Abstract
Graphite felt is a felt-like porous material made of high-temperature carbonized polymers. It is widely used in electrode materials because of its good temperature resistance, corrosion resistance, large surface area and excellent electrical conductivity. In this paper, the surface functional group modification is of graphite felt electrodes (mainly nitrogen doping modification, nitrogen–sulfur or nitrogen–boron co-doping modification) and surface catalytic modification (metal/ion surface modification and metal oxide surface modification as Main). There are two main methods and research progresses to improve the performance of graphite felt electrodes, and the comprehensive performance of surface functional group-modified graphite felt electrodes and surface catalytically modified graphite felt electrodes are compared respectively. The results show that both surface functional group modification and surface catalytic modification can improve the comprehensive performance of graphite felt electrodes. In this paper, the future development direction of graphite felt activation modification is also prospected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the increase of fossil energy consumption and the aggravation of environmental pollution, people have accelerated the utilization of some renewable new energy (solar energy, tidal energy, etc.) [1]. Due to regional and climatic conditions, renewable energy is intermittent and discontinuous, which limits its wide application [2, 3]. Therefore, it is particularly important to develop a system that is conducive to renewable energy storage [4,5,6].
Redox flow batteries (RFBS) are an electrochemical energy storage system that separates energy and power [7,8,9], which has the advantages of safety and environmental protection, long cycle life, and easy energy storage [10, 11], RFBS is an electrochemical energy storage device suitable for electrical energy storage of various scales, and has the potential to become the most promising long-term energy storage technology on the grid scale [12,13,14]. At present, many researchers at home and abroad are still conducting related research on flow batteries. In the past 40 years of research and development, only the all-vanadium system [15], the iron–chromium system [16] and the zinc–bromine system [17] are close to comprehensive. It is commercialized and has been widely used in the fields of emergency continuous power supply facilities, industrial batteries, electric traction, stand-alone applications, electric load averaging, etc. [18,19,20].
NASA conducted extensive research on Fe–Cr redox flow batteries in the 1970s, which were the world's first true flow batteries [21]. However, the problem of cross-contamination of iron-chromium flow batteries hinders its further development. Since then, NASA proposed the concept of iron–chromium mixed electrolyte and conducted further research, that is, the positive and negative electrodes are made of ferrous chloride, chromium chloride and hydrochloric acid. Mixed solution, which effectively avoids cross-contamination of active substances in the electrolyte, and the electrolyte after long-term use can be mixed to obtain a new electrolyte. A typical iron–chromium flow battery system consists of a flow circulation system, a membrane separator, two electrodes, and two external reservoirs for the dissolved active electrolyte [22, 23]. The role of iron–chromium flow batteries in energy storage is shown in Fig. 1 [24]. During the charging process, the Fe2+ of the positive electrode is oxidized to Fe3+, and the Cr3 + of the negative electrode is reduced to Cr2+. During the discharge process, the above-mentioned reactions will occur in the opposite process. The electrochemical reaction of the Fe–Cr flow battery during the charge–discharge cycle is shown in Eqs. 1–3 [25]:
The role of iron-chromium redox flow battery in energy storage [24]
The key materials of flow batteries include electrodes, membranes, electrolytes, etc. [26,27,28]. Among them, the commonly used electrode materials are carbon materials, including graphite plate, graphite felt, carbon felt, etc. [29, 30]. Mainly because carbon materials have the advantages of good electrical conductivity and corrosion resistance, they are widely used in flow batteries.
Electrochemical reactions occur on the surface of electrode materials in flow batteries. The hydrophilicity and electrochemical activity of the electrodes will have a direct impact on the electrochemical reactions, which in turn have an important impact on the energy efficiency and power density of the battery. At the same time, the surface of the electrode needs to be continuously washed by the acidic electrolyte, and the electrode needs to have certain mechanical properties, acid resistance and good chemical stability. An ideal flow battery electrode should have the characteristics of high electrochemical activity, high corrosion resistance, good hydrophilicity, and low cost.
Among many carbon electrodes, polyacrylonitrile graphite felt has the advantages of light weight, good flexibility, high carbon content, high temperature resistance, non-volatile at high temperature, corrosion resistance, small thermal conductivity, and high shape retention. The material of choice for flow batteries [31, 32]. At the same time, the polyacrylonitrile-based graphite felt is highly graphitized, poor in hydrophilicity and electrochemical activity, resulting in low battery energy efficiency and power density. Therefore, the development of electrode materials with high activity and excellent hydrophilic properties to improve the energy efficiency and power density of flow batteries has gradually become a current research hotspot.
Most of the ways to speed up the redox reaction of flow batteries, reduce electrochemical polarization, and improve energy efficiency and power density are by changing the performance of graphite felt electrodes [33,34,35]. The activation methods of graphite felt are generally divided into two methods: increasing surface functional groups and introducing surface catalytic substances [36,37,38].
The method of increasing surface functional groups is to use oxidation reaction to etch the surface of graphite felt to increase the specific surface area while introducing active functional groups, such as heat treatment [39], acid treatment [40], electrochemical oxidation [41] and chemical vapor deposition [42]. From previous research conclusions, it can be seen that functional groups can enhance the activity of graphite felt [43], while conductivity and specific surface area also affect its performance [44]. Studies have shown that the increase in the specific surface area of the graphite felt makes the electrolyte mass transfer smoother and provides more active sites for the primary reaction [45, 46]. Qiao et al. [47] introduced hydrophilic nitrogen-containing functional groups and oxygen-containing functional groups through the ammonium sulfate hydrothermal method to optimize the hydrophilicity of the graphite felt electrode. The energy efficiency of the flow battery reached 87.34%, which was 3.91% higher than the original energy efficiency. Yang et al. [48] introduced an efficient modulation of graphite felt electrodes through a boron doping method, and the resulting boron-doped graphite felt electrodes could improve the electrochemical performance of flow batteries when an appropriate amount of dopant was provided. Shanahan et al. [49] increased the surface area, oxygen-containing functional groups and electrolyte wettability of the modified graphite felt by rapidly activating the graphite felt in an acidified potassium permanganate solution for 1 h, thereby improving the vanadium redox flow. Battery performance.
