our study than aerial Pb. According to the current study, consortium BC8 increases B. juncea's phytostabilization efficiency when compared to the non-inoculated control. As a result, their bioconcentration factor value increased (> 1) and their translocation factor and bioconcentration factor decreased (< 1). This is because phytostabilization can occur through the precipitation of heavy metals or reduction in metal valence in the rhizosphere, absorption and sequestration within root tissues, absorption onto root cell walls, or adsorption onto root cell walls. In this way, HMs are less likely to be transferred from the roots to the shoots in plants with lower TF values (TF < 1.0). (Amin et al. 2018).
Conclusion for future biology
Although phytoremediation is a new, cost-effective method for bioremediating soil contaminated with heavy metals, its effectiveness is greatly hindered by the slow growth of plants and the low bioavailability of heavy metals in the soil. Additionally, it is well known that Ni and Pb have negative effects on the Brassica juncea plant's health parameters and growth. In this study, we demonstrated that inoculation with the multi-metal-tolerant rhizobacterial consortium Klebsiella variicola SMHMZ46 and Pseudomonas otitidis SMHMP23, not only protects plants from growth inhibition caused by heavy metals (Ni and Pb), but also increases plant growth, biomass production, and the bioavailability of metal in the soil with a concurrent increase in metal accumulation in root and shoot by Brassica juncea. Biofilm-forming ability is an added advantage for imparting rhizobacterial survival and stability under stressful environmental conditions in the rhizosphere. This rhizobacteria is the first to be reported as assisting metal (Ni and Pb) phytoremediation by employing B. juncea. The results revealed that the Brassica juncea amendments with the rhizobacterial consortium were more accomplished for green remediation of Ni and well suited for phytostabilization of Pb. Furthermore, the efficiency of Brassica juncea's phytoremediation capability could be improved by studying the combination of these rhizobacterial consortiums with different pH and synthetic (EDTA) and natural (citric acid) chelators. Also, Brassica juncea plant species have both economic and ecological values, as they can both remediate metal-contaminated sites and provide useful biomass, which can generate revenue. Following harvesting, the biomass could be burned to create valuable bio-ore, from which accumulated metals could be recovered. Additionally, during this process, thermal and electric energy could be produced, which could be recovered and used again to create biofuels.
References
Ahmad I, Akhtar MJ, Asghar HN, Ghafoor U, Shahid M (2016) Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regula 35(2):303–315. https://doi.org/10.1007/s00344-015-9534-5
Alaa FM (2018) Effectiveness of exopolysaccharides and biofilm forming plant growth promoting rhizobacteria on salinity tolerance of faba bean (Vicia faba L.). Afr J Microbiol Res 12(17):399–404. https://doi.org/10.5897/AJMR2018.8822
Ali A, Guo D, Li Y, Shaheen SM, Wahid F, Antoniadis V, Zhang Z (2021) Streptomyces pactum addition to contaminated mining soils improved soil quality and enhanced metals phytoextraction by wheat in a green remediation trial. Chemosphere 273:129692. https://doi.org/10.1016/j.chemosphere.2021.129692
Altaf MM, Ahmad I (2017) In vitro and in vivo biofilm formation by Azotobacter isolates and its relevance to rhizosphere colonization. Rhizosphere 3:138–142. https://doi.org/10.1016/j.rhisph.2017.04.009
Amin H, Arain BA, Jahangir TM, Abbasi MS, Amin F (2018) Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): profitable phytoremediation with biofuel crops. Geol Ecol Landscapes 2(1):51–60. https://doi.org/10.1080/24749508.2018.1452464
Ansari FA, Ahmad I (2018) Biofilm development, plant growth promoting traits and rhizosphere colonization by Pseudomonas entomophila FAP1: a promising PGPR. Adv Microbiol 8(03):235. https://doi.org/10.4236/aim.2018.83016
Antoniadis V, Shaheen SM, Stärk HJ, Wennrich R, Levizou E, Merbach I, Rinklebe J (2021) Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ Int 146:106233. https://doi.org/10.1016/j.envint.2020.106233
