Abstract
The development of low-cost and highly efficient electrocatalysts for hydrogen evolution reaction (HER) is critical to the wide-spread applications of water splitting technology. In recent years, many efforts are devoted to exploring HER catalysts with high activity and stability based on non-precious metals. Benefited from the advantages of two-dimensional (2D) materials with unique physicochemical properties along with the single-atom catalysts with high activity, excellent stability and maximized atom utility efficiency, a new category of catalysts with 2D materials confining single atoms have shown great promises as high-performance HER catalysts. In this review, MoS2, as one of the typical 2D materials, doped with various single metal atoms as HER catalysts are fully discussed, including different synthetic strategies, catalytic performances and mechanisms toward HER as well as the major challenges ahead.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the increasing consumption of fossil fuels and environmental pollution, the exploration for clean, renewable, and affordable energy sources is a critical challenge [1,2,3,4]. Hydrogen, as a zero-emission and high-density energy carrier, has attracted increasing attention due to its important role in replacing fossil fuels and solving the environmental issue [5]. At present, hydrogen was mainly produced by using carbon-based resources such as natural gas and coal, which unavoidably cause carbon emissions and the as-produced hydrogen is of low purity [6, 7]. Electrochemical water splitting is an alternative choice for hydrogen production that is advantageous for its high efficiency, non-contaminating nature and high purity of hydrogen products. Hydrogen evolution reaction (HER, 2H+ + 2e− → H2) is the half reaction of electrochemical water splitting and is considered to involve three possible reaction steps [8]. Specifically, the first step is the Volmer step: H+ + e− → Had; the second step is the Heyrovsky step: H+ + Had + e− → H2, or the Tafel step: 2Had → H2. Had represents an absorbed hydrogen atom intermediate, which exists in both cases and thus the free energy of hydrogen adsorption (ΔGH) determines the overall reaction rate [9]. In principle, an optimal HER catalyst should have ΔGH close to zero, which means that the binding energy for hydrogen is neither too strong nor too weak [10,11,12], as shown by the “volcano” plot (Fig. 1) [13, 14]. Currently, Pt is considered to be the state-of-the-art catalyst for HER, while the high cost and limited reserve hamper its wide-spread use, thus calling for alternative catalysts based on earth-abundant elements.

Reproduced with permission from Ref. [13]. Copyright 2007 American Association for the Advancement of Science
“Volcano” plot of the exchange current density (i0) as a function of the density functional theory (DFT)-calculated Gibbs free energy (ΔGH*) of adsorbed atomic hydrogen for MoS2 nanoparticulate and the pure metals.
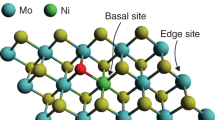
Two-dimensional (2D) materials, such as graphene, carbon nitride (C3N4), and transition metal dichalcogenides (TMDs), are good candidates for electrocatalysis due to their unique physicochemical properties [8]. Among them, molybdenum disulfide (MoS2) has been considered as a promising non-noble HER catalyst with its earth abundance, unique electronic structure and good stability [15]. However, both experimental and theoretical studies have demonstrated that only the edge sites possess high HER activity, while the basal plane of the defect-free MoS2 is catalytically inert [13]. In recent years, great efforts have been devoted to developing MoS2 with abundant active sites and thus enhanced HER activity [5, 16,17,18,19]. Toward this goal, various strategies have been developed, such as creating more edge planes [11,12,13,14], hetero-structuring [20, 21], S vacancies as surface defects [22], strain [23] or incorporation of dopant promoters [24], and phase engineering [25].
In parallel, single-atom catalysts (SACs) have attracted tremendous attentions in recent years for their great potential as highly efficient catalysts with high activity, high selectivity, high stability and maximum atom utilization efficiency [26,27,28,29,30]. SACs are typically stabilized by loading them on certain substrates, which not only serve for stabilizing function but could also provide strong metal-support interaction to tune the reactivity of the single atoms [31,32,33,34]. On the other hand, 2D materials could be an ideal support for SACs due to their unique geometric, physical, chemical and electronic properties. For example, the open structure of 2D materials allows the reactants to access the catalytic sites on both sides so that a maximized exposure of the single metal atoms and thus enhanced catalytic performances can be achieved; the geometric and electronic structure of the metal atoms embedded in 2D materials with atomic thickness can be characterized by advanced spectroscopic and imaging techniques; the metal atoms on 2D materials are more likely to be coordinatively unsaturated compared to bulk materials and, therefore, could lead to a better catalytic performance. In particular, SACs confined in 2D materials such as graphene and C3N4 have shown excellent catalytic properties toward HER with several advantageous features, including rich active sites, high intrinsic catalytic activity, well-defined catalytic centers allowing for performing theoretical calculations regarding the catalytic process or in situ characterization on catalytic processes [35]. As for SACs supported on MoS2, the support has different phases (e.g., metallic 1T phase with octahedral coordinated crystal structure and semiconducting 2H phase with trigonal prismatic coordinated crystal structure) and can provide different types of anchoring sites (Mo or S sites) for the metal atoms, thus offering degrees of freedom to tune the composition, structure and catalytic performance of MoS2 supported SACs. Also, MoS2 by itself is a good HER catalyst and metal atoms can be regarded as the promotor to further enhance the activity of MoS2. Furthermore, MoS2 with the semiconducting feature may allow SACs supported on MoS2 to be used for both electrocatalysis and photocatalysis.
In this review, we summarize recent progress on HER catalysts based on the single-atom (SA) doped MoS2. Various synthesis methods of MoS2 doped with different single metal atoms, including noble metals such as Pt and Pd and non-noble metals such as Co, Ni and W, are summarized and their applications as HER catalysts are discussed.
2 Synthesis
The successful synthesis of single metal atom-doped MoS2 is the prerequisite for exploring their structure and catalytic behaviors. However, it is still a big challenge in the controllable doping and precise anchoring of single atoms into the lattices of MoS2, mainly due to the mobility of single atoms on the surface of MoS2 and the aggregation tendency of metal atoms during the synthesis and post-treatments. Here, different synthetic strategies for single metal atom-doped MoS2 are summarized and discussed (Fig. 2a–f [36,37,38,39,40,41]).