The introduction of surface active substances [50] is to add catalysts that can promote the redox reaction of the electrode surface on the surface of the graphite felt, such as Sb, Cu, Bi, PbO2, ZrO2, CoO, carbon nanofibers and carbon nanotubes, etc. [51,52,53,54,55,56,57]. Loghavi et al. [58] found that the antimony-modified graphite felt electrode has better hydrophilicity than the bare graphite felt electrode, which has a direct impact on the improvement of electrode performance, as well as improved electro-catalysis and gassing inhibition performance. Feng et al. [59] modified the graphite felt electrode with SnO2, and the reversibility and electrochemical kinetics of the graphite felt electrode were improved. Compared with the blank battery, the SnO2-modified graphite felt electrode battery has a larger discharge capacity, higher electrolyte utilization and lower polarization. Liu et al. [60] modified a graphite felt electrode with nano-porous carbon, which has abundant porosity and large specific surface area, which promotes the two-electron oxygen reduction reaction and has a higher current response compared with the original graphite felt and lower charge transfer resistance.
Among the various modified graphite felts reported so far, polyacrylonitrile-based graphite felts have become popular in recent years. However, most researchers focus on whether the modification method improves the electrochemical activity. In fact, depending on the nature of the matrix material, these treatments can have beneficial effects.
2 Modification of surface functional groups
Using the oxidation reaction to etch the surface of the graphite felt to increase the specific surface area while introducing active functional groups can enhance the activity of the graphite felt, thereby improving its electrochemical performance. The basic overview of functional group-modified graphite felt electrodes is shown in Table 1.
2.1 Nitrogen doping modification
Studies have shown that the electrochemical performance can be improved by introducing nitrogen-containing functional groups on the surface of graphite felt [61,62,63]. Anarghya et al. [64] used nitrogen-doped carbon particles prepared from Bermuda grass to modify the surface of graphite felt electrodes. The experimental results showed that the peak values of the cathodic and anodic currents were increased, and the electrochemical properties were also changed. The data obtained from the Nyquist plot and the equivalent circuit show that the MGF has a low charge transfer resistance, the cell has an average Coulombic efficiency of 95%, a peak power density of 60 mW/cm2, and good stability over multiple cycles. Youn et al. [65] investigated a facile method to prepare nitrogen-functionalized graphite felt (GF) as electrodes for vanadium redox flow batteries. Nitrogen-functionalized graphite felts were produced by ultrasound-assisted self-polymerization and pyrolysis of dopamine (as shown in Fig. 2). After nitrogen functionalization, the electrode surface changed from hydrophobicity to hydrophilicity, the charge transfer resistance decreased, and the electrochemical performance was improved. Excellent electrochemical performance can be obtained by observing the porosity, specific surface area, etc. of the nitrogen-containing functional graphite felt. In the VRFB single-cell test, all NGF samples showed improved energy efficiency. Specifically, at a current density of 150 mA/cm2, the energy efficiency (75.5%) of the optimized sample NGF05 (containing 0.5 mM dopamine) was higher than that of GF (69.2%). In addition, the electrolyte utilization of GF is only 58.2% while that of NGF05 reaches 68.1%.
Preparation of NGF electrode via ultra-sonication-assisted self-polymerization and subsequent pyrolysis [65]
Sang et al. [66] proposed another facile method to prepare nitrogen-doped graphite felt electrodes with high electro-catalytic activity for vanadium–oxygen batteries. The surface of the graphite felt was coated with 1-ethyl-3-methylimidazole dicyanamide, and then heat-treated under N2 atmosphere to prepare nitrogen-doped graphite felt. 1-Ethyl-3-methylimidazole dicyanamide is an ionic liquid with high nitrogen content, which can be used as an effective precursor for nitrogen doping on graphite surfaces. In the charge–discharge test, the nitrogen-doped graphite felt single cell showed excellent performance. Under the condition of current density of 150 mA/cm2, the surface of graphite felt coated with 20% electrolyte solution (GF-ED20) increased its discharge capacity by 3 times, which was comparable to that of graphite felt with oxygen-containing functional groups (GF-REF) prepared by conventional heat treatment, the energy efficiency is improved by 10%. The improved performance is due to the high nitrogen content of the graphite felt, which also enhances the electro-catalytic activity for the vanadium redox reaction. Dinesh et al. [67] coated N-doped carbon spheres (NDCS) on the surface of graphite felt electrodes, which can significantly improve the performance of iron-based flow batteries. NDCS was synthesized by one-step hydrothermal method using glucose and ammonia water as precursors, and NDCS was prepared on the surface of graphite felt electrode by electrostatic spraying method. During 15 cycles at a current density of 30 mA/cm2, the cell achieved an average coulombic efficiency of 93%, a current efficiency of 72%, and an energy efficiency of 68%. Since the surface of the graphite felt is nitrided, its electrochemical activity is also improved. To improve the positive reaction kinetics, Li et al. [68] used a combination of freeze-drying and pyrolysis (as shown in Fig. 3) to prepare a nitrogen-doped reduced graphite oxide modified graphite felt (N-rGO/GF) electrode. The combined method of freeze-drying and pyrolysis can make N-rGO uniformly dispersed on the surface of glass fiber electrode (as shown in Fig. 4), with good catalytic activity. The results show that the composite electrode has good electrochemical performance at a pyrolysis temperature of 900 °C, and the catalytic activity and electrochemical reversibility of VO2+/VO2+ positive ions are significantly improved, indicating that the composite electrode has potential in improving VRFB performance. Application value.
Schematic diagram of the N-rGO/GF fabrication process [68]
Surface morphology of N-rGO-modified GF electrode (a) and detailed view of the N-rGO absorbed on GF electrode. Te red arrow in (b) points to the absorbed N-rGO [68]
Fy et al. [69] investigated a novel modified graphite felt (GF) as an electro-Fenton (EF) cathode material, which supported nitrogen-doped porous carbon (NPC) with a zeolite-type imidazolium salt framework-8 (ZIF-8) is a carbon precursor. The cathode modified with phenol (50 mg/L) by NPC can greatly remove organic carbon in wastewater. When the optimized conditions are 120 min, the mineralization rate reaches 100%, and the total organic carbon reaches 82.61%. After 5 cycles, the NPC cathode remained stable. The experimental results show that the electrochemical activity of the modified graphite felt is improved, which is due to the uniform distribution of nanoparticles on the fiber surface and the promoting effect of sp2 carbon and graphitic N between the carbon surface and oxygen molecules. Optimization of fabrication parameters, such as current, pH, and NPC loading, improved the efficiency of a novel cathode for in-situ electrochemical production of hydrogen peroxide, providing a potential material for degrading organic pollutants.
