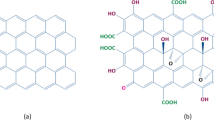
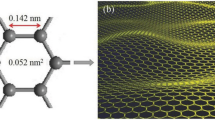
Abstract
Graphene, the wonder material has brought a revolutionary change in the field of nanotechnology owing to its tremendous properties. Though different methods for the synthesis of graphene have been reported, the chemical synthesis route offers a scalable and high-volume production of graphene. The unreliability of graphite and hydrocarbon resources to serve as steady supplies of carbon resources and further in the synthesis of graphene has led to the exploration and use of alternative low-cost carbon-rich resources (coal, graphite, rice husk, sugarcane bagasse, peanut shells, waste tyres, etc.) as precursors for graphene synthesis. The use of untraditional carbon resources reduces dependence on traditional resources (coal, graphite), reduced cost, increased reliability, and provides a way for the management of waste biomass. This review hence focuses on the synthesis of graphene by the most common approachable method, oxidation–reduction of graphite, along with the various other chemical methods of synthesis from varied carbon resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene, the carbon derivative is the simplest and thinnest material ever known. The one-atom thick planar sheets of sp2 bonded carbon atoms of graphene are densely packed giving it a honeycomb crystal lattice [1]. Geim and Novoselov [1] explained “graphene as a single atomic plane of carbon” exhibiting tremendous properties and a wide range of applications. The term graphene was first put forth by Boehm et al. [2] while designating the individual single-layer carbon foils as the final member of the graphitic series [2]. Graphite flakes, the common carbon precursor for the synthesis of graphene is a stack of graphene layers having a C–C bond length of about 0.142°A. Being the mother of all other graphitic forms of different dimensionalities (buckyballs, nanotubes, graphite, etc.), graphene has been termed the “wonder material” owing to its structure, physical, mechanical, electrical, thermal, and optical properties [3]. Defect-free graphene presents outstanding physical properties making it a remarkable material for making functional nanocomposites. The outstanding physical properties, in turn, include high intrinsic mobility and ballistic transport, high thermal conductivity and Young’s modulus, 98% optical transmittance, and a large specific surface area [4, 5]. The complete realization of graphene’s potential in applications ranging from transistor [6], energy storage devices [7], conductive inks [8] to replacement of indium-tin-oxide [9] is highly related to the different routes from which it is synthesized.
This review article focuses on the brief history and recent advancement in the chemical synthesis of graphene from varied carbon resources. Among the most common techniques established for graphene synthesis viz., mechanical cleaving, chemical exfoliation, thermal CVD, chemical synthesis, unzipping nanotube, microwave synthesis; chemical route consisting of the synthesis of graphite oxide followed by reduction with reagents (starting from graphite) became the most common and approachable method for preparation of graphene. The ability of pure graphite to act as the carbon precursor for the synthesis of graphene has been the key factor for the top to down synthesis approach. With increasing demand and the high cost of pure graphite, there occurs a dire need to search for inexpensive graphitic materials, which could serve as carbon precursors in the synthesis process. The journey toward graphene’s golden age is a slow but steady one. This, in turn, provides a possible route for inexpensive as well as reliable mass production of high-quality graphene.
2 Physico-mechanical methods for synthesis of graphene
The common physico-mechanical methods for synthesis of GO are exfoliation and reduction to form graphene, by the way of mechanical, chemical, thermal, or electrochemical routes [10]. In the mechanochemical ball-milling method, the kinetic energy of grinding media is used to separate graphite layers by overcoming the van der Waals forces thus inducing chemical reactions resulting in the functionalization of graphene. Additionally, micromechanical cleavage employs the Scotch tape method with baking and repetition several times, producing 200 nm thick multi-layered graphite or graphene nanosheets. With sonication, the yield of nanosheets can be improved from 40% to ~ 100% peeling improving electrical conductivity. This process. However, yields high-quality graphene with very few defects, low production rates, and difficulties in predicting the required number of peelings. On employing continuous mechanical cleavage from natural graphite using a three-roll-mill machine with PVC, generated nanosheets of graphene. However, the removal of PVC requires 5 h of heating at 500 °C, which is energy-intensive and contributes to the production cost [11].
Graphene exfoliation can also be performed by adding sodium cholate with sonication, however, the exfoliation yield is as low as 0.1%. Explosive exfoliation of graphite by the controlled detonation of acetylene in the presence of oxygen can produce graphene nanosheets in a combustion chamber with a yield per detonation as high as 66%. This method leads to complete disruption of graphene crystallinity but has several safety issues associated with it leading to runaway explosions [11].
These methods present several challenges as graphene synthesized by these means display inconsistent properties, namely, low production yields and poor conductivity, leading to larger adoption of chemical processes in the synthesis of graphene.
3 Chemical processes for synthesis of graphene from various carbon resources
Graphene has been a major research interest since long back. The several works reported for the exfoliation of graphite to graphene have been mainly attributed to the different works done by various researchers for the past 60 years. The various methods of preparation of graphene can be mainly split into two main categories: the top-down approach and the bottom-up approach. The top-down approach involves the separation of the individual layers of graphene from graphite by overcoming the Van der Waals forces existing between the layers [10]. On the other hand, the bottom-up approach considers carbon molecules as the building blocks from which bigger carbon structures and derivatives can be created with carbon molecules typically obtained from alternate carbon resources. Though the top-down approach faces several challenges including surface defects, agglomeration of separated sheets, tediousness in the process leads to difficulty in the production of large surface area graphene sheets. A bottom-up approach often offers the possibility of manufacturing graphene nanoribbons and graphene dots (so-called nano-flakes) in large quantities [11]. Among these various methods of preparation of graphene, the most challenging part lies in deciding one suitable method to synthesize the best quality graphene. Chemical synthesis via the redox process proves to be the most promising method for efficient synthesis of graphene owing to its easy manipulation, low cost, and high yield with the use of simpler production equipment.
