Abstract
The carbon-based nanostructures are in limelight due to their widespread applications in nano-to-micro-scale technologies. The carbon dots are known for their unique physical, electrical, optical, chemical and biological properties. The carbon dots (CDs) are being produced through several well-developed synthesis methods, one of which is the green sonochemical. This method is preferred over others because it is a green source of energy, facile, fast, low-temperature process, non-toxic and less expensive. Despite the fact of using 90% less energy than other methods, this method has been overlooked in the published literature. It is possible to prepare pure and doped CDs of low toxicity and controlled physicochemical properties through sonochemical method. In recent years, sonochemically produced CDs have been tuned and characterized for a variety of applications. This review has explored the merits and demerits of sonochemical method in comparison to the other methods for the synthesis of pure CDs and their nanocomposites. The role of multiple factors in tailoring the specific parameters of CDs for their application in antibacterial, polymerization, tissue engineering, catalysis, bio-imagining, supercapacitors, drug delivery and electric devices is also elaborated in this review. This review also concludes on future directions in the applications of sonochemically produced CDs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles are flexible materials with different chemical, optical, physical, magnetic, electrical, electrochemical and biological properties. Nanoparticles are widely categorized into inorganic and organic. Carbon allotropes, such as carbon nanotubes, fullerenes and graphene, are being used in many sophisticated technologies due to their unique properties. Carbon dots (CDs) are known for their outstanding biocompatibility, chemical inertness, low cytotoxicity, better optical properties, high solubility in water [1] and ability to get functionalized with various chemical species [2, 3]. CDs are included in zero-dimensional nanoparticles with average size less than 10 nm in all dimensions [4]. CDs were mentioned as a novel class of carbon nanoparticles in the first scientific paper on CDs, which was published in 2004 [1]. CD is a general term used for different nanoscale carbon particles, such as polymer quantum dots (PQDs), carbon quantum dots (CQD) and carbon nano-dots (CNDs) or graphene quantum dots (GQDs), as illustrated in Fig. 1 [5]. GQDs are formed of one or more sheets of graphene with chemical groups bonded on their edges. GQDs are anisotropic with lateral dimensions larger than their height. CNDs are spherical in shape and classified into carbon nanoparticles and carbon quantum dots.
General classification of fluorescent CDs [5]
The carbon nanoparticles do not possess crystal lattice but carbon quantum dots do have clear crystal lattice. PDs are crosslinked or aggregated polymers, synthesized from monomers or polymers. The core of carbon and network of polymer chains can form PDs through a self-assembly process [6]. The fluorescence is the main property of CDs, which have sp2 carbon hybridization and made up of 2–3 parallel sheets of graphene. CDs find their applications in cell imaging, bioimaging, chemiluminescence, dye degradation, catalysis, solar cells, tissue engineering, drug transportation, polymers, nano-electronic devices, photo-catalysts, gene delivery and photodynamic therapy [7]. There are many approaches for the preparation of CDs, such as combustion, pyrolysis, carbonization, laser ablation, precipitation, hydrothermal and sonochemical [8]. Each method has advantages and disadvantages in terms of performance and adaptability.
For instance, chemical-based preparation mostly involves toxic chemicals that cause cytotoxicity in CDs. The chemically synthesized CDs have limited applications in biological and environmental systems. The precipitation method as an easy approach to produce CDs, however, is difficult to eliminate large amount of solvents and formation of amorphous nanoparticles. The low product yield and toxicity are also listed drawbacks of such methods. To realize true potential of CDs in the mainstream nanotech industry, new research efforts should be committed to develop low-cost and environmental benign techniques of CD synthesis. When compared to other conventional approaches, the sonochemical procedure is a relatively greener method of producing nanomaterials [9]. Many researchers are working on production of novel nanostructures using sonochemistry [10]. However, production of CDs through sonochemical approach is not documented well. This review article covers the preparation of CDs and CD-based nanoparticles and the influence of different parameters on physical and chemical properties of CDs with their applications in different fields. This review also includes a comparison of the sonochemical method to other reported methods in terms of economic value and product yield.
