Abstract
Searching for novel complex materials with enhanced lithium-ion battery performances is one of the most challenging efforts. Many kinds of transition metal oxides and polyanionic frameworks were developed with various structures, which can improve the energy density of lithium-ion batteries. In this work, we explored 4d and 4f transition metal La–Nb–O compounds as cathode materials for lithium-ion energy storage. Orthorhombic pyrochlore LaNb5O14, orthorhombic perovskite LaNb3O9, and monoclinic LaNbO4 compounds with different metal cation coordination polyhedra were synthesized using solid-state reaction. The orthorhombic pyrochlore LaNb5O14 compound showed the highest capacity among these La–Nb–O compounds owing to its quasi‐2D network for Li‐ion incorporation. According to the electronegativity theory and ionic size, La3+ cations can form LaO12 polyhedra and hexahedral LaO8 units in different La–Nb–O compounds, which can stabilize octahedral NbO6 and/or pentahedral NbO7 and their assembled structures, resulting in easy lithium-ion diffusion. This work may provide some structure clues for the design of electrode materials for fast lithium storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The design and synthesis of transition metal compounds with targeted properties and enhanced performances is important to develop the present and future technologies [1]. For fast energy storage applications, novel electrode materials are urgent to enhance both their capacities and charge storage kinetics [2, 3]. Recently, two novel complex niobium tungsten oxides (Nb16W5O55 and Nb18W16O93) were synthesized to effectively use superstructure motifs to provide stable host structures for lithium intercalation with facile and defect-tolerant lithium diffusion and multi-electron redox [4]. At a current density of 20 C, the Nb18W16O93 material shows a capacity of ~ 150 mAh g−1. Even at a current density of 60 C (8.9 A g−1) and 100 C (14.9 A g−1), the capacities are 105 and 70 mAh g−1, respectively. For fast lithium-ion energy storage, nanostructured materials, e.g., TiO2, Li4Ti5O12, and Nb2O5, were proved to overcome poor ionic diffusion and electronic properties [5,6,7]. Among these materials, orthorhombic Nb2O5 (T-Nb2O5) was found to show the anomalously fast energy storage behavior (60 C rate) [5, 6], owing to its specific crystal structure. In the T-Nb2O5 crystal, loosely packed oxygen atomic layers and densely packed Nb–O atomic layers form a quasi‐2D network for Li‐ion incorporation, which allows direct Li-ion transport between bridging sites with very low steric hindrance [5]. Therefore, finding and designing electrode materials with specific atomic arrangements are still needed to obtain enhanced electrochemical properties [8, 9].
Niobium‐based oxides, i.e., Nb2O5, TiNbxO2+2.5x compounds, M–Nb–O (M = Cr, Ga, Fe, Zr, Mg, etc.) family, etc., showed fast charge storage performances because of their unique quasi‐2D networks for Li‐ion incorporation and both open and stable Wadsley–Roth shear crystal structures [10]. The finding and unraveling of new M–Nb–O materials systems are of great interest. Li–La–Nb–O and La–Nb–O compounds were served as solid-state electrolytes for the lithium-ion battery and fuel cell, which may show a fast Li-ion migration behavior. La–Nb–O compounds mainly contain La3NbO7, LaNbO4, LaNb3O9, LaNb5O14, La2Nb12O33, etc. [11, 12]. Most of niobium oxides consist of octahedral NbO6 structures with different degree distortions, while the tetrahedral NbO4 structure only exists in the LaNbO4 compound [13,14,15]. However, among niobium oxide compounds, the NbO4 tetrahedron is rarely found, because the large Nb5+ atom cannot fit into the oxygen-anion tetrahedron. Electronegativity is a useful guideline tool to design targeted electrode materials [9]. With applying electronegativity of metal cations, the change of redox potential and the selectivity of targeted doping element can be achieved in the design of electrode materials [16,17,18]. Ionic electronegativity values of Nb5+ and La3+ are 1.862 and 1.327, respectively. In different La–Nb–O compounds, La3+ can decrease the orbital overlap by easing some charge densities of Nb–O bonds, leading to easy enable redox reaction of Nb5+/Nb4+ and lithium-ion diffusion. With high electronegativity, tetrahedral NbO43− species often do not exist in aqueous solution [19], except with the help of La3+.