Graphite is the most commonly and conventionally used form of carbon source for graphene synthesis due to its availability in natural form. Hydrocarbons which include solids like polymethyl methacrylate, polystyrene [11]; liquids like methanol, ethanol, benzene [12]; and gases like methane, ethane, have also been used as alternative carbon source. The unreliability of these hydrocarbons as steady supplies of carbon resources is attributed to the increasing demand for natural graphite, the need for pressurized systems for hydrocarbon transport, and market fluctuations. This, in turn, creates a dire need to explore alternative low-cost carbon-rich resources to grow high-quality graphene. Such attempts will not only prevent the dependence on traditional carbon resources but will also lead to the economic, environmentally friendly, and scalable synthesis of graphene. According to the reports, researchers have converted food waste, low-grade coal, agricultural wastes to value-added products and their carbon derivatives, the details of which are discussed in the following sections.
4 Traditional carbon resources
Graphite and coal are the traditional resources for the synthesis of graphene via GO and rGO. Graphite, the ordered carbon allotrope with layered structure has been the most commonly chosen traditional carbon resource (apart from coal) as the starting material for the preparation of graphene-related carbon materials due to its availability and low cost. The various forms of natural graphite include crystalline graphite (commonly referred to as flake graphite), amorphous graphite (very fine flake graphite), and highly ordered pyrolytic graphite (graphite with an angular spread between the graphite sheets of less than 1°A). Other compounds derived from graphite include graphite powder, expanded graphite, HOPG (highest quality synthetic graphite), etc.
4.1 Utilization of graphite for the synthesis of graphene
One of the most popular approaches towards graphene synthesis is firstly the use of a strong oxidation agent on graphite to obtain graphite oxide (stacked GO) followed by exfoliation in solution to obtain graphene oxide, followed by reduction of GO to form rGO (reduced graphene oxide) or graphene. The schematic representation of the approach of preparation of rGO or graphene is depicted in Fig. 1. The common route is as follows:
4.1.1 Oxidation/exfoliation of graphite to GO
GO is a non-conductive hydrophilic carbon material. It is a non-stoichiometric molecule, which has a layer of graphene decorated with oxygen functionalities namely alkoxy, hydroxyl, carbonyl, and carboxyl groups [13]. In the wet synthesis approach, the route is achieved via oxidation/exfoliation of graphite in a strongly acidic medium (e.g., H2SO4) with the presence of an oxidant (like KMnO4), the mechanism of which is explained in the consequent section.
4.1.2 Mechanism for the formation of GO
Though the mechanism of interaction between solid graphite, liquid medium, and oxidizing agent remains unclear, a three-step conversion process [14] shown in Fig. 2 consists of:
-
First step involves the conversion of bulk graphite to a graphite intercalation compound (GIC) by an acidic oxidizing agent.
-
Second step being the rate-determining step involves the formation of pristine graphite oxide (PGO) or oxidized graphite from the first step by diffusion of the oxidizing agent into the graphite galleries.
-
Final step involves the conversion of PGO into GO upon exposure to water leading to hydrolysis of covalent sulfates, loss in interlayer structure along with modification in oxygen functionalities.
4.1.2.1 Common methods for the preparation of GO
The extent of graphite oxidation largely varies based on the procedures used, reaction conditions, and graphite precursor, which is evident from the C:O ratio of different methods given in Table 1. The oxidation of graphite increases the interlayer spacing by weakening their interaction facilitating the exfoliation of graphite oxide into individual layers upon sonication or mechanical stirring, in turn, inducing lattice defects. This exfoliation process can happen in water medium or polar solvents including NMP, DMF, etc. to produce stable graphene oxide suspension, the details of which are reviewed elsewhere [28].
4.1.2.2 Different synthetic methods of oxidation of graphite
Brodie [21] first synthesized graphite oxide by adding the stoichiometric amount of potassium chlorate (KClO3) to the slurry of graphite in fuming nitric acid [21]. In 1898, Staudenmaier used a mixture of concentrated sulfuric acid and fuming nitric acid (to increase acidity) followed by the gradual addition of chlorate to the reaction mixture to prepare graphite oxide [22]. Even though the variation in procedure led to the production of highly oxidized graphite oxide, however, both methods generated toxic ClO2 gas, which rapidly decomposed in the air resulting in explosions. Hofmann et al. [23] improvised Staudenmaier’s method by replacing fuming nitric acid with 68% nitric acid to reduce the amount of acid fog produced [23]. To improve the overall reaction safety, Hummers and Offeman [26] replaced KClO3 with KMnO4 and fumed HNO3 with NaNO3 in concentrated H2SO4 to completely avoid the formation of acid fog [26]. The highly efficient graphite oxide prepared by this method was adopted as the most common method to synthesize graphite oxide as shown in Fig. 3. Although the oxidation procedure adopted by Hummers and Offeman [26] attracted increased attention, it led to the difficulty in the separation of Na+, NO3− ions from the water formed during the synthesis, purification of obtained graphite oxide, and evolution of toxic gases (like NO2, N2O4). As a result, large variations and optimization approaches were practiced with Hummers’ method to improve the overall yield and quality of the product (Fig. 4).