2.2 Nitrogen-sulfur, nitrogen–boron co-doping modification
In previous reports, it was found that the synergistic effect of heteroatoms in nitrogen–sulfur or nitrogen–boron co-doped structures increased the electrochemical activity of graphite felt. Li et al. [70] used thiourea as the sulfur and nitrogen source, and modified double nitrogen-doped graphene on graphite felt electrodes by hydrothermal method for vanadium redox flow battery. The results show that the introduction of nitrogen and sulfur on the surface of graphite felt can introduce functional groups and increase the surface area for its reaction. At a current density of 80 mA/cm2 (as shown in Fig. 5), the energy efficiency, power density, discharge capacity and retention capacity were significantly improved. These results indicate that nitrogen–sulfur co-doped graphene well improves the activity of graphite felt electrodes, providing an effective way to enhance the performance of vanadium redox flow batteries.
The energy efficiency for different samples in the current density range of 80–160 mA/cm2 [70]
Mcd et al. [71] used sulfur/nitrogen co-doped graphene quantum dots (GQDS) to modify graphite felt electrodes to improve the electrochemical performance of vanadium redox flow batteries. The average diameter of S/N co-doped GQDs is 6.2 nm, and various performances of vanadium redox flow batteries are improved to varying degrees under the action of S/N co-doped GQDs. This is mainly due to the introduction of GQDs doped with O functional groups, lattice N atoms and S. Under their combined action, the charge transfer in the electrolyte is accelerated, and the catalytic activity of the vanadium redox surface is enhanced. Thus, the multi-level S/N co-doped GQD/GF electrodes paves the way for engineered electrode nanostructures that enhance the catalytic activity and durability of redox flow batteries.
To promote the generation of dimethyl phthalate (DMP), Ding et al. [72] developed a modified graphite felt cathode doped with nitrogen and boron and applied it in a hydrogen peroxide-coagulation system. After simple modification, the cathode H2O2 yield was increased from 9.39 to 152.8 mg/l, and the current efficiency was increased from 1.61 to 70.3%. Under the optimal conditions of pH 5, cathode potential of − 0.69 V, and electrode spacing of 1 cm, DMP was completely degraded within 2 h, and the TOC removal rate reached 80%. Through quenching experiments, the possible mechanism of the synergistic effect of electro-reagents and electro-coagulation in the peroxide-coagulation system was revealed (as shown in Fig. 6). The application prospect of the system in landfill leachate and domestic sewage treatment was studied. The removal rate of the system reached 50% and 61% respectively within 2 h. The system adopts a simple modified cathode and has a good application prospect.
The proposed mechanisms for DMP degradation in PC system with modified cathode material [72]
3 Modification of surface catalytic materials
Modification of graphite felt by metal or metal oxide can improve its electrochemical performance. The basic overview of surface catalytic material modification is shown in Table 2
Vanadium redox flow batteries stand out among various electrochemical energy storage technologies due to their good operational flexibility and scalability. However, traditional electrodes with low electrochemical activity, such as carbon felt and graphite felt, hinder the interfacial charge transfer process, resulting in considerable over-potential loss, which greatly reduces the energy efficiency and voltage efficiency of the electrode.
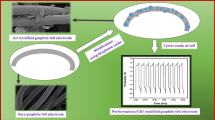
Xia et al. [73] used a simple electrodeposition technique to prepare the composite electrode, and successfully obtained high electrochemical activity. The Pb/CF electrode has good electrochemical activity for the VO2+/VO2+ redox couple in the battery. At 100 mA/cm2, the battery voltage efficiency reaches 90% and the energy efficiency also reaches 88%. In addition, due to the uniform distribution of lead particles on the surface of the graphite felt, the long-term stability of the graphite felt battery with the Pb/CF electrode is significantly better than that of the pristine graphite felt battery due to the redox-mediated catalysis. Furthermore, three single cells were combined into a stack to estimate the reliability of the redox-mediated Pb/CF electrode application in a large scale (as shown in Fig. 7). This technique of preparing electrodes from high-performance and facile redox-mediated reactions is expected to provide new ideas for industrial electrode design.
VRB cell stack. A Photograph of a VRB cell stack consisting of three single cells (16 cm.2) and B cycling stability of the VRB cell stack with the CF and PB/CF electrodes, respectively [73]
Among various metal halide redox flow batteries, poly-halide-based hybrid redox flow batteries are widely used in the field of long-term fixed charge storage due to their long cycle life and high energy density. Zinc–bromine flow battery systems have attracted much attention due to their reasonable battery voltage, energy density, and lifetime. However, the main reason why the performance of the battery cannot be improved is because of the generation of bromine vapor during the charging process, and the poor kinetics of the bromine oxidation reaction on the electrode is related to this flow system. To improve the reaction kinetics of the zinc–bromine battery, Mariyappan et al. [74] introduced noble metal platinum particles on the graphite felt electrode, and the platinum particles accelerated the electron transfer rate in the electrode. The graphite felt was modified by a simple synthesis process. The pt content was optimized by improving the reaction kinetics of br2/br−. Mariyappan et al. [75] also used low-loaded platinum as the electro-catalyst (as shown in Fig. 8), and the br2/br− reaction kinetics were better improved due to the deposition of platinum particles. The improvement of the kinetics of the br2/br− redox coupling reaction was studied by introducing platinum as the electrode material on the surface of the graphite felt. This method greatly affects the kinetics of the bromine/bromide redox reaction. A Coulombic efficiency of 99.96% is the best average efficiency. Under the condition of 50 mA/cm2, the energy efficiency of the zinc–bromine-based flow battery was measured to be 88%. Therefore, graphite felt electrodes modified with low loadings of platinum are used as materials to improve br2/br− kinetics in most studies.
Exploited view of the Flow cell configuration used in the present investigation [75]
To improve the catalytic activity of graphite felt (GF) electrodes, Wang et al. [76] proposed to modify the surface of GF with metal oxides. In supported oxides, the presence of nanoparticles significantly enhances mass transfer and reactivity. X-ray photoelectron spectroscopy and contact angle measurements showed that the content and hydrophilicity of oxygen-containing groups increased significantly. After assembling the electrodes in the battery, the laser/glass fiber electrodes greatly improved the performance of the battery, significantly increasing the capacity and efficiency of the battery. When the current is 50 mA/cm2, the laser/glass fiber electrode increases the energy efficiency of the battery from 54.76 to 61.37%. In addition, the cyclability test of the system shows that there is no obvious fading phenomenon after 100 cycles, indicating that the LaSrOx/GF electrode has good stability.
The low efficiency of cerium-based redox flow batteries is mainly due to the limitation of the kinetics of the cerium redox reaction. Therefore, it is very important to develop electrode materials that can accelerate the redox reaction. Na et al. [77] proposed an activation method that utilizes surface functionalization to significantly improve the electrochemical performance of commercial graphite felts. Activated graphite felt as an electrode for cerium redox reaction has good catalytic activity and durability for Ce(IV)/Ce(III) redox coupling. It is found that the combination of oxygen functional groups, especially O–C=O functional groups, on the active graphite felt electrode accelerates the charge transfer rate, thereby improving the performance of the electrode.