Synthetic strategies. a Synthesis illustration of the SA-Ru MoS2 electrocatalysts. Reproduced with permission from Ref. [36]. Copyright 2019 Wiley-VCH. b Synthesis of single-layered MoS2 and single transition-metal atom doped MoS2. Reproduced with permission from Ref. [37]. Copyright 2018 The Royal Society of Chemistry (RSC). c Fabrication of Co-in-MoS2 catalyst on carbon paper. Reproduced with permission from Ref. [38]. Copyright 2020 Wiley-VCH. d Synthesis of metal SAs on a cogenetic 2D monolayer by cold hydrogen plasma reduction. Reproduced with permission from Ref. [39]. Copyright 2020 American Chemical Society (ACS). e Synthetic method for SA Co-D 1T MoS2. Reproduced with permission from Ref. [40]. Copyright 2019 Nature Publishing Group. f SA electrochemical deposition on the MoS2/GP electrode. Reproduced with permission from Ref. [41]. Copyright 2019 ACS
2.1 Wet chemical impregnation
For the impregnation strategy, it is a fast and inexpensive method for the preparation of supported catalysts, typically including three steps [42]: (1) immerse the supports into the impregnation solution, (2) dry out the mixed solution and (3) activate the catalysts by following heating treatment. An appropriate support with large surface area and good stability and the proper control of the operating conditions such as temperature and precursor concentrations are important for the formation of the final catalysts with desired composition and structure.
Recently, this method has been applied in the preparation of several SA-doped MoS2 catalysts. For example, Zhang et al. [36] reported an SA-Ru doped MoS2 electrocatalyst that was formed using a one-step impregnation method. As shown in Fig. 2a, the MoS2 ultrathin nanosheets were used as the support and the RuCl3 solution was selected as the impregnation solution. By adjusting the volume and the concentration of RuCl3 solution, a series of SA-Ru doped MoS2 catalysts with the loading amounts of Ru ranging from 0.2 to 7.5 wt% were achieved. The resulted 5.0 wt% SA-Ru doped MoS2 sample exhibited the best HER performance. By applying the same synthesis strategy, SA-Ni doped MoS2 was reported by using a hydrothermal synthesized MoS2/carbon cloth as the support and NiCl2 in ethanol as the impregnation solution [43]. The loading amount of Ni atoms on supported MoS2 was about 1.8 at%, determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). According to their study, the annealing temperature and duration during synthesis were important for the type of Ni structures formed on the supports. SA-Ni was anchored on MoS2 composites when the annealing was performed at 300 °C for 1 h under the 10% H2/Ar atmosphere, while Ni clusters were found if the annealing condition changed to 600 °C for 2 h. Besides, by using MoS2/carbon matrix as the support and Ni(NO3)2 as the impregnation solution, SA-Ni atoms anchored on the basal plane of hierarchical MoS2 nanosheets were reported by Zhang et al. [44]. Similarly, the annealing process was suggested to be essential for the interaction between the decorated Ni atoms and the in-plane S atoms. Instead of annealing the composite at relatively low temperature, 600 °C was applied by the authors to strengthen the interaction between Ni and S atoms as well as to increase the crystallinity of MoS2. Furthermore, SA-Pd doped MoS2 and SA-Pt doped MoS2 were also reported [45, 46]. For SA-Pd doped MoS2, it was achieved by impregnating MoS2 nanosheets with an aqueous solution of Pd(OAc)2 and heating at 60 °C for 12 h. Interestingly, the doping of Pd resulted in a phase transition of MoS2 from 2H to 1T, which contributed to the improved catalytic activity. For SA-Pt doped MoS2, K2PtCl6 was applied as the metal precursor and the mass loading of Pt reached 7.5 wt% while still maintaining its atomic dispersion in the MoS2 lattice. What is more, a SA loading amount on MoS2 was reported by Deng et al. [47], where the SA-Co content was as high as 16.7 wt% and might be attributed to the porous nature of the MoS2 foam.
2.2 Hydrothermal/solvothermal method
Hydrothermal or solvothermal method is the most commonly used approach for the preparation of SA doped MoS2 [35]. SA-Pt doped MoS2, which was first synthesized by Deng et al. [48], was prepared via a hydrothermal method. (NH4)6Mo7O24·4H2O, H2PtCl6 and CS2 solution were selected as the Mo, Pt, S precursors, respectively. The solution mixture contained in an autoclave was treated under Ar atmosphere and maintained at 400 °C for 4 h. Few-layered SA-Pt MoS2 was characterized by high-angle annular dark field-scanning transmission electron microscope (HAADF-STEM) and the images revealed that the single Pt atoms occupied the positions of the Mo atoms (Fig. 3a–d). The loading amount of Pt was about 1.7 wt% measured by ICP-OES. For SA-Co doped MoS2, it was prepared by using the same Mo precursor, but with CH4N2S as the S source and Co(NO3)2·6H2O as the Co source [49]. A uniform distribution of Co over the MoS2−x lattice was observed from the energy-dispersive X-ray spectroscopy (EDS) mapping (Fig. 3e), and the concentration of Co atoms was determined to be around 6 wt%. In addition to the substitution of Mo sites by Co atoms, the replacement of the in-plane S atoms in MoS2 was also reported. For example, Liu et al. [50] prepared single Co atom-doped MoS2 catalysts through a hydrothermal treatment with Co(thiourea)42+ complexes and few-layer MoS2 prepared from Li-assisted exfoliation as precursors. A very low concentration of the Co complex was used to prevent the Co atom from self-nucleation. It was suggested that the thiourea-Co complex can refill some of the surface sulfur vacancy sites of MoS2 and the single Co atoms were mainly anchored on the S sites of MoS2, which were demonstrated by extended X-ray absorption fine structure (EXAFS) and HAADF-STEM mages (Fig. 3f). The similar synthesis strategy has been applied by the same group to other transition metals, including Fe, Ni, Pd and Ag (Fig. 2b) [37]. For SA-Ni doped MoS2, the modification of the interlayer spacing of MoS2 introduced by Ni atoms was found by other groups [51]. By using Na2MoO4·2H2O, NiSO4·6H2O, and L-cysteine as precursors, SA-Ni MoS2 was formed under 200 °C for 24 h inside the autoclaves. According to their study, the interlayer spacing was increased from 0.66 nm of the pristine MoS2 to 0.95 nm of 1.0Ni-MoS2, indicating that Ni doping leads to the expansion of the interlayer spacing of MoS2. A similar phenomenon was also observed in the SA-W doped MoS2 nanosheets [52]. MoCl5, WCl6 and CH3CSNH2 were used as precursors and by adjusting the growth parameters Mo0.75W0.25S2 nanosheets with an interlayer spacing as large as 1.035 nm were synthesized. The combined effects of heteroatoms and NH4+ intercalation were found to be responsible for the larger spacing. In another study, a porous 1T MoS2 that was integrated with the atomic doping of Cu atoms was reported [53]. MoO3 was used as the Mo precursor, Cu(NO3)2 as doping agent and N,N-dimethylformamide (DMF) as solvent to obtain SA-Cu MoS2 via the solvothermal method. The composite was composed of flowerlike 2D nanosheets, and the optimal sample had a Cu content of 2.21 wt%. Very recently, single Fe atoms confined in MoS2 were prepared by using FeSO4·7H2O, Na2MoO4·2H2O, and L-cysteine (C3H7NO2S) as Fe, Mo, S sources [54]. The thermal decomposition of C3H7NO2S could release H2S that reduced MoO42− to MoS2, and the collision between Fe2+ and S of MoS2 may allow the incorporation of Fe atoms into MoS2. More importantly, the atomic ratio of Fe to Mo can be easily tuned by adjusting the ratio of each precursor during the hydrothermal synthesis.