Chen et al. [29] reported the production of highly oxidized GO without the use of sodium nitrate and obtained similar results as the Hummers method, indicating the very little effect of NaNO3 in the process. Shao et al. [19] reported that the oxidation degree of graphite should be as low as to obtain single-layer rGO upon reduction [19]. As a piece of evidence to it, Xu et al. [30] demonstrated the production of mildly oxidized GO by lowering the ratio of KMnO4 from 3:1 (Hummers method) to 1:1 during oxidation and reported the conductivity values of the produced rGO to be 2.7–3 times more than the conventionally produced rGO, indicating the stringent effect of chemical reduction on the former owing to its fewer oxygen functionalities [30]. These variations largely show the differences in the oxidation of graphite with different reaction conditions and procedures used. On the other hand, it was reported that the size of parent graphite is detrimental in determining the structure of GO and its influence on the difficulties of product formation [29, 30]. As a result, Jasim et al. [31] investigated the role of the starting graphitic material on the yield of the rGO obtained and studied the structural characteristics of the GO sheets produced [31]. The different forms of graphite namely flake graphite, ground graphite, and powder graphite were subjected to modified Hummer’s method to produce their respective GO’s. Owing to its larger surface area, GO produced from powder graphite yielded 70 mg of GO (from 0.4 g feed), which was double the quantity as produced by flakes and ground graphite. Since size modification of the graphite crystal in different forms affect intercalation and exfoliation rates, Sun et al. [32] used expanded graphite as the starting precursor to produce GO [32] as shown in Fig. 5. This commercially available pre-expanded graphite (d50:15 μm) with a larger inter-layer space, showed spontaneous expansion with complete intercalation. This water-free oxidation method proves a promising method for the scalable synthesis of graphene. The different types of graphite/graphene oxide produced by various methods vary in properties, giving rise to a wide range of future use applications. Hence there lies no exhaustive method to produce GO.
4.1.3 Reduction of GO to form rGO or graphene
GO has emerged as a material of intense research interest due to its ability to undergo reduction to form graphene-like sheets by removing the oxygen functionalities on its basal structure along with recovery of the conjugated graphitic lattice. The obtained reduced GO (rGO) is generally considered as one form of chemically derived graphene, chemically converted graphene, reduced graphene, or graphene by itself depending upon the reduction process to which it is subjected. The difference in reduction processes affects the final properties and performance of the materials derived from rGO.
The two main reduction strategies to reduce GO are achieved by thermal treatment or chemical treatment. The rapid heat treatment (> 2000 °C/min) to stacked layers of GO, makes the oxygen-containing functional groups on the carbon plane decompose into gases (CO/CO2) and hence increasing the pressure between the stacked layers. This, in turn, produces a dual effect-exfoliation of graphite oxide and reduction of functionalities on the carbon basal plane to produce graphene or rGO. Though thermal treatment appears to be a good strategy to produce graphene, the small quantity of graphene sheets produced with wrinkled and distorted carbon planes paves the way to the chemical reduction techniques. Since chemical reduction could be achieved at moderate heating without any requirement for critical equipment and environment, it is considered a cheaper and easily available way for mass production of graphene.
4.1.3.1 Mechanism of reduction
Since the difference in the structure of GO and graphene largely lies in the number of functional groups attached to the carbon plane and structural defects, the reduction mechanism mainly targets the elimination of those functional groups and healing of the structural defects, as depicted in Fig. 6. The chemical reduction of GO shows selectivity in the chemical deoxygenation process depending upon the reducing agent used. The mechanism to the reduction with hydrazine was first proposed by Stankovich et al. [33], after which Li et al. [20] noticed the preservation of carboxyl groups after reduction of GO with hydrazine [33, 34]. Since chemical reduction preserved the structure of the carbon plane and thermal treatment at high temperature facilitated desorption of functional groups, the combination to obtain rGO gave a C:O ratio of 246 [34]. In this multi-step reduction, the deoxygenation process was carried with sodium borohydride, dehydration process with concentrated sulfuric acid, and finally thermal annealing. Since chemical treatment could accomplish the reduction to the maximum extent which was solely not possible by thermal annealing (even at temperatures greater than 2000 °C), the biggest challenge of chemical reduction lies in the choice of a suitable reducing agent. The reduction of GO to prepare graphene-like rGO is being achieved by several reduction methods with varied reducing agents to attain controllable functionalization which can alter the application of graphene in various fields. Though complete reduction to graphene is on the verge of accomplishment by research, partial reduction of GO is rather carried out with a range of chemical reductants.
Table 2 gives evidence and details to the process of reduction using conventional, non-conventional/alternative, and green reagents.