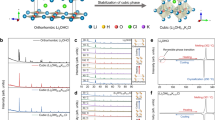
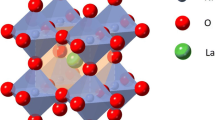
In this work, we synthesized orthorhombic pyrochlore LaNb5O14, perovskite LaNb3O9, and monoclinic LaNbO4 compounds. In LaNb5O14, each Nb atom is surrounded by six or seven oxygen atoms to form a NbO6 octahedron or tilted pentagonal bipyramids, while in LaNb3O9, each Nb atom forms a NbO6 octahedron and 2/3 of La cation sites are vacant (Fig. 1). In the LaNbO4 compound, a La cation is eightfold coordination and a Nb cation and 4 O anions form an NbO4 tetrahedron. Served as electrode materials for lithium-ion batteries, the electrochemical performances of these La–Nb–O compounds were systematically studied.
2 Experimental
2.1 Material synthesis
La–Nb–O samples were synthesized with a one-step solid-state reaction. The stoichiometric amounts of Nb2O5 and La2O3 were weighed (Table 1) and milled in a mortar for 1 h. Then, the mixed powder was pressed into pellets under a pressure of 30 MPa and calcined in air at 1080 °C for 10 h. After that, the pellets were crushed and milled for further usage.
2.2 Material characterization
The phase, image, and element mappings of La–Nb–O samples were analyzed by X-ray diffraction (XRD) with a D8 Focus X-ray diffractometer (Bruker) and field-emission scanning electron microscope (SEM, Hitachi S-4800). Raman spectra were measured in a Jobin–Yvon Horiba T64000 Raman triple grating spectrometer (Horiba Ltd., France) with a green line of Ar+ laser (514.5 nm radiation).
2.3 Electrode preparation
The La–Nb–O sample was mixed with carbon black and polyvinylidene fluoride (PVDF) with a mass ratio of 70:15:15 to form slurry and coated on an Al foil, which was served as the cathode. The assembled half-cells used lithium metal as the anode. The cathode and anode were assembled into a CR2032 coin cell in an Ar-filled glovebox. The separator is a polypropylene film (Celgard 2400) and the electrolyte is 1 mol L−1 LiPF6 in ethylene carbonate/dimethyl carbonate/diethyl carbonate (EC/DMC/DEC, 1:1:1 vol%).
2.4 Electrochemical measurement
A charge–discharge cycling test of the assembled half-cell was performed on a LAND CT2001A system in the voltage range of 1.0–3.0 V (vs. Li+/Li) at different current densities. Cyclic voltammogram (CV) curves were measured at an electrochemical workstation (CHI 660E).
3 Results and discussion
Different La–Nb–O compounds were synthesized by solid-state reaction. Figure 2 show XRD patterns of as-synthesized La–Nb–O compounds. When the atom ratios of Nb:La vary from 7:1 to 4:1, the main phase of samples is orthorhombic pyrochlore LaNb5O14 (JCPDS No. 76-263) with the trace phase of monoclinic Nb2O5 (JCPDS No. 72-1121). Orthorhombic pyrochlore LaNb5O14 is constituted by corner and edge-sharing NbO6 and NbO7 polyhedra, which also includes loosely packed La–O atomic layers and densely packed Nb–O atomic layers, forming a quasi‐2D network for Li‐ion insertion (Fig. 1b). When the atom ratios of Nb:La are between 3:1 and 2:1, the main phase of samples is orthorhombic perovskite LaNb3O9 (JCPDS No. 72-2080) with the trace phase of monoclinic LaNbO4. Pure phase monoclinic LaNbO4 (JCPDS No. 22-1125) was obtained at the atom ratio 1:1 of Nb:La. Monoclinic LaNbO4 includes isolated NbO4 tetrahedra which are interlinked by eightfold coordination LaO8 (Fig. 1c, d). SEM shows that the as-obtained samples have large particles with several micrometer and uniform distribution of La, Nb, and O elements (Fig. 3).