a TEM image of Pt-MoS2. b, c HAADF-STEM images of Pt-MoS2 showing that the single Pt atoms (marked by red circles in b) uniformly disperse in the 2D MoS2 plane. d Magnified domain with the red dashed rectangle in c. Reproduced with permission from Ref. [48]. Copyright 2015 RSC. e EDS mapping of elements in Co-doped MoS2−x. Reproduced with permission from Ref. [49]. Copyright 2019 ACS. f EXAFS spectra of Co-doped single-layer molybdenum disulfide (Co-SMoS2), Co(thiourea)4 and Co foil and HAADF-STEM image of Co-SMoS2 The scale bar is 0.5 nm. Reproduced with permission from Ref. [50]. Copyright 2017 Nature Publishing Group
2.3 Post-treatment synthesis
For the substitution of the Mo sites of MoS2 by metal dopants, thermal annealing has been frequently applied. For example, a hybrid Pt-MoS2 system was achieved by annealing a CVD-grown monolayer MoS2 together with H2PtCl6 under an Ar gas flow at 350 °C [55]. By using the same Pt precursor of H2PtCl6, Guan et al. [56] prepared SA-Pt doped MoS2 composite by thermal treatment with the condition of 400 °C for 200 min under Ar atmosphere, and the loading of Pt atoms was as high as 1.8 at%. Besides Pt, SA-Co doped MoS2 was developed through thermal annealing treatment [38]. MoS2 nanosheets were first grown on hydrophilic carbon paper via a low-temperature hydrothermal reaction; then CoPc was encapsulated into MoS2 layers by an electrochemical co-interaction to expand the interlayer spacing for the diffusional intercalation of CoPc, and finally the CoPc was converted into Co by thermal annealing in Ar at 600 °C (Fig. 2c). STEM images and EXAFS spectrums (Fig. 4a-c) confirmed the uniform distribution of individual Co atoms in-between MoS2 layers. An increased interlayer spacing from 0.6 to 1.1 nm was confirmed by X-ray diffraction (XRD) patterns (Fig. 4d).
a Atomic‐resolution STEM‐HAADF image of Co-in-MoS2. The scale bar is 1 nm. b Co K-edge XANES spectra and c Fourier transform of the EXAFS (FT‐EXAFS) spectra of various catalysts. Dotted lines represent the fitting of EXAFS spectra. d XRD patterns of various catalysts. Reproduced with permission from Ref. [38]. Copyright 2020 Wiley-VCH. e SA-Pt distribution on MoS2, with red circles (Pt on Mo-vacancy) and yellow circles (Pt on S-vacancy). The surface doping level is ~ 1.1 at%. The scale bar is 2 nm. f Atomic resolution image of Pt-MoS2 with extended simulated atomic structures. The scale bar is 0.5 nm. g HAADF-STEM image of Au-2H-MoS2. The scale bar is 1 nm. Red circles represent Au on Mo-vacancy and yellow circles represent Au on S-vacancy. h HAADF-STEM image of Pd-2H-MoS2. The scale bar is 1 nm. Similarly, red circles represent Pd on Mo-vacancy, yellow circles represent Pd on S-vacancy and purple circles represent mobile Pd atoms. Reproduced with permission from Ref. [41]. Copyright 2019 ACS
In addition to thermal annealing, plasma treatment is another effective way for introducing single atoms, which shows a strong reducibility and the ability to dissociate chemical bonds between metal cations and anions at a low temperature in a metal complex to generate metal single atoms. For example, Luo et al. [39] reported the synthesis of single metal atoms confined in MoS2 monolayers by a cold hydrogen plasma reduction method (Fig. 2d). By controlling the temperature, pressure, time and radio frequency power of hydrogen plasma, SA-Mo doped MoS2 was formed, and the loading amounts of single atoms was 2.2 wt%. Interestingly, when Ar plasma was applied instead of hydrogen plasma, a decreased degree of Mo reduction was found, which indicated that hydrogen was essential for the dissociation of Mo–S bonds.
2.4 Other synthetic strategies
With the fast development of SA-doped MoS2 and other TMD catalysts, some other intelligently designed synthesis strategies have been reported. For example, sonication was reported to dope single-layer 1T MoS2 with various transition metal precursors [57]. The chemically exfoliated 1T MoS2 and H2PtCl4 were used as precursors, and SA-Pt doped MoS2 was formed after a 24-h sonication treatment. Due to the strong adsorption of Pt species, the isolated Pt atoms with ~ 3 wt% could be chemically attached to the S sites of 1T MoS2. The authors also prepared five different transition metal elements as dopant atoms on 1T MoS2 with the same sonication method, including Fe, Ni, Co, Pt and Pd. With the same assistance of sonication, Qi et al. [40] achieved an interface catalyst consisting of atomic cobalt arrays covalently bound to distorted 1T MoS2 nanosheets. As can be seen in Fig. 2e, MoS2 nanosheets were first synthesized by a standard solvothermal method, and Co nano-disks were synthesized by a standard air-free procedure. Then the sonication at 4 °C for 24 h was applied for the assembly of Co nano-disks on MoS2. Finally, the SA-Co doped 1T MoS2 was achieved by an electrochemical leaching between 0.1 and − 0.4 V for 50 cycles. By assembly/leaching process, the SA-Co doped 1T MoS2 catalysts were formed. The amount of Co loading was highly dependent on the sonication power, in which the phonons at ultrasonic power may help to closely contact Co nano-disks with MoS2 nanosheets and provide the energy for the Co–S bond formation through ultrasonic activation. The Co mass loading amount of SA-Co doped 1T MoS2 was about 3.54 wt% and the single Co atoms were uniformly bound to the top of Mo atoms on the MoS2 slab according to both the HAADF-STEM image and EELS spectrums. In addition, using the same strategy, the authors successfully prepared SA-Ni and SA-Fe doped MoS2 catalysts, indicating that this method is universal and can be extended to other 2D metals or metal oxides.