Arnow LE (1937) Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem 118(2):531–537
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Castric PA (1975) Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol 21(5):613–618. https://doi.org/10.1139/m75-088
Christensen GD, Simpson WA, Bisno AL, Beachey EH (1982) Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 37(1):318–326. https://doi.org/10.1128/iai.37.1.318-326.1982
Czaky T (1948) Estimation of bound hydroxylamine in biological materials. Acta Chem Scand 21:450–454. https://doi.org/10.3891/acta.chem.scand.02-0450
Din BU, Rafique M, Javed MT, Kamran MA, Mehmood S, Khan M, Chaudhary HJ (2020) Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: a biochemical analysis. Plant Physiol Biochem 146:249–258. https://doi.org/10.1016/j.plaphy.2019.11.010
Ekoa Bessa AZ, Ngueutchoua G, Kwewouo Janpou A, El-Amier YA, Njike Njome Mbella Nguetnga OA, Kankeu Kayou UR, Armstrong-Altrin JS (2021) Heavy metal contamination and its ecological risks in the beach sediments along the Atlantic Ocean (Limbe coastal fringes, Cameroon). Earth Syst Environ 5(2):433–444. https://doi.org/10.1007/s41748-020-00167-5
Farid M, Ali S, Akram NA, Rizwan M, Abbas F, Bukhari SAH, Saeed R (2017) Phyto-management of Cr-contaminated soils by sunflower hybrids: physiological and biochemical response and metal extractability under Cr stress. Environ Sci Pollut Res 24:16845–16859. https://doi.org/10.1007/s11356-017-9247-3
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99(3):259–278. https://doi.org/10.1016/S0168-1656(02)00218-3
Freeman DJ, Falkiner FR, Keane CT (1989) New method for detecting slime production by coagulase negative staphylococci. J Clin Path 42(8):872–874. https://doi.org/10.1136/jcp.42.8.872
Goswami D, Vaghela H, Parmar S, Dhandhukia P, Thakker JN (2013) Plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. J Plant Interact 8(4):281–290. https://doi.org/10.1080/17429145.2013.768360
Halstead RL, Finn BJ, MacLean AJ (1969) Extractability of nickel added to soils and its concentration in plants. Can J Soil Sci 49(3):335–342. https://doi.org/10.4141/cjss69-046
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Alyemeni MN, Ahmad P (2018) Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S Afr J Bot 119:1–10. https://doi.org/10.1016/j.sajb.2018.08.003
Hoque M, Hannan A, Imran S, Paul NC, Mondal M, Sadhin M, Rahman M, Bristi JM, Dola FS, Hanif M, Ye W, Brestic M, Rhaman MS (2022) Plant growth-promoting rhizobacteria-mediated adaptive responses of plants under salinity stress. J Plant Growth Regul 28:1–20. https://doi.org/10.1007/s00344-022-10633-1
IBM Corp. (2017) IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp. Retrieved from https://hadoop.apache.org
Itusha A, Osborne WJ, Vaithilingam M (2019) Enhanced uptake of Cd by biofilm forming Cd resistant plant growth promoting bacteria bioaugmented to the rhizosphere of Vetiveria zizanioides. Int J Phytoreme 21(5):487–495. https://doi.org/10.1080/15226514.2018.1537245
Jeyasundar PGSA, Ali A, Azeem M, Li Y, Guo D, Sikdar A, Zhang Z (2021) Green remediation of toxic metals contaminated mining soil using bacterial consortium and Brassica juncea. Environ Pollut 277:116789. https://doi.org/10.1016/j.envpol.2021.116789
Jiang CY, Sheng XF, Qian M, Wang QY (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72(2):157–164. https://doi.org/10.1016/j.chemosphere.2008.02.006
Kaur G, Asthir BJBP (2015) Proline: a key player in plant abiotic stress tolerance. Biol Plant 59:609–619. https://doi.org/10.1007/s10535-015-0549-3
Kaur R, Bhatti SS, Singh S, Singh J, Singh S (2018) Phytoremediation of heavy metals using cotton plant: a field analysis. Bull Environ Contam Toxicol 101(5):637–643. https://doi.org/10.1007/s00128-018-2472-8
Khanna K, Kohli SK, Ohri P, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ahmad P (2019) Microbial fortification improved photosynthetic efficiency and secondary metabolism in Lycopersicon esculentum plants under Cd stress. Biomolecules 9:581. https://doi.org/10.3390/biom9100581