Some researchers have found that some inexpensive metal oxides can be used as catalysts for flow battery electrode materials. Mahanta et al. [78] used a low-temperature hydrothermal method to prepare thermally activated graphite felt with Co3O4 nanostructures (as shown in Fig. 9). Functional groups on TGF can nucleate Co3O4 particles, creating covalent bridges between them. The bridge improves the tunneling of electrons across the electrolyte/electrolyte interface and reduces the over-potential of the vanadium redox reaction. The EEs of the two battery packs after 50 cycles are 84% and 89% for their initial capacity, respectively, and the 3% loss of EE compared to a single cell is mainly due to the additional contact resistance due to cell coupling.
Schematic illustration of the fabrication of Co3O4-modified electrodes [78]
Xiang and Daoud [79] attached cobalt oxide to the surface of graphite felt in the form of a coating. Through electrochemical detection, it was found that the modified graphite felt has good electro-catalytic activity and reversibility, and after 50 cycles of repeated experiments has stability. The coulombic efficiency, voltage efficiency, energy efficiency and discharge capacity of the battery detected by charging and discharging are 89.5%, 77.6%, 69.4% and 373.9mAh, respectively. Compared with the graphite felt battery, the energy efficiency is increased by 12.7% and the discharge capacity is increased by 101.7%. Polarization curve analysis shows that the limiting current density and maximum power density are significantly increased at 95% of the charge state due to the suppression of ohmic polarization. The results show that the modification of light cobalt has a good and stable catalytic effect on improving the electrochemical performance of graphite felt in vanadium redox batteries.
Iron-based flow batteries are of great interest due to their economic viability and environmentally friendly electrolytes. The electrodes and electrolytes used in iron-based redox flow batteries (IRFBs) play a crucial role in the performance of electrochemical energy storage devices. Therefore, it is very necessary to design suitable electrodes and optimize the electrolyte composition. Graphite is one of the suitable electrodes in flow batteries, but it must be modified to improve its electrical properties. Dinesh et al. [80] used WO3 nanoparticles (WON) for the first time to modify graphite felt electrodes for IRFBs applications. The electrochemical activity of the modified graphite felt electrode was increased by electrochemical detection. Faggiano et al. [81] studied the heat treatment time of copper-based redox flow battery graphite felt, and the polyacrylonitrile-based carbon felt showed excellent stability and the best electrochemical performance after heat treatment at 400 °C for 6 h. Wang et al. [82] prepared a simple and environmentally friendly graphite felt active electrode by introducing vanadium oxide on the surface of the graphite felt. On the activated graphite felt surface, the surface porosity and roughness increased when the calcination temperature and surface area reached 350 °C to 400 °C and 17.11 m2/g, respectively. At a charge transfer resistance of 0.27 Ω, the polarization of the activated graphite felt decreases with decreasing charge transfer resistance.
Yan et al. [83] designed a simple one-step activated graphite felt containing nitrate to improve the energy storage performance and cycling durability of batteries by increasing the number of oxygen-containing functional groups and mesoporous. Under the condition of 4 mA/cm2, its area capacity is 1.26 mAh/cm2, and the cycle capacity retention rate is 99.8%. Furthermore, at a power density of 3.7 mW/cm2, the cell provides a remarkable energy density of 1.82 mWh/cm2. This modification strategy and material have great potential for wear-resistant, flexible devices.
For the application of vanadium redox flow battery (VRFB), Chen [84] proposed a simple and effective method for roughening graphite felt (GF) using manganese oxide as an etchant (as shown in Fig. 10). At different current densities, VRFBs with R-GF electrodes exhibit strongly enhanced electrochemical performance in terms of energy efficiency and electrolyte utilization. The large specific surface area of R-GF with abundant oxygen-containing functional groups is extremely favorable for improving VRFB kinetics.
Schematic of the fabrication process of roughened graphite felt [84]
Qi et al. [85] used a one-step hydrothermal method to in-situ grow transition metals Cu and Fe on the surface of a graphite felt electrode without a polymer binder. The electrode exhibits low metal ion leaching rate and good stability. Jiang et al. [86] used metal–organic frameworks (MOFs) to prepare metal oxides and porous carbon nanocomposites for the first time, and used the in-situ growth method for ultra-uniform surface modification of graphite felts. Using a hydrothermal synthesis method, the graphite felt was modified with UiO-66 (Zr-MOF) nanoparticles, which were then converted into porous nanocomposites (ZrO2@C) by high-temperature carbonization (as shown in Fig. 11). In the 500-cycle test, the flow battery modified with ZrO2@C/GF has better stability and higher electrolyte utilization than the primary battery. The performance of the studied cells is superior to that reported in previous metal oxide-related studies.
Schematic representation of the procedure for preparing graphite felt electrode decorated with in-situ grown porous ZrO2@C nanoparticle derived from metal–organic framework [86]
In terms of current efficiency and mass transfer, the current of flow electrodes generally exceeds that of conventional parallel plate electrodes. The high cost and complex fabrication of high-performance electrode materials hinder their widespread application. Chen et al. [87] used graphite felt as the electrode of a flow battery to study the ability of the electrode to electro-reduce Cr(VI) in solution. Tests under acidic conditions show that the reduction efficiency of Cr(VI) is very high and can reach 95–100%. The electro-reduction of Cr(VI) is due to the low flow rate and high current in the cell, while the conductivity of Cr(VI) in dilute solution is enhanced by the presence of other metal ions in the solution. Low-valent ions can reduce Cr(VI) better at low flow rate, but these ions reduce the reduction efficiency of Cr(VI) at fast flow rate. In addition, the surface of the graphite felt is hindered from forming an insoluble layer in an acidic environment, which reduces the energy loss of the battery and improves the durability of the graphite felt.
To improve the electro-catalytic activity of pristine graphite felt (GF) for V3+/V2+ redox reaction and reduce the influence of hydrogen evolution reaction on battery performance, Xiao et al. [88] used hydrothermal method to load cadmium oxide (CdO) nanoparticles on graphite felt On the surface, modified graphite felt (CdO/GF) was prepared as a high-performance vanadium battery anode. Compared with GF, CdO/GF effectively inhibited the activity of hydrogen evolution reaction. CdO/GF significantly improved the electrochemical activity and reversibility of V3+/V2+ redox reaction, and the charge transfer resistance was also significantly reduced, compared with GF, the discharge capacity decay rate of CdO/GF decreased significantly, and the voltage efficiency and energy efficiency increased by about 5% at a current density of 90 mA/cm2. The catalytic performance of CdO/GF shows good stability during multiple charge–discharge cycles. Li et al. [89] used a combination of impregnation and high-temperature calcination to deposit non-noble metal chromium oxides on the surface of graphite felt to modify the graphite felt electrode (as shown in Fig. 12). In the charge–discharge cycle test, the energy efficiency of the nanopowder assembled with the chromium oxide modified electrode was 87.2%. This provides a possible route for deep eutectic solvent (DES) electrolytes to enhance the properties of nanofibers.