Furthermore, electroplating method was another strategy that had been applied for the synthesis of SA-doped MoS2. This atomic level electroplating method was first achieved by Xuan et al. [41]. In a three-electrode electrochemical cell, the graphite paper with full coverage of MoS2 worked as the counter electrode, a Pt foil as the working electrode, and mercurous sulfate works as the reference electrode. By the chronoamperometric method, Pt atoms migrated in the form of Pt(II) when the working electrode voltage was above 1.1 V vs reference hydrogen electrode (RHE), and then the Pt species moved toward the counter electrode and anchored on MoS2, as shown in Fig. 2f. The Pt doping level at the surface of MoS2 was around 1.1 at%. Based on the HAADF-STEM intensity analysis, Pt atoms can be found both in Mo vacancy and S vacancy (Fig. 4e, f). In addition to Pt, Au atom and Pd atom doping on MoS2 were also realized and the concentration of the doped atoms can be adjusted by the deposition time and the electrode voltage. What is more, it was found that the ratio of the Au atoms on Mo vacancy to S vacancy is about 2, and its behavior was similar to Pt. However, the Pd atoms on 2H MoS2 behave differently with Pt and Au. Rather than existing as fixed Pd atoms anchored on the MoS2 surface, many Pd atoms were directly captured by the electron beam (Fig. 4g, h).
3 Electrocatalytic application toward hydrogen evolution reaction
3.1 Single precious metal dopants in MoS2
The substitution of Mo by Pt atoms of MoS2 was first achieved by Deng et al. [48]. Based on the HER measurement, SA-Pt doped MoS2 showed a reduced overpotential of about 60 mV relative to few-layer MoS2 at a current density of 10 mA·cm−2 and exhibited high stability after 5000 cycles between − 0.31 and 0.57 V at a current density of 1 mA·cm−2 (Fig. 5a, b). The enhanced HER activity was attributed to the fact that the substitution of Pt to Mo atom triggered the in-plane S of the inert basal plane to be catalytically active. According to the DFT calculation, the adsorption-free energy of H (\(\Delta {G}_{\text{H}}^{0}\)) on the in-plane S of singe Pt atom doped MoS2 was close to 0 eV, while the edge S of pure MoS2 was about 0.1 eV (Fig. 5c), which indicated that the in-plane S with SA-Pt doped MoS2 can possess higher HER activity than the edge S of pure MoS2 and matched well with the experimental results. Furthermore, the electronic properties and chemical origins of HER performance on the SA-Pt doped MoS2 was also calculated. According to the density of states (DOS) calculation in Fig. 5d, part of electronic states was saturated and disappeared above the Fermi level when an H atom was absorbed on the S atom nearby doped Pt atoms of Pt-MoS2. S atoms in the Pt-MoS2 also showed the corresponding changes. The electronic state of in-plane S sites below the Fermi level was increased, and it was comparable with the edge S atoms of pure MoS2. Thus, the enhanced H adsorption ability of in-plane S atoms of Pt-MoS2 can be attributed to the introduction of Pt atoms and caused by the increased electronic states around the Fermi level. Furthermore, the high durability of Pt-MoS2 also can be explained by the good stability of doping configuration of Pt atoms in MoS2. Detailed structure analysis of SA-Pt confined in MoS2 was also explored by another group [55]. Interestingly, a sequence of ADF-STEM images showed that Pt atoms preferred to bind to S vacancies rather than to substitute Mo sites (Fig. 6a–h). Their DFT calculations indicated that metal atoms were prone to be trapped by S vacancies, which had a detrimental effect on the catalytic activity for HER and needed to be avoided. However, Pt atoms that located in Mo vacancy as the primary Pt species were recently reported by Xuan et al. [41], and a systematic study on HER activity of SA-Pt doped MoS2 was performed. By an electroplating method, a precisely controlled Pt atoms (~ 1.1 at%) anchored on pure MoS2 was achieved. The SA-Pt doped MoS2 not only exhibited a lower overpotential than pure MoS2, but also much better stability and extraordinary CO tolerance. The electronic structure of the doped MoS2 was studied by Kelvin probe force microscope and a decreased work function of MoS2 was detected (from 4.635 to 4.610 eV) (Fig. 6i–k), which could be attributed to the extra electrons donated from Pt atoms that could benefit the HER activity. What is more, the good CO tolerance of Pt doped MoS2 further demonstrated that Pt substituted Mo rather than S. According to the DFT study, the Pt will be poisoned by CO if Pt substituted the S site due to the strong binding between CO and Pt (1.50 eV), while Pt will be “buried” under the S layer if substituting Mo and HER performance will not be influenced by the CO tolerance measurement, which matched well with the experimental measurement. SA-Au and SA-Pd doped MoS2 as HER catalysts have also been explored by the authors and enhanced HER activity was observed, consistent with other reports [58, 59]. Furthermore, other precious metal atoms were doped into the lattices of MoS2 for HER application and showed an enhanced performance, including Pd [57], Rh [59] and Ru [36].

Reproduced with permission from Ref. [48]. Copyright 2015 RSC
a HER polarization curves (current density versus potential) for Pt-MoS2 in comparison with blank glassy carbon (GC) electrode, bulk MoS2, FL-MoS2, and 40% Pt/C. b Comparison of the polarization curves (current density versus potential) of Pt-MoS2 before and after cycling test. c Calculated hydrogen adsorption free energy on S and Pt atoms of different sites. d HER process on a Pt-MoS2 catalyst. The top views are shown in the insets.