Raman spectroscopy is a powerful tool for probing chemical and structural properties of La–Nb–O materials. As shown in Fig. 4, the band in the range of 450–850 cm−1 can be assigned to the Nb–O-stretching modes. The O–Nb–O-bending modes appear below 450 cm−1, which are strongly interacted with the O–La–O-bending and La–O-stretching modes [19, 20]. The 670 cm−1 band originates from the symmetric stretching mode of NbO6 octahedra (Fig. 4d). Both LaNb5O14 and LaNb3O9 compounds include the NbO6 octahedral structure with different degree distortions. Therefore, LaNb5O14 and LaNb3O9 compounds show similar Raman bands. However, only the NbO4 tetrahedral structure exists in the LaNbO4 compound. The Raman bands centered at 810 and 320 cm−1 are due to the Nb–O symmetric modes of NbO4 tetrahedral structure.
Electrochemical performances of La–Nb–O compounds were further studied as electrode materials for lithium-ion batteries. CV was used to demonstrate their electrochemical reaction mechanisms. Figure 5 shows CV curves of La-Nb–O compounds with different atom ratios of Nb:La at scan rate of 0.5 mV s−1 and potential range of 1.0–3.0 V. These La–Nb–O electrodes have high working voltages of > 1.0 V, which can suppress the formation of the solid electrolyte interface film and lithium dendrites, ensuring the safety of working batteries [10]. When the atom ratios of Nb:La were changed from 7:1 to 5:1, one couple of main redox peaks centered at 1.59 and 1.77 V and weak redox peaks of 2.05/1.90 V were appeared. The redox peaks are assigned to the redox reaction of Nb5+/Nb4+ couple in different chemical environments of NbO6 and NbO7 [21]. Beginning from 4:1 of the Nb:La atom ratio, positions of main redox peaks were changed to 1.16 and 1.29 V, while the intensity of redox peaks of 1.59 and 1.77 V decreased. Only NbO6 and NbO4 exist in LaNb3O9 and LaNbO4, respectively. The above results show that rare earth La3+ can change the thermodynamic potential of Nb5+/Nb4+ redox reaction by changing the crystal structure and Nb coordination condition.
Furthermore, we studied the charge–discharge performances of these La–Nb–O compounds. The first discharge capacities at the current density of 100 mA g−1 are 175, 160, 116, 112, 138, 122, and 60 mAh g−1 for different samples (Table 1, Fig. 6 and 7). The La–Nb–O compound with the Nb:La atom ratio of 7:1 shows the highest capacity among 1–7# La–Nb–O compounds. The capacity contribution originates from Nb5+/Nb4+ redox reaction coupled with lithium-ion intercalation. Thus, LaNb5O14 with a quasi‐2D network structure can favor the insertion of Li‐ion (Fig. 1b) to obtain a high capacity. The rate capability was tested at various current densities from 0.1 to 2 A g−1 (Fig. 6a). The La–Nb–O compound with the Nb:La atom ratio of 2:1 exhibits a higher capacity especially at a discharge rate of 2 A g−1 than that of 1–5# and 7# La–Nb–O compounds. The NbO6 framework structure and many La vacant sites in LaNb3O9 provide a stable host for fast lithium intercalation. Owing to high electronegativity of tetrahedral NbO43− species in LaNbO4, this compound only displays the smallest capacity and bad rate performance than that of 1–6# La–Nb–O compounds [19, 22]. Electrochemical impedance spectra are used to analyze the resistance during electrochemical reaction. Figure 6d shows Nyquist plots of as-synthesized La–Nb–O compounds. The calculated charge transfer resistances of most La–Nb–O compounds have small values of < 50 Ω, which indicate that La–Nb–O electrodes have low resistances.