SEM images of PGF (a) and TGF (b) and CrTGF (c, d) [89]
Zhang et al. [90] prepared an active electrode by introducing magnesium oxide (MgO/GF) catalyst into the surface of graphite felt by isometric impregnation method. Mechanistic studies show that MgO/GF has high activity in the ozonation of BPA. Lou et al. [91] developed a three-dimensional porous Ag–Bi electrode to realize the highly selective reduction of CO2 to formate (as shown in Fig. 13). A galvanic displacement reaction was used to deposit silver particles on the surface of double-layer graphite felt. Compared with the double-coated graphite felt, the reduction selectivity of the silver-double electrode is enhanced. Formic acid is converted from CO2 under the action of the bimetallic catalytic system, and its selectivity can reach 88% at − 1.6 V. The highly porous structure and large surface area of the electrode facilitates the mass transfer of the electrode, which enables the electrode surface current density to reach 76 mA/cm2, and the yield is also improved.
Proposed mechanism for the selective reduction of CO2 at the bimetallic electrode [91]
Lou et al. [92] studied the reduction and removal of the organochlorine herbicide alachlor by nano-silver modified nickel-coated graphite felt (Ag/Ni@GF). A new type of graphite felt electrode is prepared by coating the surface of the graphite felt with a layer of metallic nickel and then modifying it with silver ions. Through Raman scattering detection, it can be known that the content of PVP as a material for controlling the deposition of silver nanoparticles will not affect the catalytic activity of silver. And the prepared composite electrode is very stable, so it can be reused many times.
In the heterogeneous electro-Fenton (EF) process, transition metal oxide-based carbon composite electrodes with high stability and high activity have broad application prospects in environmental governance. Cui et al. [93] synthesized Cu/CuFe2O4(CCFO) with different Cu0 ratios in one step by solvothermal method. These materials were then used to manufacture modified graphite felts by polytetrafluoroethylene (PTFE) bonding techniques. Importantly, the hydrophobicity of PTFE effectively inhibits metal leaching from the cathode and improves oxygen utilization. During the degradation of EF-based tetracycline (TC), the cathode performance increases with the increase of Cu0 ratio. Cu0 enhances the selectivity of the 2-electron oxygen reduction reaction and endows the cathode with abundant electron-rich centers, accelerating the regeneration of active Fe(II), resulting in the rapid conversion of H2O2 to hydroxyl radicals. A possible TC mineralization pathway was deduced by liquid chromatography–mass spectrometry analysis, and a catalytic mechanism for the heterogeneous EF process was proposed. The most efficient cathode also showed high stability at pH 3 (0.84 ± 0.11 mg/L Fe and 1.35 ± 0.17 mg/L Cu leaching), with TC removal close to 80% after 5 oxidation cycles, indicating its potential for efficient and durable wastewater treatment in heterogeneous EF technology. Lu et al. [94] designed a three-dimensional bimetallic carbon-based electrode CuNi/multi-walled carbon nanotubes/graphite felt (CuNi/M/GF) for the electrochemical reduction of nitrate by adjusting the electrodeposition potential (As shown in Fig. 14) CuNi/M/GF exhibited excellent corrosion resistance and stability in cycling tests. Yang et al. [95] proposed a batch catalyst electrodeposition method to resolve the conflict between electrodeposition current density and catalyst distribution, and achieved a more uniform and dispersed catalyst distribution inside the porous electrode, thus, compared with the optimized, has higher voltage efficiency and electrolyte utilization. Nariyama et al. [96] introduced redox mediators in the no aqueous electrolyte, and the active materials were charged and discharged without direct contact with the electrodes. A novel high-capacity and high-pressure redox flow battery was fabricated.
SEM images of a GF, b M/GF, c CuNi–0.7/M/GF, d CuNi–1.3/M/GF [94]
4 Conclusion and outlook
Redox flow batteries and to a lesser extent hybrid flow batteries have the advantages of flexible layout (due to separation of power and energy components), long cycle life (because there is no solid–solid phase transition), fast reaction times, and no need for “equilibration” Charging (overcharging of the battery to ensure all cells have the same charge) and no harmful radiation. Some types also offer simple state-of-charge determination (charge-dependent via voltage), low maintenance and tolerance for overcharge/over-discharge. These technical advantages make redox flow batteries ideal for large-scale energy storage.
Electrode materials are one of the key materials that need to be broken through in flow batteries. Graphite felt is widely used as an electrode material for redox flow batteries due to its stable electrochemical performance, high mechanical strength, and large surface area. However, the graphite felt material has poor hydrophilicity and insufficient electrochemical activity, and needs to be modified before being used as a battery electrode.
This paper introduces the surface functional group modification of graphite felt electrode (mainly nitrogen doping modification, nitrogen–sulfur or nitrogen–boron co-doping modification) and surface catalytic modification (metal/ion surface modification and metal oxide surface modification as the main Main) two main methods and research progresses to improve the performance of graphite felt electrodes, mainly focus on improving the specific surface area, hydrophilicity and electrical conductivity of graphite felt, so as to achieve the purpose of speeding up the reaction rate and improving the electrochemical performance of graphite felt electrodes. Judging from the research results obtained so far, the graphite felt electrode still needs to further improve its electrochemical activity to improve the performance of the electrode. At the same time, with the development of the redox flow battery industry, low-cost, high-performance graphite felt modification method suitable for industrial production is still an important development direction of redox flow battery electrode research. In view of this, future research on graphite felt electrodes will mainly focus on the following work:
-
1.
At present, the metals and metal oxides introduced on the surface of the graphite felt are mainly sub-group (B) metal elements. This is an accidental or inevitable conclusion, and no one has given a definite conclusion, so it needs to be systematically studied to clarify;
-
2.
In terms of functional group modification, nitrogen doping modification, nitrogen–sulfur or nitrogen–boron co-doping modification is mainly used, and organic functional groups, such as carbonyl group and carboxyl group, are also used for modification, but this modification is due to the introduction of radicals. The lone pair of electrons in the group is caused by other factors, and this kind of modification mechanism has not yet formed a unified theory, so further systematic research is needed;
-
3.