a–f Sequence of ADF-STEM images (false color LUT “fire”, color LUT “fire” is used to highlight the contrast associated with the Pt atom.) capturing the movement of a single Pt atom on MoS2. White circles indicate the prior location. g Magnified ADF-STEM image of the Pt atom in a, showing the Pt sitting in the S site location and the presence of a S vacancy. h Schematic illustration of Pt migration on a MoS2 surface. Reproduced with permission from Ref. [55]. Copyright 2017 ACS. i, j Surface potential difference before and after the single-atom Pt deposition on MoS2. k Band diagram evolution before and after the single-atom Pt deposition on MoS2. Reproduced with permission from Ref. [41]. Copyright 2019 ACS
3.2 Single Co dopants in MoS2
In addition to precious metal dopants, non-precious Co dopant was also predicted and demonstrated as a good candidate for tuning the catalytic activity of MoS2 [40, 43, 57, 60, 61]. SA-Co doped mesoporous MoS2 foam prepared via an impregnation procedure was reported by Deng et al. [47]. The substitution of Co atoms to Mo sites in the MoS2 matrix can enhance the intrinsic activity of in-plan S atoms for electrocatalysis [48]. More importantly, the relationship between HER activity and Co atom doping level was thoroughly explored. According to the electrocatalytic measurements, the mesoporous MoS2 foam with an optimal doping content of 16.7 wt% Co atoms showed the highest HER activity with an overpotential of 156 mV at a current density of 10 mA·cm−2 and superior stability (Fig. 7a, b). DFT studies indicate that a very high Co content caused the excessive decrease of electron on S atoms and led to the strong interaction between H atoms and S atoms, while a moderate Co atom doping level could adjust the hydrogen adsorption energy on MoS2 to an optimal value and maintained its good stability to enhance the HER performance to reach an optimum value. Besides, SA-Co doped on monolayer MoS2 was studied, which could benefit the fundamental mechanism study of SA-doped MoS2 for HER [37]. Among the HER performances on Co-doped monolayered, few layered and bulk MoS2 samples, the authors found the activity was dependent on the thickness of MoS2 that the monolayer MoS2 exhibited the lowest onset potential of 220 mV at a current density of 10 mA·cm−2. The HADDF-STEM images directly showed the bonding sites of Co on the MoS2 basal plane that the single Co atom can be observed both on Mo atop sites and on S vacant sites (Fig. 8a, b). The EXAFS spectrum demonstrated that the majority of the Co atoms were on the Mo atop sites of the MoS2 basal plan and further suggested that the Co dopants could influence the neighbored S sites by a Co–S interaction (Fig. 8c), which was predicted as the dominant contribution for the enhancement of HER activity by DFT calculation. In another study, a phase transition from 2H to 1T MoS2 was observed after the SA-Co doping and a Pt-like activity toward HER was achieved [40]. The atomic dispersion of Co and the phase transition of MoS2 could be observed by HAADF-STEM, and EXAFS spectrum further indicated the Co single atom was located right on the top of Mo atom instead of the replacement of Mo and the contact layer Co was covalently bonded with S atom on the surface of MoS2 (Fig. 8d–i). DFT calculation was performed to understand the mechanism of the phase transition of MoS2 after Co doping and indicated that the phase transition was driven by the external force. The strain induced by the lattice mismatch between metallic Co and pristine 2H MoS2 was identified as the major cause for 2H to 1T phase transition (Fig. 8j–l). Notably, besides the phase transition, the ensemble effect by the synergy of Co adatom and S of MoS2 by tuning hydrogen binding mode at the interface could also lead to enhanced HER activity. According to the HER measurement, the SA-Co doped 1T MoS2 catalyst exhibited a low overpotential of 42 mV at a current density of 10 mA·cm−2, low Tafel slope of 32 mV·dec−1 and superior long-term stability, which is very close to commercial Pt (Fig. 9).

Reproduced with permission from Ref. [47]. Copyright 2017 Nature Publishing Group
a HER polarization curves (current densities versus potential) for mesoporous Co-doped MoS2 foam (mPF-Co-MoS2) with different Co-doping contents in comparison with mPF-MoS2 and 40% Pt/C. b Current densities at overpotentials of 150, 200 and 250 mV for mPF-Co-MoS2 with different Co-doping contents compared with mPF-MoS2.

Reproduced with permission from Ref.[40]. Copyright 2019 Nature Publishing Group
a, b HAADF-STEM images of Co-SMoS2. c Fourier transforms of the k3-weighted Co K-edge of extended EXAFS spectra of Co-SMoS2. Reproduced with permission from Ref. [37]. Copyright 2018 RSC. d Aberration-corrected HAADF-STEM image of SA Co doped distorted 1T MoS2 (Co-D 1T MoS2). The scale bar is 1 nm. e Enlarged HAADF-STEM image in the red square area of d. The scale bar is 0.2 nm. f Theoretical model and g simulated STEM images using QSTEM simulation software. h FT-EXAFS spectra of SA Co-D 1T MoS2 and bulk cobalt foil at the Co K-edge. i Co K-edge XANES of SA Co-D 1T MoS2 and fitted curve. The scale bar is 0.2 nm. j 2H and k 1T atomic structures of MoS2 assembled with Co atomic layer calculated by first principles. l Energies of 2H MoS2 and 1T MoS2 assembled with the Co atomic layer as a function of Co–Co distance calculated by the first-principles method.

Reproduced with permission from Ref. [40]. Copyright 2019 Nature Publishing Group
HER performance of SA-Co 1T MoS2. a Polarization curves of different catalysts tested in Ar-saturated 0.5 mol·L−1 H2SO4. b Tafel plots for the catalysts derived from a. c Polarization curves of the SA Co-D 1T MoS2 after 10,000 CV cycles. d Time dependence of the current density for SA Co-D 1T MoS2 at a static overpotential of 100 mV vs. RHE.
3.3 Single Ni dopants in MoS2
Single Ni atom is another non-precious metal dopant that has been explored for the enhancement of doped MoS2 as HER catalysts. However, early studies indicated that Ni was not as an effective metal dopant as Co for HER improvement due to their structure difference [48]. Indeed, SA-Ni doped MoS2 that lowered the HER rate was demonstrated and their structure–activity relationship was explored [37]. The Ni-doped MoS2 nanosheets had a high onset potential of 353 mV at a current density of 10 mA·cm−2 compared to the onset potential of 300 mV of non-doped MoS2. According to the HAADF-STEM images, Ni atoms could occupy both the atop sites and substitute S vacant sites, which was similar to Co dopants (Fig. 10a, b). However, according to the EXAFS study, besides the Ni–S bonding, the Ni–Mo interaction was also found in the Ni-doped MoS2 (Fig. 10c). According to the DFT calculation, the Ni–Mo interaction caused a direct electronic modification of Mo sites and induced the deviation of Gibbs free energy of hydrogen adsorption from zero, leading to the decrease in HER activity. Recently, more studies on the Ni dopant with MoS2 were explored, and those studies indicated that the isolated Ni atoms could form new electronic states to tune the adsorption behavior of H atoms on the coordinated S atoms and consequently adjust the HER performance [43, 44, 62]. In another study, a series of Ni-doped MoS2 with different Ni/Mo ratios were synthesized and the related catalytic activities were evaluated [51]. As shown in Fig. 10d, e, the 1.0 Ni-MoS2 (1.0 means Ni/Mo = 1/1) catalysts exhibited an enhanced HER activity with an overpotential of around 173 mV at a current density of 10 mA·cm−2, while the pure MoS2 showed an overpotential of around 300 mV and 0.5 Ni-MoS2, 2.0 Ni-MoS2 had an overpotential of around 248 mV and 225 mV, respectively. The enhanced HER activity was attributed to the expansion of interlayer spacing and the electronic structure change after Ni doping. According to DFT study, the position shift of Ni atoms and the average offset distance between Ni atoms to the original Mo lattice sites would be increased if the doping ratio was increased, which matched well with the HAADF-STEM observations in Fig. 10g, h. In addition, the amounts of coordinately unsaturated S atoms that were induced by the Ni atom deviation were also increased with the increased amounts of Ni dopants. However, NiS was formed with excessive Ni dopants and thus the solubility of Ni in MoS2 was limited, which explained the decreased catalytic activity of 2.0 Ni-MoS2 in comparison with 1.0 Ni-MoS2.