4.1.3.2 Conventional reductants
H2S was used as the first known reducing agent to produce reduced GO [35]. Since then a large number of reductants have been used to produce rGO, one of which was hydrazine. Stankovich et al. [33] first reported the use of hydrazine to obtain chemically derived graphene which opened an easy way to accept hydrazine (in solid, colloidal, or vapor form) and its derivatives (hydrazine hydrate, dimethylhydrazine), as good chemical reagents to produce rGO which resembled as pristine graphite to a larger extent [33, 43]. Hydrazine could obtain rGO with a maximum C:O ratio of 12.5 [43]. However, due to its toxic nature and harmful effects, there was an imperative need to find alternative reducing agents with characteristics comparable to hydrazine.
4.1.3.3 Alternative chemical reductants
Shin et al. [45] demonstrated the effectiveness of sodium borohydride as a replacement for hydrazine [46]. Since NaBH4 was only most effective at removing the carbonyl groups, moderately effective at removing epoxy, and carboxyl groups, a dehydration step using concentrated H2SO4 at 180 °C after reduction with NaBH4 was tested to increase the reduction efficiency of the remaining hydroxyl groups too and obtained a C:O ratio of the rGO to be 8.6 after the two-step treatment. Pei et al. [44] reported another strong reducing agent more effective than hydrazine, which could be used to reduce GO films [45]. They reported that the C:O ratio was much better maintained as 15 using hydroiodic acid (HI) as the reducing agent. The GO film reduced by HI showed greater flexibility and improved tensile strength, whereas hydrazine vapor reduced GO films became too rigid to be rolled showing an increase in the thickness of the film by three times whereas hydrazine monohydrate and sodium borohydrate reduced GO films to split up. Other reducing agents (inferior to the strong reductants as reported earlier) to reduce GO included hydroquinone, hydroxylamine, potassium hydroxide, pyrogallol, etc.
4.1.3.4 Green reductants
The toxic and explosive nature of these chemical reagents has led to a pathway of the “green reduction” approach. This approach would mainly concern two factors apart from environmental and safety issues. Firstly, the green reductant should be as effective as hydrazine, and secondly, there should be no precipitation of the green reductant after reduction. Deionized water at 95 °C for 4 days was used as the media without the use of a reducing agent [46]. Though this method proved efficient in terms of environmental friendliness, the first condition of similar effectiveness of hydrazine was not achieved. An exhaustive comparison of the reducing ability of several different reducing agents (hydrazine monohydrate, sodium borohydride, pyrogallol, and vitamin C) towards deoxygenation of graphene oxide suspensions has been evaluated [38]. The GO suspensions were dispersed in the reductants (0.1 mg ml−1) at 95 °C. The findings suggested that vitamin C [ascorbic acid(AA)]—a natural antioxidant, food additive, matched the reduction efficiency with that of hydrazine, where the reduction can be made in water as well as common polar solvents like N, N-dimethyl formamide (DMF) and N-methyl-2-pyrrolidone (NMP). These findings together with the non-toxicity of this natural product prove that ascorbic acid can an ideal substitute for hydrazine in the large-scale production of solution-processable graphene. Ascorbic acid also minimizes the risk of the introduction of heteroatoms in the reduced products owing to the presence of carbon, oxygen, and hydrogen. Scalable preparation of RGO films was done by vacuum-assisted self-assembly method with sodium citrate as the reductant [39]. Caffeic acid (CA), another organic acid reductant that was used to synthesize rGO, could be applied in gas sensors and supercapacitors [41]. Lemon juice can also serve as an efficient reductant for GO with a C/O ratio of 8.2 [44]. Recently, a binary reducing system of acetic acid and sodium citrate has been tested for the green synthesis of rGO [42, 47, 48].
4.2 Utilization of coal in graphene synthesis
The utilization of coal (carbon enriched abundantly available traditional resource) as an alternative carbon source has opened a new pathway for inexpensive, reliable, and scalable synthesis of graphene owing to its poly-aromatic structures that are quite similar to sp2 bonding characteristics of graphene.
Sharma et al. [48] reported a method to synthesize graphene from coke coal by mixing the crushed powder with 60 ml HNO3 (95%) and 20 ml H2SO4 (68%) followed by subsequent sonication for 6 h [49]. The black suspension left to stand overnight was centrifuged (after diluting it with deionized water till pH 5) to obtain a black precipitate which was further heated to 100 °C for 1 h to obtain grown graphene. Though direct transformation from coal to graphene was reported, the synthesis route of graphene via the formation of graphene oxide was most commonly employed. Synthesis of GO from coal was investigated using NaNO2, H2SO4 without the use of dangerous chemicals (KMnO4 and H2O2) by stirring coal powder first at room temperature for ~ 16 h followed by stirring at 80 °C for 6 h [50]. The GO gets sedimented after centrifuging the diluted mixture with 1 M HNO3 at room temperature. This solution of GO was subjected to thermal reduction (argon flow at 1100 C for 3 h) on a quartz surface to obtain the graphene film. This common method of synthesis via GO formation and subsequent reduction follows multiple steps and the use of concentrated acids gives rise to health hazards and disposal problems. This issue was in turn addressed by the use of leonardite extracted humic acid as an equivalent to GO obtained by the Hummers method [50]. Leonardite is low-grade lignite coal extracted in a basic solution (pH ~ 10) with a yield of 75%. The solution containing leonardite was then precipitated by acidification to obtain humic acid (HA). The powder form of HA possessed a comparable C:O ratio as GO produced by acid oxidation of graphite (Fig. 7) and hence proved to be a material very similar to GO with a large number of oxygenated groups around the edges of the graphene-like core. It also shows quite a similar response for chemical reduction to form graphene in comparison to the GO formed by the Hummers method, which is evident from Fig. 8. The extent to which humic acid matches the characteristics of GO as produced by the Hummers method in terms of morphology, carbon/oxygen ratios, Raman spectroscopy, etc. are compared in Table 3. This is a much less expensive method of using HA as the starting precursor (rather than graphite) for producing GO and ultimately conjugated graphene-like analogs proves noteworthy.