In terms of functional group modification, in addition to single functional group modification, multi-element doping can be introduced (such as –OH doping-SH; –COO-doping-CSS-, etc.). Or whether the co-introduction of multiple functional groups (such as carbonyl + carboxyl) can increase the electrochemical activity of the graphite felt electrode is also worth investigating;
-
4.
The current research on the activation of graphite felt electrodes is limited to experimental research. In the context of digitalization and big data, whether it is possible to establish mathematical models, carry out simulation experiments, and combine theory and experiments to improve graphite felt electrodes. Electrochemical activity;
-
5.
The problem of expanding the application field of subsequent graphite felt modified materials. That is to say, in addition to being used as an active electrode in energy storage systems, the modified graphite felt should also be considered as a cathode material in other applications such as degrading pollutants in water.
References
Rong C, Jxa B, Jza B et al (2020) Facile segmented graphite felt electrode for iron-vanadium redox flow batteries with deep eutectic solvent (DES) electrolyte. J Power Sour 483:229200
Mankge NS, Madito MJ, Hlongwa NW et al (2021) Review of electrochemical production of doped graphene for energy storage applications. J Energy Storage 46:103527
Hargreaves JJ, Jones RA (2020) Long term energy storage in highly renewable systems. Front Energy Res. https://doi.org/10.3389/fenrg.2020.00219
Ani VA (2021) Development of an intelligent power management system for solar PV-wind-battery-fuel-cell integrated system. Front Energy Res 9:613958. https://doi.org/10.3389/fenrg.2021.613958
Züttel A, Gallandat N, Dyson PJ et al (2022) Future Swiss Energy Economy: the challenge of storing renewable energy. Front Energy Res. https://doi.org/10.3389/fenrg.2021.613958
Qiao L, Fang M, Liu S et al (2022) New-generation iron-titanium flow batteries with low cost and ultrahigh stability for stationary energy storage. Chem Eng J 434:134588
Chen Z, Liu Y, Yu W et al (2021) Cost evaluation and sensitivity analysis of the alkaline zinc-iron flow battery system for large-scale energy storage applications. J Energy Storage 44:103327
Zhang H, Sun C (2021) Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: a review. J Power Sour 493:229445
Emmett RK, Roberts ME (2021) Recent developments in alternative aqueous redox flow batteries for grid-scale energy storage. J Power Sour 506:230087
Song Y, Li X, Yan C et al (2020) Unraveling the viscosity impact on volumetric transfer in redox flow batteries. J Power Sour 456:228004
Rodby KE, Perry ML, Brushett FR (2021) Assessing capacity loss remediation methods for asymmetric redox flow battery chemistries using levelized cost of storage. J Power Sour 506:230085
Lai Q, Zhang H, Li X et al (2013) A novel single flow zinc-bromine battery with improved energy density. J Power Sour 235:1–4
Ke G, Ma X, Conforti KM et al (2015) A zinc-iron redox-flow battery under $100/kWh of system capital cost. Energy Environ Sci 8(10):2941–2945
Raja M, Khan H, Sankarasubramanian S et al (2021) Binder-free thin graphite fiber mat sandwich electrode architectures for energy-efficient vanadium redox flow batteries. Catal Today 370:181–188
Liu J, Duan H, Xu W et al (2021) Branched sulfonated polyimide/s-MWCNTs composite membranes for vanadium redox flow battery application. Int J Hydrog Energy 46:34767–34776
Sun CY, Zhang H (2019) Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int J Energy Res 43(14):8739–8752
Venkatesan N, Archana KS, Suresh S et al (2018) Boron-doped graphene as efficient electrocatalyst for zinc-bromine redox flow battery. ChemElectroChem 6:1107–1114
Zeng YK, Zhao TS, An L et al (2015) A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J Power Sour 300:438–443
Fraunholz C, Kraft E, Keles D et al (2021) Advanced price forecasting in agent-based electricity market simulation. Appl Energy 290:116688
Reynard D, Girault H (2021) Combined hydrogen production and electricity storage using a vanadium-manganese redox dual-flow battery. Cell Rep Phys Sci 2:100556
Bartolozzi M (1989) Development of redox flow batteries. A historical bibliography. J Power Sour 27(3):219–234
Chen N, Zhang H, Luo XD et al (2020) SiO2-decorated graphite felt electrode by silicic acid etching for iron-chromium redox flow battery. Electrochim Acta 336(6058):135646
Zhang C, Guo L, Deng C et al (2022) Semi-solid reactive interfaces based on ZnO@C core-shell materials for zinc-iron flow batteries. Chem Eng Sci 250:117402
Su Y, Chen N, Ren H et al (2022) Preparation and properties of indium ion modified graphite felt composite electrode. Front Chem. https://doi.org/10.3389/fchem.2022.899287
Zhang H, Yi T, Li J et al (2017) Studies on properties of rayon and polyacrylonitrile-based graphite felt electrodes affecting Fe/Cr redox flow battery performance. Electrochim Acta 248:603–613
Ho YG, Kim MG, Kim GH et al (2022) Study on electrochemical properties of Pb(BF4)2 electrolyte for improvement of cycle lifetime and efficiency in soluble lead flow batteries. J Saudi Chem Soc 26(3):101472
Wang S, Xu Z, Wu X et al (2020) Analyses and optimization of electrolyte concentration on the electrochemical performance of iron-chromium flow battery. Appl Energy 271:115252
Pahlevaninezhad M, Pahlevani M, Roberts EPL (2022) Effects of aluminum, iron, and manganese sulfate impurities on the vanadium redox flow battery. J Power Sour 529:231271
Zhang H, Tan Y, Li JY et al (2017) Studies on properties of rayon and polyacrylonitrile-based graphite felt electrodes affecting Fe/Cr redox flow battery performance. Electrochim Acta 248:603–613
Yue L, Li WS, Sun FQ et al (2010) Highly hydroxylated carbon fibers as electrode materials of all-vanadium redox flow battery. Carbon 48:3079–3090
Li Z, Guo L, Chen N et al (2022) Boric acid thermal etching graphite felt as a high-performance electrode for iron-chromium redox flow battery. Mater Res Express 9(2):25601
Yang S, Cheng Y, Xiao X et al (2020) Development and application of carbon fiber in batteries. Chem Eng J 384:123294