Reproduced with permission from Ref. [51]. Copyright 2019 RSC
a, b HAADF-STEM images of Ni-SMoS2. c EXAFS spectra of Ni-SMoS2. Reproduced with permission from Ref. [37]. Copyright 2018 RSC. d–f Electrochemical HER measurements of pristine MoS2, Ni-MoS2 and Pt catalysts. d Polarization curves and e corresponding Tafel plots. f Deconvoluted HAADF-STEM image of 1.0 Ni-MoS2 catalysts. The scale bar is 0.5 nm. g Simulated HAADF-STEM image. h Intensity line profiles along the green rectangle in image f and along the red dashed rectangle in image g.
3.4 Other metal dopants in MoS2
He et al. [52] synthesized nanosized, ultrathin MoxW1−xS2 nanosheets by a simple one-step wet-chemistry approach and the Mo/W ratio could be adjusted by the synthetic conditions. The incorporation of SA-W resulted in an increased interlayer spacing and a high metallic phase concentration of about 90%. When tested as HER catalysts in acid, the optimal sample exhibited a low overpotential of about − 155 mV at a current density of 10 mA·cm−2 and a small Tafel slope of 67 mV·dec−1. The enhanced catalytic activity originated from the synergy of metallic conductivity and large exposed active surface area. In another study, Ji et al. [53] synthesized porous 1T MoS2 doped with atomic Cu by a facile solvothermal method. Benefited from the synergistic effects of the metallic phase, single Cu metal doping and abundant vacancies, the SA-Cu doped MoS2 catalysts exhibited considerably high HER performance with an overpotential of 131 mV at a current density of 10 mA·cm−2, a small Tafel slope of 51 mV·dec−1 and long-term stability.
4 Conclusion and perspectives
This is an exciting research area, combining TMDs and SACs together for catalytic applications. While SACs share both merits of homogeneous and heterogeneous catalysts, 2D materials like MoS2 process unique geometric/electronic structure, high surface area and atomic thickness that are attractive for catalysis. This review highlights the state-of-the-art research progress of single metal atom-doped MoS2 toward the technologically important catalytic reaction of HER. This type of catalysts has been successfully synthesized by different synthetic approaches such as the wet impregnation method, hydrothermal/solvothermal method, post-treatment by thermal annealing and plasma, sonication and electroplating,. The metal atom doping could enhance the catalytic properties of MoS2 toward hydrogen evolution to a large extent, resulting from an increased number of active sites, enhanced intrinsic activity, improved surface area, higher electrical conductivity due to phase transition or the combined effects of these factors.
Although single metal atom-doped MoS2 materials as hydrogen evolution electrocatalysts have achieved significant achievements, more efforts are still needed for them to meet practical requirements. First, like other SA catalysts, the metal atom doping amounts are relatively low and it remains a challenge to further increase the loading without forming metal clusters. Careful control on the synthetic conditions and rational design of catalysts with abundant anchoring sites would be important to achieve this goal. Second, the understanding of the correlation between structural characteristics after atom doping and catalytic properties as well as the catalytic mechanisms is still in the infancy stage, and further efforts with atomic-scale ex situ and in situ characterizations would be needed to better establish such correlation. Third, although the application of MoS2 confined with single atoms for HER have been widely studied, there are fewer reports on their application toward other more demanding catalytic processes such as oxygen evolution, oxygen reduction, CO2 reduction and N2 reduction. Fourth, similar to MoS2, other 2D TMDs such as MoSe2 and WS2 have been reported as efficient catalysts in many catalytic processes but rarely studied for the single atom confinement. As these TMD materials have quite different electronic and catalytic properties, it is, therefore, of great interests to explore the applicability of more TMD materials as single-atom supports and study their potential application for hydrogen evolution and other important catalytic processes. Despite the challenges ahead, enormous increases of research efforts have been witnessed in the relevant areas in the past few years. The continued efforts in the synthesis and structural studies will certainly facilitate the development of single metal atom-doped TMD catalysts with high performance towards wide ranges of electrocatalytic and other heterogeneous catalytic processes.