To study the fast rate performance, we studied the charge storage kinetics of La–Nb–O compounds using CV curves at different scan rates. The relationship of the peak current and scan rate satisfies the following equation [23, 24]:
where b = 0.5 indicates that the electrochemical reaction is controlled by a semi-infinite diffusion process, whilst b = 1 indicates a capacitive behavior. The b values for the La–Nb–O compounds are between 0.6 and 0.8, indicating a hybrid diffusion and capacitive processes (Fig. 6e, f) [25, 26]. The above results show that La–Nb–O compounds with specific crystal structures can provide the lithium diffusion pathway for fast lithium intercalation reaction. The design of novel complex compounds with targeted performance needs the control of the crystal structure and ionic electronegativity of component elements [27].
To study the energy storage mechanism of these electrode materials, XRD and Raman analyses were used to measure the electrode after electrochemical reaction (Fig. 8). LaNb5O14, LaNb3O9, and LaNbO4 phases are not changed after lithium insertion reaction (Fig. 8a). The existence of the peak at 805 cm−1 indicates the retaining of the NbO4 structure in the LaNbO4 compound. Raman peaks of La–Nb–O compounds with Nb:La atomic ratios of 7:1 and 3:1 are at 649 and 647 cm−1, which are consistent with Nb–O-stretching vibration of NbO6 or NbO7 structures [19, 20]. The results show that the La–Nb–O compound is an intercalation-type material for lithium storage.
4 Conclusion
In conclusion, orthorhombic pyrochlore LaNb5O14, perovskite LaNb3O9, and monoclinic LaNbO4 compounds were obtained among La–Nb–O compounds. Pyrochlore LaNb5O14 showed the highest discharge capacities of 175 mAh g−1 at the current density of 100 mA g−1, when used as cathode materials for fast lithium-ion energy storage. Perovskite LaNb3O9 displayed the highest discharge capacities of 51 mAh g−1 at current density of 2 A g−1. In both LaNb5O14 and LaNb3O9, La cation is 12-fold coordination and Nb cations form a sixfold and sevenfold coordination polyhedra, which construct the quasi-2D lithium-ion diffusion network. LaNbO4 with a NbO4 tetrahedral structure showed bad electrochemical performances. The results showed that the enhanced performances of cathode materials originated from appropriate three-dimensional oxide crystal structures, which might be identified and selected through ionic electronegativity scale.
Change history
21 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42864-021-00115-4
References
Harada JK, Charles N, Poeppelmeier KR, Rondinelli JM. Heteroanionic materials by design: progress toward targeted properties. Adv Mater. 2019;31(19):1805295.
Freire M, Kosova NV, Jordy C, Chateigner D, Lebedev OI, Maignan A, Pralong V. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat Mater. 2016;15:173.
Chen K, Song S, Liu F, Xue D. Structural design of graphene for use in electrochemical energy storage devices. Chem Soc Rev. 2015;44(17):6230.
Griffith KJ, Wiaderek KM, Cibin G, Marbella LE, Grey CP. Niobium tungsten oxides for high-rate lithium-ion energy storage. Nature. 2018;559:556.
Chen D, Wang JH, Chou TF, Zhao B, El-Sayed MA, Liu M. Unraveling the nature of anomalously fast energy storage in T-Nb2O5. J Am Chem Soc. 2017;139(20):7071.
Griffith KJ, Forse AC, Griffin JM, Grey CP. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J Am Chem Soc. 2016;138(28):8888.
Odziomek M, Chaput F, Rutkowska A, Świerczek K, Olszewska D, Sitarz M, Lerouge F, Parola S. Hierarchically structured lithium titanate for ultrafast charging in long-life high capacity batteries. Nat Commun. 2017;8:15636.
Chen K, Xue D. Crystallization of transition metal oxides within 12 seconds. Cryst Eng Comm. 2017;19:1230.
Chen K, Xue D. Materials chemistry toward electrochemical energy storage. J Mater Chem A. 2016;4(20):7522.
Deng Q, Fu Y, Zhu C, Yu Y. Niobium-based oxides toward advanced electrochemical energy storage: recent advances and challenges. Small. 2019;15(32):1804884.