Jiang HR, Shyy W, Wu MC et al (2019) A bi-porous graphite felt electrode with enhanced surface area and catalytic activity for vanadium redox flow batteries. Appl Energy 233:105–113
Zhao C, Li Y, He Z et al (2019) KHCO3 activated carbon microsphere as excellent electrocatalyst for VO2+ /VO2+, redox couple for vanadium redox flow battery. J Energy Chem 29:103–110
Liu YC, Shen Y, Yu LH et al (2018) Holey-engineered electrodes for advanced vanadium flow batteries. Nano Energy 43:55–62
Ahn Y, Moon J, Park SE et al (2021) High-performance bifunctional electrocatalyst for iron-chromium redox flow batteries. Chem Eng J 421:127855
Sawant TV, Yim CS, Henry TJ et al (2021) Harnessing interfacial electron transfer in redox flow batteries. Joule 5(2):360–378
Shi X, Esan OC, Huo X et al (2021) Polymer electrolyte membranes for vanadium redox flow batteries: fundamentals and applications. Prog Energy Combust Sci 85:100926
Tossaporn J, Bhupendra S, Apisada C et al (2021) Characteristics of graphite felt electrodes T reated by atmospheric pressure plasma jets for an all-V anadium redox flow battery. Materials 14:3847
Jiang B, Wang Y, Wang D et al (2019) Modifying graphite felt cathode by HNO3 or KOH to improve the degradation efficiency of electro-Fenton for landfill leachate. Water Sci Technol 80(12):2412–2421
Anantha MS, Anarghya D, Hu C et al (2021) Electrochemical performance of graphene oxide modified graphite felt as a positive electrode in all-iron redox flow batteries. J Appl Electrochem 51(2):331–344
Elahi Davaji H, Shamoradi F, Panjepour M et al (2022) Preparation and characterization of carbon felt/carbon composites by chemical vapor infiltration process. Carbon Lett 32(1):201–215
Yuan Z, Duan Y, Liu T et al (2018) Toward a low-cost alkaline zinc-iron flow battery with a polybenzimidazole custom membrane for stationary energy storage. Iscience 3:40–49
Li Q, Bai A, Zhang T et al (2020) Dopamine-derived nitrogen-doped carboxyl multiwalled carbon nanotube-modified graphite felt with improved electrochemical activity for vanadium redox flow batteries. R Soc Open Sci 7(7):200402
Wang R, Li Y, Wang Y et al (2020) Phosphorus-doped graphite felt allowing stabilized electrochemical interface and hierarchical pore structure for redox flow battery. Appl Energy 261:114369
Huang X, Yao S, Zhou R et al (2022) Study on the effect of hydrogen evolution reaction in the zinc-nickel single flow battery. J Energy Storage 50:104246
Qiao L, Fang M, Guo J et al (2022) Nitrogen-doped carbon felt as an electrode material for vanadium flow batteries. ChemElectroChem. https://doi.org/10.1002/celc.202200292
Yang I, Lee S, Jang D et al (2022) Enhancing energy efficiency and long-term durability of vanadium redox flow batterywith catalytically graphitized carbon fiber felts as electrodes by boron doping. Electrochim Acta S0013–4686(22):01190–01192
Shanahan B, Seteiz K, Heizmann PA et al (2021) Rapid wet-chemical oxidative activation of graphite felt electrodes for vanadium redox flow batteries. RSC Adv 11(51):32095–32105
Bae J, Hong J-Y (2022) Fabrication of nitrogen-doped porous carbon nanofibers for heavy metal ions removal. Carbon Lett 31:1339–1347
Shittu E, Suman R, Ravikumar MK et al (2022) Life cycle assessment of soluble lead redox flow battery. J Clean Prod 337:130503
Chen P, Cheng R, Meng G et al (2021) Performance of the graphite felt flow-through electrode in hexavalent chromium reduction using a single-pass mode. J Hazard Mater 416:125768
Zeng YK, Zhou XL, An L et al (2016) A high-performance flow-field structured iron-chromium redox flow battery. J Power Sour 324:738–744
Wang H, Li D, Xu J et al (2021) An unsymmetrical two-electron viologens anolyte for salt cavern redox flow battery. J Power Sour 492:229659
Wang Q, Chen W, Zhao C et al (2022) Analysis of overpotential in discharge process associated with precipitation for vanadium-manganese flow battery. J Power Sour 517:230717
Lu M, Yang W, Tang X et al (2021) Asymmetric structure design of a vanadium redox flow battery for improved battery performance. J Energy Storage 44:103337
Yogeeshwari RT, Krishna RH, Adarakatti PS et al (2022) Ultra-trace detection of toxic heavy metal ions using graphitic carbon functionalized Co3O4 modified screen-printed electrode. Carbon Lett 32(1):181–191
Loghavi MM, Zarei-Jelyani M, Niknam Z et al (2022) Antimony-decorated graphite felt electrode of vanadium redox flow battery in mixed-acid electrolyte: promoting electrocatalytic and gas-evolution inhibitory properties. J Electroanal Chem 908:116090
Feng X, Yang Y, Ren Y et al (2022) Anion doping enabling SnO2 superior electrocatalytic performances for vanadium redox reactions. Int J Green Energy. https://doi.org/10.1080/15435075.2022.2044331
Liu J, Jia J, Yu H et al (2022) Graphite felt modified by nanoporous carbon as a novel cathode material for the EF process. New J Chem 46(26):11272–12696
Rubio-Garcia J, Cui J, Parra-Puerto A et al (2020) Hydrogen/Vanadium Hybrid Redox Flow Battery with enhanced electrolyte concentration. Energy Storage Mater 31:1–10
Lee W, Kwon BW, Jung M et al (2019) Iron-vanadium redox flow batteries with polybenzimidazole membranes: high coulomb efficiency and low capacity loss. J Power Sour 439:227079
Noh C, Serhiichuk D, Malikah N et al (2021) Optimizing the performance of meta-polybenzimidazole membranes in vanadium redox flow batteries by adding an alkaline pre-swelling step. Chem Eng J 407:126574
Anarghya D, Anantha MS, Venkatesh K et al (2020) Bermuda grass derived nitrogen-doped carbon as electrocatalyst in graphite felt electrode to increase the efficiency of alliron redox flow batteries. J Electroanal Chem 878:114577
Youn C, Song SA, Kim K et al (2019) Effect of nitrogen functionalization of graphite felt electrode by ultrasonication on the electrochemical performance of vanadium redox flow battery. Mater Chem Phys 237:121873–121873
Sang JY, Kim S, Dong KK et al (2020) Ionic liquid derived nitrogen doped graphite felt electrodes for vanadium redox flow batteries. Carbon 166:131–137