Kushwaha A, Hans N, Kumar S, Rani R (2018) A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol Environ Saf 147:1035–1045. https://doi.org/10.1016/j.ecoenv.2017.09.049
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Ma Y, Rajkumar M, Rocha I, Oliveira RS, Freitas H (2015) Serpentine bacteria influence metal translocation and bioconcentration of Brassica juncea and Ricinus communis grown in multi-metal polluted soils. Front Plant Sci 5:757. https://doi.org/10.3389/fpls.2014.00757
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918. https://doi.org/10.3389/fpls.2016.00918
Mendoza-Hernández JC, Vázquez-Delgado OR, Castillo-Morales M, Varela-Caselis JL, Santamaría-Juárez JD, Olivares-Xometl O, Pérez-Osorio G (2019) Phytoremediation of mine tailings by Brassica juncea inoculated with plant growth-promoting bacteria. Microbiol Res 228:126308. https://doi.org/10.1016/j.micres.2019.126308
Mishra N, Sundari SK (2017) A `six-step-strategy’ to evaluate competence of plant growth promoting microbial consortia. Curr Sci 113(1):63–70
Ndeddy Aka RJ, Babalola OO (2016) Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes faecalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int J Phytoreme 18(2):200–209. https://doi.org/10.1080/15226514.2015.1073671
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Prajapati K, Prajapati M, Panchal R, Sharma S, Saraf MS (2022) Application of microorganisms for bioremediation of heavy metals. Acta Sci Environ Sci 1(1):01–09
Pramanik K, Mitra S, Sarkar A, Soren T, Maiti TK (2018) Characterization of a Cd2+-resistant plant growth promoting rhizobacterium (Enterobacter sp.) and its effects on rice seedling growth promotion under Cd2+-stress in vitro. Agric Nat Res 52(3):215–221. https://doi.org/10.1016/j.anres.2018.09.007
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71(5):834–842. https://doi.org/10.1016/j.chemosphere.2007.11.038
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574. https://doi.org/10.1016/j.biotechadv.2012.04.011
Reddy KR, Chinthamreddy S (2000) Comparison of extractants for removing heavy metals from contaminated clayey soils. J Soil Contam 9(5):449–462. https://doi.org/10.1080/10588330091134347
Rochlani A, Dalwani A, Shaikh NB, Shaikh N, Sharma S, Saraf MS (2022) Plant growth promoting rhizobacteria as biofertilizers: application in agricultural sustainability. Acta Sci Microbiol 5(4):12–21. https://doi.org/10.31080/ASMI.2022.05.1028
Rostami S, Azhdarpoor A (2019) The application of plant growth regulators to improve phytoremediation of contaminated soils: a review. Chemosphere 220:818–827. https://doi.org/10.1016/j.chemosphere.2018.12.203
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Saraf M, Sharma S, Thakkar A (2017) Production and optimization of siderophore from plant growth promoting Rhizobacteria. Braz J Microbiol 43:639–648
Schwyn B, Neilands JB (1987) Siderophores from agronomically important species of the Rhizobiacae. Comm Agric Food Chem 1(2):95–114
Shah RK and Saraf M (2019) Exploring inorganic phosphate solubilizing trait of halotolerant rhizobacteria isolated from Cuminum cyminum. Int J Res Advent Techno ISSN: 2321- 9637. http://ijrat.org/downloads/Vol-7/may-2019/752019107.pdf
Shaikh NB, Shaikh N, Rochlani A, Dalwani A, Sharma S, Saraf MS (2022) Rhizobacteria that promote plant growth and their impact on root system architecture, root development, and function. Acta Sci Microbiol 5(4):53–62. https://doi.org/10.31080/ASMI.2022.05.1035
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Sharma S, Saraf M (2022) Isolation, screening and biochemical characterizations with multiple traits of heavy metal tolerant rhizobacteria from mining area and landfill site. Adv Biores 13(1):147–156. https://doi.org/10.15515/abr.0976-4585.13.1.147156
Sharma S, Shah RK, Rathod ZR, Jain R, Lucie KM, Saraf M (2020) Isolation of heavy metal tolerant rhizobacteria from Zawar Mines Area, Udaipur, Rajasthan, India. Biosci Biotechnol Res Commu 13(1):233–238. https://doi.org/10.21786/bbrc/13.1specialissue/01
Sharma S, Rathod ZR, Saraf MS (2021) Elucidate the influence of heavy metal on bacterial growth isolated from a mining location and a waste dump: using their inducible mechanism. Curr Trends Biomedi Eng Biosci 20(2):001–006. https://doi.org/10.19080/CTBEB.2021.20.556034