5 Non-traditional carbon resources
The approaches to synthesize graphene from non-traditional (rice husk, bagasse, etc.) has been discussed in the following section.
5.1 Rice husk
Rice husks (RHs), the protective coating of grains of rice, is a by-product of the milling of rice and renewable waste, constituting 16–25 wt% of the dry weight of paddy rice. Due to the low density and less commercial interest of RH, its high-value applications are often left unnoticed except for silica nanoparticle synthesis [51]. Rice husk ash (RHA)—the byproduct of burnt RH at ambient atmosphere has wide applications in the synthesis of silica, activated carbon, zeolites, graphene, etc. The use of RH and RHA in domestic and industrial processing not only provides an alternative solution to the disposal problem but also addresses the issue of burning or landfilling and further merits in converting them into value-added products.
Owing to the very good properties of rice husk, several works have been carried out to synthesize graphene from it, the summary of which is given in Table 4. Out of the two different processes adopted to synthesize graphene, one method uses Rice Husk Ash (RHA) as the starting material. RHA was formed by washing the rice husk (RH) repetitively (to remove silica and other contaminants), followed by combustion in the air. The obtained RHA was chemically activated by treating it with KOH (1:5 ratio of RH: KOH). This mixture was compacted in a porcelain crucible covered with ceramic wool and was kept in a larger crucible. The larger crucible was covered with a sufficient amount of powder (either carbon black or RHA) to provide a barrier against oxidation of the sample inside the porcelain crucible. The crucible was annealed at 1123 K for 2 h in a muffle furnace followed by washing with deionized water to remove excess KOH and other impurities. Finally, the filtered sample was dried at 373 K for 24 h to obtain rice husk-derived graphene. The use of RHA in process-2 itself as the protective coating was adopted to avoid oxidation instead of carbon black in process-1 [52, 53]. This approach further improved the cost-effectiveness and reliability of the process and produced nano-sized silica along with graphene. The properties of silica combined with graphene could be exploited for use in Li-ion battery-based energy storage devices. In another method of development of graphene, the rice husk was subjected to a four-stage treatment, which includes carbonization of RH, desilication of CRH, chemical activation using alkali, followed by exfoliation of CRH (Fig. 9) with high yield [51].
5.2 Sugarcane bagasse
Among the various important agricultural crop residues, sugarcane bagasse is one of the main agricultural waste, which could serve as a major substrate for the production of fuel, alcohol, chemicals, protein for food due to its global availability throughout the year. The Indian sugar industry produces ~ 75–90 million tonnes of wet bagasse annually from 600 operational sugar mills [54]. Bagasse, a fibrous residue of the cane stalk left after crushing and extraction of the juice mainly consists of cellulose (27%), pentosans (30%), lignin (20%), and ash (3%) [55].
Instead of simply disposing of the million-ton agro waste, a simple, low-cost, and non-toxic process was adopted to synthesize graphene oxide by oxidizing sugarcane bagasse under a muffled atmosphere [55]. This method seemed to be more environmentally friendly and less sophisticated as compared to the traditional Hummers or CVD process. In the typical process, 0.5 g of crushed and finely grounded sugarcane bagasse powder (taken in a crucible) was mixed with 0.1 g of ferrocene. The mixture was directly introduced into the muffle furnace at a temperature of 300 °C for 10 min. The black solid product obtained was found to be fully oxidized graphene oxide with an interlayer distance of 0.79 nm and a well-graphitized structure as verified by SEM, TEM, XRD, and FTIR.
5.3 Peanut shells
Peanut shells, the inexpensive, globally known waste biomass can be efficiently and economically converted into few-layer graphene-like nanosheets without the use of any chemical graphitizing agent [56]. The peanut shell-derived graphene possessed remarkably high specific surface area (2070 m2 g−1) making it an ideal choice to be used in supercapacitors. Purification of the peanut waste was done by washing it with distilled water followed by sun drying for a few days to remove dust and other interfering particles attached. The moisture content was removed by vacuum drying the shells in an oven at 80 °C overnight. The finely crushed powder was pyrolyzed in a tubular furnace at 1073 K under argon atmosphere for 2 h. The organic depositions in the carbonized peanut shell were removed by washing it thoroughly with isopropanol. This carbon powder was subjected to chemical activation by KOH (1:3 ratio) to produce a homogenous mixture, which was heated at 1073 K in an argon atmosphere to get an activated carbon material. The peanut shell activated carbon material was exfoliated in 10% sulfuric acid by sonication for 1 h with a power of 15 W with a 5 s pulse. The obtained product after subjecting to repeated washing was centrifuged and dried at 80 °C overnight; to obtain peanut shell-derived few-layer graphene sheets (Fig. 10).