Dinesh A, Shankaranarayana AM, Srid SM et al (2021) Nitrogen-doped carbon spheres-decorated graphite felt as a high-performance electrode for Fe based redox flow batteries. Diam Relat Mater 116:108413
Li Q, Liu J, Bai A et al (2019) Preparation of a nitrogen-doped reduced graphene oxide-modified graphite felt electrode for VO2+/VO2+ reaction by freeze-drying and pyrolysis method. J Chem 2019:1–9
Fy A, Ling TB, Tc B (2019) High yield of hydrogen peroxide on modified graphite felt electrode with nitrogen-doped porous carbon carbonized by zeolitic imidazolate framework-8 (ZIF-8) nanocrystals. Environ Pollut 255:113119
Li Q, Bai A, Xue Z et al (2020) Nitrogen and sulfur co-doped graphene composite electrode with high electrocatalytic activity for vanadium redox flow battery application Science Direct. Electrochim Acta 362:137223
Mcd A, Sg B, Dsa A et al (2020) Decorating sulfur and nitrogen co-doped graphene quantum dots on graphite felt as high-performance electrodes for vanadium redox flow batteries. J Power Sour 477:228709
Ding J, Dong L, Geng Y et al (2020) Modification of graphite felt doped with nitrogen and boron for enhanced removal of dimethyl phthalate in peroxi-coagulation system and mechanisms. Environ Sci Pollut Res Int 27(15):18810–18821
Xia L, Ting L, Li LW et al (2020) Highly stable vanadium redox-flow battery assisted by redox-mediated catalysis. Small 16(38):2003321
Mariyappan K, Ragupathy P, Ulaganathan M (2021) Enhancement of bromine kinetics using Pt@graphite felt and its applications in Zn-Br 2 redox flow battery. J Electrochem Soc 168(9):090566
Mariyappan K, Velmurugan R, Subramanian B et al (2021) Low loading of Pt@Graphite felt for enhancing multifunctional activity towards achieving high energy efficiency of Zn-Br 2 redox flow battery. J Power Sour 482(4):228912
Wang H, Li D, Chen L et al (2020) La and Sr composite oxides-modified graphite felt for aqueous organic redox flow batteries. Chem Res Chin Univ 36:1255–1260
Na Z, Sun X, Wang L (2018) Surface-functionalized graphite felts: Enhanced performance in cerium-based redox flow batteries. Carbon S0008–6223(18):30702–30704
Mahanta V, Raja M, Khan H et al (2020) Drastic improvement in capacity-retention and polarization of vanadium redox flow battery with hydrophilic CO3O4 nanostructure modified activated graphite felt electrodes. J Electrochem Soc 167(16):160504
Xiang Y, Daoud WA (2019) Investigation of an advanced catalytic effect of cobalt oxide modification on graphite felt as the positive electrode of the vanadium redox flow battery. J Power Sour 416(MAR.15):175–183
Dinesh A, Anantha MS, Priya MG et al (2020) Improved performance of iron-based redox flow batteries using WO3 nanoparticles decorated graphite felt electrode. Ceram Int 47(7):10250–10260
Faggiano L, Lacarbonara G, Badenhorst WD et al (2022) Short thermal treatment of carbon felts for copper-based redox flow batteries. J Power Sour 520:230846
Wang YH, Hung IM, Wu CY (2021) V2O5-activated graphite felt with enhanced activity for vanadium redox flow battery. Catalysts 11:800
Yan C, Tong X, Qu Y et al (2021) Porous manganese dioxide nanosheets on modified graphite felt for cathodes in high-capacity flexible Zinc–MnO2 batteries. Vacuum 1(1):110353
Chen L (2019) Roughened graphite felt electrode with enhanced electrochemical activity for vanadium redox flow batteries. Int J Electrochem Sci. https://doi.org/10.20964/2019.06.67
Qi HQ, Sun XP, Sun ZR (2021) Cu-doped Fe2O3 nanoparticles/etched graphite felt as bifunctional cathode for efficient degradation of sulfamethoxazole in the heterogeneous electro-Fenton process. Chem Eng J 427(2022):131695
Jiang Y, Cheng G, Li Y et al (2021) Promoting vanadium redox flow battery performance by ultra-uniform ZrO2@C from metal-organic framework. Chem Eng J 415(22):129014
Chen P, Cheng R, Meng G et al (2021) Performance of the graphite felt flow-through electrode in hexavalent chromium reduction using a single-pass mode. J Hazard Mater 17:125768
Xiao QH, Wa Ng L, Dan LI et al (2019) CdO-modified graphite felt as a high-performance negative electrode for a vanadium redox flow battery. Chin J Inorgan Chem 35:1678–1686
Li JZ, Qiang M, Qian X et al (2021) Performance improvement of non-aqueous iron-vanadium flow battery using chromium oxide–modified graphite felt electrode. Ionics 27:4315–4325
Zhang XF, Shen TD, Ding YL et al (2019) Graphite felt supported MgO catalytic ozonation of bisphenol A. Ozone Sci Eng 41:541–550
Lou Y, Fu D, Fabre B et al (2021) Bismuth coated graphite felt modified by silver particles for selective electroreduction of CO2 into formate in a flow cell. Electrochim Acta 371:137821
Lou YY, He W, Verlato E et al (2019) Journal pre-proof Ni-coated graphite felt modified with Ag nanoparticles: a new electrode material for electro-reductive dechlorination. J Electroanal Chem 849(C):113357
Cui L, Li Z, Jing W (2020) Cu/CuFe2O4 integrated graphite felt as a stable bifunctional cathode for high-performance heterogeneous electro-Fenton oxidation. Chem Eng J 420:127666
Lu C, Lu X, Yang K et al (2021) Cu, Ni and multi-walled carbon-nanotube-modified graphite felt electrode for nitrate electroreduction in water. J Mater Sci 56(12):7357–7371
Yang Z, Wei Y, Zeng Y et al (2021) Effects of in-situ bismuth catalyst electrodeposition on performance of vanadium redox flow batteries. J Power Sour 506:230238
Nariyama H, Ito S, Okada Y et al (2022) High energy density 3V-class redox flow battery using LiFePO4 and graphite with organic bifunctional redox mediators. Electrochim Acta 409:139915
Funding
This work is funding by the National Key Research and Development Program (2020YFC1909300), Regional joint fund project of Liaoning Provincial Department of science and technology (2020-YKLH-27), the Foundation of Liaoning Key Laboratory of Chemical Additive Synthesis and Separation (ZJNK2001) and the Yingkou Institute of Technology Innovation Team Project (TD201901).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, Y., Chen, N., Ren, Hl. et al. Application of modified graphite felt as electrode material: a review. Carbon Lett. 33, 1–16 (2023). https://doi.org/10.1007/s42823-022-00414-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-022-00414-x