Sharma S, Rathod ZR, Saraf MS (2022) Exploring the biotic stress tolerance potential of heavy metal tolerate rhizobacteria isolated from mines area and landfill site. Acta Sci Microbiol 5(2):31–37. https://doi.org/10.31080/ASMI.2022.05.1000
Sharma P, Bakshi P, Kaur R, Sharma A, Bhardwaj R, El-Sheikh MA, Ahmad P (2023a) Inoculation of plant-growth-promoting rhizobacteria and earthworms in the rhizosphere reinstates photosynthetic attributes and secondary metabolites in Brassica juncea L. under chromium toxicity. Plant Soil 483:573–587. https://doi.org/10.1007/s11104-022-05765-y
Sharma S, Rathod ZR, Jain R, Goswami D, Saraf M (2023b) Strategies to evaluate microbial consortia for mitigating abiotic stress in plants. Sustain Agrobiol Des Dev Microb Consort 43:177–203. https://doi.org/10.1007/978-981-19-9570-5_9
Sharma I (2020) Bioremediation techniques for polluted environment: concept, advantages, limitations, and prospects. In: Trace metals in the environment-new approaches and recent advances. IntechOpen pp. 221–235
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enolo Viticult 16(3):144–158
Stepanović S, Vuković D, Hola V, Bonaventura GD, Djukić S, Ćirković I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x
Sullivan TS, Gadd GM (2019) Metal bioavailability and the soil microbiome. Adv Agronomy 155:79–120. https://doi.org/10.1016/bs.agron.2019.01.004
Tank N, Saraf M (2009) Enhancement of plant growth and decontamination of nickel-spiked soil using PGPR. J Basic Microbiol 49(2):195–204. https://doi.org/10.1002/jobm.200800090
Wang Q, Shaheen SM, Jiang Y, Li R, Slaný M, Abdelrahman H, Zhang Z (2021) Fe/Mn-and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J Hazar Mater 403:123628. https://doi.org/10.1016/j.jhazmat.2020.123628
Wang Y, Huang W, Li Y, Yu F, Penttinen P (2022) Isolation, characterization, and evaluation of a high-siderophore-yielding bacterium from heavy metal–contaminated soil. Environ Sci Pollut Res 29(3):3888–3899. https://doi.org/10.1007/s11356-021-15996-8
Wuana RA, Okieimen FE, Imborvungu JA (2010) Removal of heavy metals from a contaminated soil using organic chelating acids. Int J Environ Sci Tech 7(3):485–496. https://doi.org/10.1007/BF03326158
Ying R, Xia B, Zeng X, Qiu R, Tang Y, Hu Z (2021) Adsorption of cadmium by Brassica juncea (L.) Czern. and Brassica pekinensis (Lour.) rupr in pot experiment. Sustainability 14(1):429. https://doi.org/10.3390/su14010429
Yu S, Teng C, Bai X, Liang J, Song T, Dong L, Qu J (2017) Optimization of siderophore production by Bacillus sp. PZ-1 and its potential enhancement of phytoextraction of Pb from soil. J Microbiol Biotechnol 27(8):1500–1512. https://doi.org/10.4014/jmb.1705.05021
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica. Chemosphere 64(6):991–997. https://doi.org/10.1016/j.chemosphere.2005.12.057
Zainab N, Din BU, Javed MT, Afridi MS, Mukhtar T, Kamran MA, Chaudhary HJ (2020) Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol Biochem 152:90–99. https://doi.org/10.1016/j.plaphy.2020.04.039
Zhang X, Su C, Liu X, Liu Z, Liang X, Zhang Y, Feng Y (2020) Effect of plant-growth-promoting rhizobacteria on phytoremediation efficiency of Scirpus triqueter in pyrene-Ni co-contaminated soils. Chemosphere 241:125027. https://doi.org/10.1016/j.chemosphere.2019.125027
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
I am grateful to my guide for her support and encouragement, as well as the facilities offered by the DST-FIST-sponsored Department of Microbiology and Biotechnology, University School of Sciences, Gujarat University, Gujarat, India.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The writers have no beyond reconciliation circumstances in the arrangement of this material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Saraf, M. Biofilm-forming plant growth-promoting rhizobacterial consortia isolated from mines and dumpsites assist green remediation of toxic metal (Ni and Pb) using Brassica juncea. BIOLOGIA FUTURA 74, 309–325 (2023). https://doi.org/10.1007/s42977-023-00179-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-023-00179-y