Miruszewski T, Winiarz P, Dzierzgowski K, Wiciak K, Zagórski K, Morawski A, Mielewczyk-Gryń A, Wachowski S, Strychalska-Nowak J, Sawczak M, Gazd M. Synthesis, microstructure and electrical properties of nanocrystalline calcium doped lanthanum orthoniobate. J Solid State Chem. 2019;270:601.
Wachowski S, Mielewczyk-Gryn A, Zagorski K, Li C, Jasinski P, Skinner SJ, Haugsrud R, Gazda M. Influence of Sb-substitution on ionic transport in lanthanum orthoniobates. J Mater Chem A. 2016;4:11696.
Brunckova H, Medvecky L, Kovalcikova A, Fides M, Mudra E, Durisin J, Sebek M, Kanuchova M, Skvarla J. Structural and mechanical properties of La1/3NbO3 and La1/3TaO3 thin films prepared by chemical solution deposition. J Rare Earths. 2017;35(11):1115.
Brunckova H, Medvecky L, Hvizdos P, Girman V. Effect of solvent on phase composition and particle morphology of lanthanum niobates prepared by polymeric complex sol–gel method. J Sol Gel Sci Technol. 2014;69(2):272.
Brunckova H, Medvecky L, Hvizdos P, Durisin J, Girman V. Structural and mechanical properties of sol–gel prepared pyrochlore lanthanum niobates. J Mater Sci. 2015;50(22):7197.
Li K, Shao J, Xue D. Site selectivity in doped polyanion cathode materials for Li-ion batteries. Funct Mater Lett. 2013;6(4):1350043.
Li K, Xue D. Estimation of electronegativity values of elements in different valence states. J Phys Chem A. 2006;110(39):11332.
Melot BC, Tarascon JM. Design and preparation of materials for advanced electrochemical storage. Acc Chem Res. 2013;46(5):1226.
Jehng JM, Wachs IE. Structural chemistry and Raman spectra of niobium oxides. Chem Mater. 1991;3(1):100.
Ishii K, Morita N, Nakayama K, Tsunekawa S, Fukuda T. Raman spectra of LaNbO4 in the ferroelastic phase and the relaxation after the state shift. Phys Status Solidi A. 1989;112(1):207.
Griffith KJ, Senyshyn A, Grey CP. Structural stability from crystallographic shear in TiO2–Nb2O5 phases: cation ordering and lithiation behavior of TiNb24O62. Inorg Chem. 2017;56(7):4002.
Cao Y, Duan N, Yan D, Chi B, Pu J, Jian L. Enhanced electrical conductivity of LaNbO4 by A-site substitution. Int J Hydrogen Energy. 2016;41(45):20633.
Deng T, Zhang W, Arcelus O, Kim JG, Carrasco J, Yoo SJ, Zheng W, Wang J, Tian H, Zhang H, Cui X, Rojo T. Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat Commun. 2017;8:15194.
Chen K, Xue D. How to high-efficiently utilize electrode materials in supercapattery? Funct Mater Lett. 2019;12(1):1830005.
Lukatskaya MR, Kota S, Lin Z, Zhao MQ, Shpigel N, Levi MD, Halim J, Taberna PL, Barsoum MW, Simon P, Gogotsi Y. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat Energy. 2017;2:17105.
Liang X, Chen K, Xue D. A flexible and ultrahigh energy density capacitor via enhancing surface/interface of carbon cloth supported colloids. Adv Energy Mater. 2018;8(16):1703329.
Sun C, Xue D. Multisize and multiweight effects in materials science and engineering. Sci China Technol Sci. 2019;62(4):707.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21601176), CAS-VPST Silk Road Science Found 2018 (Grant No. GJHZ1854), the Youth Innovation Promotion Association, CAS (Grant No. 2018262), Jilin Province Youth Talent Lifting Project (Grant No. 181901), and the Youth Talent Development Program of the State Key Laboratory of Rare Earth Resource Utilization (Grant No. RERUY2017004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, K., Yin, S. & Xue, D. Active La–Nb–O compounds for fast lithium-ion energy storage. Tungsten 1, 287–296 (2019). https://doi.org/10.1007/s42864-019-00029-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-019-00029-2