Change history
21 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42864-021-00115-4
References
Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature 2012;488(7411):294
Panwar NL, Kaushik SC, Kothari S. Role of renewable energy sources in environmental protection: A review. Renew Sustain Energy Rev 2011;15(3):1513
Stolley RM, Helm ML. Light-harvesting materials: soft support for energy conversion. Nat Chem 2014;6(11):949
Wang JY, Ouyang T, Li N, Ma T, Liu ZQ. S, N co-doped carbon nanotube-encapsulated core-shelled CoS2@Co nanoparticles: efficient and stable bifunctional catalysts for overall water splitting. Sci Bull 2018;63(17):1130
Benck JD, Hellstern TR, Kibsgaard J, Chakthranont P, Jaramillo TF. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal 2014;4(11):3957
Cai Z, Liu B, Zou X, Cheng HM. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem Rev 2018;118(13):6091
Staszak-Jirkovský J, Malliakas CD, Lopes PP, Danilovic N, Kota SS, Chang KC, Genorio B, Strmcnik D, Stamenkovic VR, Kanatzidis MG, Markovic NM. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat Mater 2016;15(2):197
Kibler LA. Hydrogen electrocatalysis. Chem Phys Chem 2006;7(5):985
Trasatti S. Work function, electronegativity, and electrochemical behaviour of metals. J Electroanal Chem 1972;39(1):163
Hinnemann B, Moses PG, Bonde J, Jorgensen KP, Nielsen JH, Horch S, Chorkendorff I, Nørskov JK. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J Am Chem Soc 2005;127(15):5308
Greeley J, Stephens IEL, Bondarenko S, Johansson TP, Hansen H, Jaramillo TF, Rossmeisl J, Chorkendorff I, Nørskov JK. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat Chem 2009;1(7):552
Greeley J, Jaramillo TF, Bonde J, Chorkendorff IB, Nørskov JK. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater 2006;5(11):909
Jaramillo TF, Jørgensen KP, Bonde J, Nielsen JH, Horch S, Chorkendorff I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007;317(5834):100
Nørskov JK, Bligaard T, Logadottir A, Kitchin JR, Chen JG, Pandelov S, Stimming U. Trends in the exchange current for hydrogen evolution. J Electrochem Soc 2005;152(3):J23
Montoya JH, Seitz LC, Chakthranont P, Vojvodic A, Jaramillo TF, Nørskov JK. Materials for solar fuels and chemicals. Nat Mater 2016;16(1):70
Kibsgaard J, Chen Z, Reinecke BN, Jaramillo TF. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat Mater 2012;11(11):963
Kibsgaard J, Jaramillo TF, Besenbacher F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2- clusters. Nat Chem 2014;6(3):248
Benck JD, Chen Z, Kuritzky LY, Forman AJ, Jaramillo TF. Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: insights into the origin of their catalytic activity. ACS Catal 2012;2:1916
Xie JF, Zhang H, Li S, Wang RX, Sun X, Zhou M, Zhou JF, Lou XW, Xie Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater 2013;25(40):5807
Jaramillo TF, Nayak AK, Pradhan D, Pal A, Pal T. Fabrication of MoS2 decorated reduced graphene oxide sheets from solid Mo-precursor for electrocatalytic hydrogen evolution reaction. Electrochim Acta 2019;313:341
Deng DH, Novoselov KS, Fu Q, Zheng NF, Tian ZQ, Bao XH. Catalysis with two-dimensional materials and their heterostructures. Nat Nanotechnol 2016;11:218
Ye GL, Gong YJ, Lin JH, Li B, He YM, Pantelides ST, Zhou W, Vajtai R, Ajayan PM. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett 2016;16(2):1097
Li H, Tsai C, Koh AL, Cai LL, Contryman AW, Fragapane AH, Zhao J, Han HS, Manoharan HC, Abild-Pedersen F, Nørskov K, Zheng XL. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat Mater 2016;15(1):48
Zhang HY, Tian Y, Zhao JX, Cai QH, Chen ZF. Small dopants make big differences: enhanced electrocatalytic performance of MoS2 monolayer for oxygen reduction reaction (ORR) by N- and P-doping. Electrochim Acta 2017;225:43
Lin YC, Dumcenco DO, Huang YS, Suenaga K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat Nanotechnol 2014;9(5):391
Zhu CZ, Fu SF, Shi QR, Du D, Lin YH. Single-atom electrocatalysts. Angew Chem Int Ed 2017;56(45):13944
Qu YT, Wang LG, Li ZJ, Li P, Zhang QH, Lin Y, Zhou FY, Wang HJ, Yang ZK, Hu YD, Zhu MZ, Zhao XY, Han X, Wang CM, Xu Q, Gu L, Luo J, Zheng LR, Wu YE. Ambient synthesis of single-atom catalysts from bulk metal via trapping of atoms by surface dangling bonds. Adv Mater 2019;31(44):1904496
Zhou SQ, Shang L, Zhao YX, Shi R, Waterhouse GIN, Huang YC, Zheng LR, Zhang TR. Pd single-atom catalysts on nitrogen-doped graphene for the highly selective photothermal hydrogenation of acetylene to ethylene. Adv Mater 2019;31(18):1900509
Yang HZ, Shang L, Zhang QH, Shi R, Waterhouse GIN, Gu L, Zhang TR. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat Commun 2019;10:4585
Wang XQ, Li ZJ, Qu YT, Yuan TW, Wang WY, Wu YE, Li YD. Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chem 2019;5(6):1486
Li X, Lee JK, Lu Y, Gerkman MA, Kengmana ES, Fonseca MV, Warner JH, Han GG. Precursor design for high density single Pt atom sites on MoS2: enhanced stability at elevated temperatures and reduced 3D clustering. Chem Mater 2020;32(6):2541
Chen YJ, Ji SF, Chen C, Peng Q, Wang DS, Li YD. Single-atom catalysts: synthetic strategies and electrochemical applications. Joule 2018;2(7):1242
Qu YT, Li ZJ, Chen WX, Lin Y, Yuan TW, Yang ZK, Zhao CM, Wang J, Zhao C, Wang X, Zhou FY, Zhuang ZB, Wu YE, Li YD. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat Catal 2018;1(10):781
Zhang LL, Wang YJ, Niu ZQ, Chen J. Single atoms on graphene for energy storage and conversion. Small Methods 2019;3(9):1
Wang Y, Mao J, Meng XG, Yu L, Deng DH, Bao XH. Catalysis with two-dimensional materials confining single atoms: concept, design, and applications. Chem Rev 2018;118(13):6019
Zhang JM, Xu XP, Yang L, Cheng DJ, Cao DP. Single-atom Ru doping induced phase transition of MoS2 and S vacancy for hydrogen evolution reaction. Small Methods 2019;3(12):1900653