5.4 Natural and industrial carbonaceous waste
High-quality GO and rGO sheets can be synthesized from various natural and industrial carbonaceous wastes like wood, leaf, bagasse, fruit wastes, animal wastes (bone and cow dung), semi-industrial waste (newspaper), and industrial waste (soot powders) [57]. Natural carbonaceous wastes were subjected to imperfect burning at 400–500 °C for 5 days to obtain charcoal. Graphitization of the carbonized materials was done by mixing 1 equivalent of wastes with 0.5 equivalent of ferric chloride with the addition of distilled water. The mixture was stirred at 60 °C for 5 h and dried at 100 °C for 5 h to produce a black solid material. The prepared charcoal or soot powders were used as a carbon precursor to synthesize GO by modifying Hummers’ method.
Recently, MIT researchers discovered a potentially scalable way to mass-produce graphene [58,59,60]. Utilizing an industrial manufacturing process known as a roll-to-roll method, combined with chemical vapor deposition, the researchers were able to produce a graphene foil at 5 cm per min. After their machine had run for 4 h, they had produced 10 m of graphene. If this process continues to prove viable, we may begin to see the mass production of graphene become a reality.
5.5 Waste tyres
Approximately 1000 million used tires will emerge worldwide each year, and the quantity continually grows as the vehicle development [61]. Numerous tires are inevitably discarded, and thus their disposal is a worldwide issue. Apart from the pyrolysis methods practiced in a few undeveloped and developing countries, utilization of waste tyres to convert to graphene is the most recent innovation and thus finding wide applications.
Bonnia et al. [62] synthesized graphene oxide from waste carbon tyre using modified Hummer’s method [62]. Beyond this initial development, Wang et al. [61] could directly convert waste tyres into 3D graphene through an alkaline-assisted one-step pyrolysis process at ~ 1000 °C [61]. The method could transform waste tyre existing as amorphous carbon nanospheres to monolithic carbon conglomeration, followed by wrinkled graphene, and finally to vertical 3D graphene which was proven to have excellent capacitive behaviors with both high rate performance and superstable cyclic life.
Okan et al. [63] produced graphene nanoplatelets (GNP) from recycled carbon black obtained from the pyrolysis of waste tyres [63]. These GNPs were used to reduce glass fiber amount for the production of polyamide 66 (PA66) based automotive part, a cost-effective and light-weight automotive plastic composite part by extrusion and injection techniques and providing 10% weight reduction. Additionally, new hybrid compounds can be used in engine components (e.g. oil pan), plastic structural parts (e.g. FEM, pedals), metal-plastic hybrid structural parts (e.g. traverses, brackets), and underhood components (e.g. engine undercover) in the automotive industry. Similar structural stability components of GNPs with cement grouts were prepared by Berktas et al. [64]. In presence of functionalized silica nanoparticles, the dispersion and solubility of carbon material improved and thus the hydrolyzable groups of silane coupling agents were attached to the silica surface yielding hybrid structures. Based on the addition of GNPs from 3 to 5%, water uptake reduced resulting in the enhancement in thermal conductivity by 29% [64].
Flash Joule heating (FJH) is an effective method to synthesize graphene by Advincula et al. [65]. Using turbostratic flash graphene (tFG) into Portland cement results in a significant increase in the compressive strength of the composite. They blended 0.1 wt% tyre-derived graphene with 0.05 wt% for carbon black and shredded tires along with Portland cement-produced concrete cylinders which upon curing for 7–28 days, improved the compressive strength (~ 30%) [65]. It would thus leave a good scope of research using this material and synthesis of low-cost graphene.
6 Conclusions and future directions of research
The different approaches (Table 5) to synthesizing graphene from top-down or bottom-up methods have resulted in different grades of graphene with significant properties demanding the use in various specific applications. The difference in synthetic methods of preparation affects the shape, dimensions, and quality of the obtained graphene, thereby challenging its upscale synthesis. This is where chemical techniques prove more advantageous compared to other methods of preparation.
The intention of achieving both high quality and scalable graphene is often accomplished by the controlled oxidation of the most widely used precursor-graphite to yield highly reducible graphene oxide, the fabrication of which is detailed reviewed in this article. The combination of chemical reduction and thermal annealing has been proved the well-designed reduction strategy to remove functionalization in GO sheets with low defects giving rise to graphene or rGO. With a growing demand for greener approaches, the proposed growth mechanisms of production of graphene from untraditional sources like rice husk, coal, peanut shells, tyres, have significantly assisted in the optimization techniques and have helped in reducing the pressure on the traditional resources thereby minimizing environmental pollution. This review is a result of the efforts to pile up information regarding different carbon resources from which graphene can be prepared.
Further, a detailed study on the controlled oxidation and reduction combined with a deeper understanding of the mechanism involved in the formation of graphene would facilitate the application of graphite in new emerging fields. Recent applications of graphene and its derivates have been off-late realized due to their application in storage devices like batteries, supercapacitors, etc. for high charge retention-transfer [66,67,68]. Other applications include biomedical modifications useful in drug delivery, coatings, electrochemical sensors, gas adsorption, and water purification membranes, catalysts, solar cells, etc. Extraction of graphene and its derivates from the discussed low-cost resources can be very useful for commercial production and applicability in aforesaid applications. Furthermore, the application of graphene in battery manufacturing is an important avenue wherein the cheap raw material for anode production shall be a useful improvement in process economics.