Lau THM, Lu XW, Kulhavý J, Wu SS, Lu LL, Wu TS, Kato RC, Foord JS, Soo YL, Suenaga K, Tsang SCE. Transition metal atom doping of the basal plane of MoS2 monolayer nanosheets for electrochemical hydrogen evolution. Chem Sci 2018;9(21):4769
Chen ZX, Liu CB, Liu J, Li J, Xi SB, Chi X, Xu HS, Park IH, Peng XW, Li X, Yu W, Liu XW, Zhong LX, Leng K, Huang W, Koh MJ, Loh KP. Cobalt single-atom-intercalated molybdenum disulfide for sulfide oxidation with exceptional chemoselectivity. Adv Mater 2020;32(4):1906437
Luo YT, Zhang SQ, Pan HY, Xiao SJ, Guo ZL, Tang L, Khan U, Ding BF, Li M, Cai ZY, Zhao Y, Lv W, Feng QL, Zou XL, Lin JH, Cheng HM, Liu BL. Unsaturated single atoms on monolayer transition metal dichalcogenides for ultrafast hydrogen evolution. ACS Nano 2020;14(1):767
Qi K, Cui XQ, Gu L, Yu SS, Fan XF, Luo MC, Xu S, Li NB, Zheng LR, Zhang QH, Ma JY, Gong Y, Lv F, Wang K, Huang HH, Zhang W, Guo SJ, Zheng WT, Liu P. Single-atom cobalt array bound to distorted 1T MoS2 with ensemble effect for hydrogen evolution catalysis. Nat Commun 2019;10(1):5231
Xuan NN, Chen JH, Shi JJ, Yue YW, Zhuang PY, Ba K, Sun YY, Shen JF, Liu YY, Ge BH, Sun ZZ. Single-atom electroplating on two dimensional materials. Chem Mater 2019;31(2):429
Perego C, Villa P. Catalyst preparation methods. Catal Today 1997;34(3–4):281
Wang Q, Zhao ZL, Dong S, He DS, Lawrence MJ, Han SB, Cai C, Xiang SH, Rodriguez P, Xiang B, Wang ZG, Liang YY, Gu M. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018;53:458
Zhang HB, Yu L, Chen T, Zhou W, Lou XW. Surface modulation of hierarchical MoS2 nanosheets by Ni single atoms for enhanced electrocatalytic hydrogen evolution. Adv Funct Mater 2018;28(51):1807086
Luo ZY, Ouyang YX, Zhang H, Xiao ML, Ge JJ, Jiang Z, Wang JL, Tang DM, Cao XZ, Liu CP, Xing W. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat Commun 2018;9(1):1
Li HL, Wang LB, Dai YZ, Pu ZT, Lao ZH, Chen YW, Wang ML, Zheng XS, Zhu JF, Zhang WH, Si R, Ma C, Zeng J. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat Nano 2018;13(5):411
Deng J, Li H, Wang SH, Ding D, Chen MS, Liu C, Tian ZQ, Novoselov KS, Ma C, Deng DH, Bao XH. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nat Commun 2017;8(1):14430
Deng J, Li HB, Xiao JP, Tu YC, Deng DH, Yang HX, Tian HF, Li JQ, Ren PJ, Bao XH. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ Sci 2015;8(5):1594
Zhang J, Tian XY, Liu MJ, Guo H, Zhou JD, Fang QY, Liu Z, Wu Q, Lou J. Cobalt-modulated molybdenum-dinitrogen interaction in MoS2 for catalyzing ammonia synthesis. J Am Chem Soc 2019;141(49):19269
Liu GL, Robertson AW, Li MMJ, Kuo WCH, Darby MT, Muhieddine MH, Lin YC, Suenaga K, Stamatakis M, Warner JH, Tsang SCE. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat Chem 2017;9(8):810
Luo RC, Luo M, Wang ZQ, Liu P, Song SX, Wang XD, Chen MW. The atomic origin of nickel-doping-induced catalytic enhancement in MoS2 for electrochemical hydrogen production. Nanoscale 2019;11(15):7123
He Q, Wan YY, Jiang HL, Wu CQ, Sun ZT, Chen SM, Zhou Y, Chen HP, Liu DB, Haleem YA, Ge BH, Wu XJ, Song L. High-metallic-phase-concentration Mo1–xWxS2 nanosheets with expanded interlayers as efficient electrocatalysts. Nano Res 2018;11(3):1687
Ji L, Yan PF, Zhu CH, Ma CY, Wu WZ, Wei C, Shen YL, Chu SQ, Wang JO, Du Y, Chen J, Yang XN, Xu Q. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction. Appl Catal B Environ 2019;251:87
Huang LZ, Wei XL, Gao EL, Zhang CB, Hu XM, Chen YQ, Liu ZZ, Finck N, Lützenkirchen J, Dionysiou DD. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: a biomimetic approach towards efficient radical generation. Appl Catal B Environ 2019;268:118459
Li HS, Wang SS, Sawada H, Han GGD, Samuels T, Allen CS, Kirkland AI, Grossman JC, Warner JH. Atomic structure and dynamics of single platinum atom interactions with monolayer MoS2. ACS Nano 2017;11(3):3392
Guan YX, Feng YY, Wan J, Yang XH, Fang L, Gu X, Liu RR, Huang ZY, Li J, Luo J, Li CM, Wang Y. Hydrogen evolution reaction: ganoderma-like MoS2/NiS2 with single platinum atoms doping as an efficient and stable hydrogen evolution reaction catalyst. Small 2018;14(27):1870125
Lau THM, Wu S, Kato R, Wu TS, Kulhavý J, Mo JY, Zheng JW, Foord JS, Soo YL, Suenaga K, Darby MT, Tsang SCE. Engineering monolayer 1T-MoS2 into a bifunctional electrocatalyst via sonochemical doping of isolated transition metal atoms. ACS Catal 2019;9(8):7527
Zhang L, Liu HS, Liu SH, Norouzi Banis M, Song ZX, Li JJ, Yang LJ, Markiewicz M, Zhao Y, Li RY, Zheng M, Ye SY, Zhao ZJ, Botton GA, Sun XL. Pt/Pd single-atom alloys as highly active electrochemical catalysts and the origin of enhanced activity. ACS Catal 2019;9(10):9350
Lou Y, Zheng YP, Li X, Ta N, Xu J, Nie YF, Cho K, Liu JY. Pocketlike active site of Rh1/MoS2 single-atom catalyst for selective crotonaldehyde hydrogenation. J Am Chem Soc 2019;141(49):19289
Hwang J, Noh SH, Han B. Design of active bifunctional electrocatalysts using single atom doped transition metal dichalcogenides. Appl Surf Sci 2019;471:545
Dai XP, Du KL, Li ZZ, Liu MZ, Ma YD, Sun H, Zhang X, Yang Y. Co-doped MoS2 nanosheets with the dominant CoMoS phase coated on carbon as an excellent electrocatalyst for hydrogen evolution. ACS Appl Mater Interfaces 2015;7(49):27242
Jin HY, Liu X, Chen SM, Vasileff A, Li LQ, Jiao Y, Song L, Zheng Y, Qiao SZ. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett 2019;4(4):805
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51902099)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, R., Fei, HL. & Ye, GL. Recent advances in single metal atom-doped MoS2 as catalysts for hydrogen evolution reaction. Tungsten 2, 147–161 (2020). https://doi.org/10.1007/s42864-020-00045-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-020-00045-7