References
Geim AK, Novoselov KS (2017) The rise of graphene. Nat Mater 6:183–191
Boehm HP, Setton R, Stumpp E (1994) Nomenclature and terminology of graphite intercalation compounds. Pure Appl Chem 66:1893–1901
Barnard A, Snook K (2010) Size and shape-dependence of the graphene to graphane transformation in the absence of hydrogen. J Mater Chem 20:10459–10464
Rao C, Biswas K, Subrahmanyam K, Govindaraja A (2009) Graphene-the new nanocarbon. J Mater Chem 19:2457–2469
Soldana C, Mahmood A, Dujardin E (2010) Production properties and potential of graphene. Carbon 48:2127–2150
Schwierz F (2010) Graphene transistors. Nat Nanotechnol 5:487–496
Liang M, Zhi L (2009) Graphene-based electrode materials for rechargeable lithium batteries. J Mater Chem 19(33):5871–5878
Huang L, Huang Y, Liang J, Wan X, Chen Y (2011) Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res 4:675–684
Segal M (2009) Selling graphene by the ton. Nat Nanotechnol 4:612–614
Edwards RS, Coleman KS (2013) Graphene synthesis: relationship to applications. Nanoscale 5:38–51
Yan Y, Nashath FZ, Chen S, Manickam S, Lim SS, Zhao H, Lester E, Wu T, Pang CH (2020) Synthesis of graphene: potential carbon precursors and approaches. Nanotechnol Rev 9:1284–1314
Li Z, Wu P, Wang C, Fan X, Zhang W, Zhai X, Zeng C, Li Z, Yang J, Hou J (2011) Low-temperature growth of graphene by chemical vapor deposition using solid and liquid carbon sources. ACS Nano 5(4):3385–3390
Sun Z, Yan Z, Yao J, Beitler E, Zhu Y, Tour JM (2010) Growth of graphene from solid carbon sources. Nature 468:549–552
Botas C, Alvarez P, Blanco C, Santamaria R, Granda M, Ares P, Rodriguez-Reinoso F, Menendez R (2012) The effect of parent graphite on the structure of graphene oxide. Carbon 50(1):275–282
Wu ZS, Ren WC, Gao LB, Liu BL, Jiang CB, Cheng HM (2009) Synthesis of high-quality graphene with a pre-determined number of layers. Carbon 47(2):493–499
He H, Klinowski J, Forster M, Lerf A (1998) A new structural model for graphite oxide. Chem Phys Lett 287:53–56
Dimiev AM, Tour JM (2014) Mechanism of graphene oxide formation. ACS Nano 8(3):3060–3068
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Shao G, Lu Y, Wu F, Yang C, Zeng F, Wu Q (2012) Graphene oxide: the mechanisms of oxidation and exfoliation. J Mater Sci 47:4400–4409
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3(2):101–105
Brodie BC (1859) On the atomic weight of graphite. Phil Trans R Soc Lond 149:249–259
Staudenmaier L (1898) Verfahren zur darstellung der graphitsäure. Ber Dtsch Chem Ges 31(2):1481–1487
Hofmann U, Konig E (1937) Studies on graphite oxide. J Anorg Allg Chem 234(4):311–336
Hofmann U, Hoist R (1939) About the acidification and methylation of graphite oxide. Ber Dtsch Chem Ges 72(4):754–771
Loryuenyong V, Totepvimarn K, Eimburanapravat P, Boonchompoo W, Buasri A (2013) Preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Adv Mater Sci Eng 2013:1–5
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Amer Chem Soc 80(6):1339
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814
Sun L, Fugetsu B (2013) Mass production of graphene oxide from expanded graphite. Mater Lett 109:207–210
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229
Xu Y, Sheng K, Li C, Shi G (2011) Highly conductive chemically converted graphene prepared from mildly oxidized graphene oxide. J Mater Chem 21:7376–7380
Jasim DA, Lozano N, Kostarelos K (2016) Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater 3:014006
Sun J, Yang N, Sun Z, Zeng M, Fu L, Hu C, Hu S (2015) Fully converting graphite into graphene oxide hydrogels by pre-oxidation with impure manganese dioxide. ACS Appl Mater Interf 7:21356–21363
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Hoffmann U, Frenzel A (1934) The reduction of graphite oxide by hydrogen sulfide. Kolloid.-Z 68:149–151
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11(3):771–778
Huitao Y, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide-based on improved Hummers method. Sci Rep 6:36143
Pendolino F, Armata N, Masullo T, Cuttitta A (2015) Temperature influence on the synthesis of pristine graphene oxide and graphite oxide. Mater Chem Phys 164:71–77
de Silva KKH, Huang HH, Joshi RK, Yoshimura M (2017) Chemical reduction of graphene oxide using green reductants. Carbon 119:190–199
Bo Z, Shuai X, Mao S, Yang H, Qian J, Chen J, Yan J, Chen K (2014) Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci Rep 4:4684
Chong SW, Lain CW, Abdul Hamid SB (2015) Green preparation of reduced graphene oxide using a natural reducing agent. Ceram Inter 41(8):9505–9513
Ji-Li T, Hua-Yu Z (2017) Preparation and characterization of reduced graphene oxide using ascorbic acid and sodium citrate as binary reductant. Fuller Nanotub Carbon Nanostr 25(1):17–22
Fernandez-Merino MJ, Guardia L, Paredes JI, Villar-Rodil S, Solıs-Fernandez P, Martınez-Alonso A, Tascon JMD (2010) Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J Phys Chem C 114:6426–6432
Zhu C, Guo S, Fang Y, Dong S (2010) Reducing sugar: new functional molecules for the green synthesis of graphene nanosheets. ACS Nano 4(4):2429–2437
Pei S, Zhao J, Du J, Ren W, Cheng HM (2010) Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 48(15):4466–4474
Shin HJ, Kim KK, Benayad A, Yoon SM, Park HK, Jung JS (2009) Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Func Mater 19(12):1987–1992
Wang X, Tang H, Huan S, Zhu L (2014) Fast and facile microwave-assisted synthesis of graphene oxide nanosheets. RSC Adv 4:60102–60105
Powell C, Beall GW (2015) Graphene oxide and graphene from low-grade coal: Synthesis, characterization, and applications. Curr Opin Colloid Interf Sci 20:362–366
Sharma S, Mahajan D (2016) Cost-effective synthesis and properties of graphene. Ind J Sci Technol 9(48):1–4
Wu Y, Ma Y, Wang Y, Huang L, Li N, Zhang T, Zhang Y, Wan X, Huang Y, Chen Y (2013) Efficient and large scale synthesis of graphene from coal and its film electrical properties studies. J Nanosci Nanotechnol 13:929–932
Singh P, Bahadur J, Pal K (2017) One-step one chemical synthesis process of graphene from rice husk for energy storage applications. Graphene 6:61–71
Muramatsu H, Kim YA, Yang KS, Cruz-Silva R, Toda I, Yamada T, Terrones M, Endo M, Hayashi T, Saitoh H (2014) Rice husk-derived graphene with nano-sized domains and clean edges. Small 10:2766–2770
Azizovna SM, Ivanovich CD, Aimuhametovich MZ, Di Capua R (2017) Development of a method of obtaining graphene layers from rice husk. Func Nanostr Proc 1:6–8
Somanathan T, Prasad K, Ostrikov K, Saravanan A, Mohana KV (2015) Graphene oxide synthesis from agro waste. Nanomaterials 5:826–834
Thiyagarajan P (2021) A review on three-dimensional graphene: synthesis, electronic and biotechnology applications—the unknown riddles. IET Nanobiotechnol 15:1–10
Purkait T, Singh G, Singh M, Kumar D, Dey RS (2017) Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci Rep 7:15239
Akhavan O, Bijanzad K, Misepah A (2014) Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv 4:20441–20448
Peng L, Xu Z, Liu Z, Wei Y, Sun H, Li Z, Zhao X, Gao C (2015) An iron-based green approach to 1-h production of single-layer graphene oxide. Nat Commun 6:5716
Yang D, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD (2009) Chemical analysis of graphene oxide films after the heat and chemical treatments by X-ray photoelectron and micro-Raman spectroscopy. Carbon 47(1):145–152
Wang X, Zhi L, Mullen K (2008) Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett 8(1):323–327
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA (2006) Graphene-based composite materials. Nature 442(7100):282–286
Wang C, Li D, Zhai T, Wang H, Sun Q, Li H (2019) Direct conversion of waste tires into three-dimensional graphene. Energy Storage Mater 23:499–507
Bonnia NN, Zanuri AZ, Asli NA, Masdar NA, Ratim S, Yahaya SM, Mahat MM, Ramli R (2018) Synthesis of graphene oxide from waste carbon tyre using modified Hummer’s method. Int J Engg Technol 7:352–355
Okan BS, Menceloğlu Y, Ozunlu BG, Yagci YE (2020) Graphene from waste tire by recycling technique for cost-effective and light-weight automotive plastic part production. AIP Conf Proc 2205:020046. https://doi.org/10.1063/1.5142961
Berktas I, Ghafar AN, Fontana P, Caputcu A, Menceloglu Y, Okan BS (2020) Facile synthesis of graphene from waste tire/silica hybrid additives and optimization study for the fabrication of thermally enhanced cement grouts. Molecules 25:886. https://doi.org/10.3390/molecules25040886
Advincula PA, Luong DX, Chen W, Raghuraman S, Shahsavari R, Tour JM (2021) Flash graphene from rubber waste. Carbon 178:649–656
Jiang J, Li N, Zou J, Zhou X, Eda G, Zhang Q, Zhang H, Li L-J, Zhai T, Wee ATS (2019) Synergistic additive-mediated CVD growth and chemical modification of 2D materials. Chem Soc Rev 48:4639–4654
Hiltunen VM, Koskinen P, Mentel KK, Manninen J, Myllyperkiö P, Pettersson M, Johansson A (2021) Ultrastiff graphene. npj 2D Mater Appl 5(78):1–15
Li N, Peng J, Ong WJ, Ma T, Arramel ZP, Jiang J, Yuan X, Zhang C (2021) MXenes: an emerging platform for wearable electronics and looking beyond. Matter 4:377–407
Acknowledgements
Ms. Swetha. V. express her gratitude to the IASc-NASI-INSA for the Summer Research Fellowship that helped to carry out this work at CSIR-National Metallurgical Laboratory, Jamshedpur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Research involved with human or animal participants
The research doesn’t involve harm to any human or animal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abhilash, Swetha, V. & Meshram, P. An overview on chemical processes for synthesis of graphene from waste carbon resources. Carbon Lett. 32, 653–669 (2022). https://doi.org/10.1007/s42823-021-00313-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-021-00313-7