2 Preparation of carbon dots
The synthesis of CDs is divided into top–down and bottom–up methods. The top–down method involves the cutting or decomposing of the bulk source of carbon into nano-level particles. Usually, CDs are synthesized by cutting carbon rods, graphite powder, carbon nanotubes, candle soot, carbon black and carbon fibers [11]. Chemical oxidation, laser ablation, electrochemical oxidation and arc-discharge techniques are the frequently used top–down routes to synthesize CDs. In bottom–up method, small polymers and molecules go through dehydration and then carbonization to make polymer dots (PDs) and carbon nanodots (CNDs). The molecules have –NH2, –C = O and –COOH groups that dehydrate at high temperatures. Bottom–up methods include the sonochemical, electrochemical carbonization, direct pyrolysis, solvothermal/hydrothermal treatment, supported route approaches and reverse-micelle method and microwave irradiation [12,13,14]. Each method has some merits and drawbacks as well, which limits the use of CDs in some of the most sophisticated applications. The bottom–up approaches show advantages of uniform size distribution, convenience of surface passivation and controlled morphology. The top–down strategies commonly have the benefits of using large raw materials for mass production of CDs. However, these routes involve complex and special treatments to refine the produced CDs [15, 16]. The advantages and disadvantages of these routes are described in Table 1. Some disadvantages in the synthesis of CDs have been solved recently like agglomeration of the CDs, which can be minimized using appropriate surfactants. The polydisperse size distribution can be achieved through dialysis, gel electrophoresis and centrifugation [17].
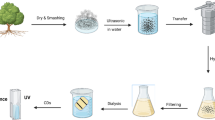
The sonochemical preparation method has applications in organometallic chemistry, industrial production methods and organic synthesis [26]. In a standard sonochemical method, the carbon precursors in solvent undergo a sonication process. The energetic radiations, used for carbonization and condensation, result in the synthesis of CDs. Li et al. [27] used acid or alkali assisted sonochemical treatment of glucose to produced CDs. Wei et al. [28] designed a sonochemical technique by employing pyrolysis of the precursors of carbon (ethylene diamine and citric acid) under the collapsing of hot bubbles. They prepared nitrogen-doped CDs (N@CDs) by using this technique. Kumar et al. [29] successfully synthesized CDs in one-step process using ultrasound radiations without introducing any acid, base or catalyst in the reaction. They took 12 mL of polyethylene glycol (PEG 400) in a test tube and immersed in water/silicon oil. An ultrasonic transducer was dipped in oil up to 10 mm for ultrasound irradiation of the solution. Figure 2 shows the steps involved in the production of pure CDs and metal-doped CDs (Sn@CDs) [30].
Steps involved in the synthesis of pure and metal-doped carbon dots [30]
The sonochemical method is preferred over other methods when it comes to the synthesis of metal@CDs. The doping of CDs with low melting point metals (Bi, Pb, Ga, In, Sb, Cd, Sn, and Zn) can easily be done through this method. Kumar et al. [29] analyzed the influence of various experimental factors on CDs, such as sonication time, temperature and amplitude of ultrasonic radiations. It was revealed that the CDs yield increases with the reaction temperature, power and sonication time. The size of synthesized CDs was reported in the range of 2–7 nm. The carbon dots in Fig. 2 exhibited 16% quantum yield [29]. The bare CDs showed less quantum yield, fluorescence and stability. So, it is a matter of concern to synthesize fluorescence CDs with maximum quantum yield and better colloidal stability. Different stabilizers are used to prepare highly stabilized CDs. However, stabilizers cannot improve the quantum yield. Different investigations have been made to enhance the quantum yield and results showed that the doping of elements or metals could be best routes to enhance the quantum yield [31]. To synthesize the doped CDs, it is recommended to consider the precursors of doping metals or elements in appropriate concentrations. Different metallic and nonmetallic precursors have been utilized for doping of CDs, such as phosphorous, nitrogen, boron, sulfur, silver, gold, tin, gallium and lead [30]. It is convenient to dope CDs with nitrogen as compared to others doping agents due to resemblance in molecular, atomic, crystal, physical and chemical properties of carbon and nitrogen that makes nitrogen as a suitable doping element [31]. Kumar et al. [30] prepared a class of CDs by metallic doping, such as silver, gold, tin, lead, gallium, with one-step sonochemical synthesis method without adding any catalyst and stabilizer. Limited effect of doping on optical properties of CDs was reported, however, physicochemical properties undergo notable changes on doping through sonication process [32]. Different preparation techniques, precursors for CDs preparation, emission properties and quantum yield are summarized in Table 2.
2.1 Quantum yield
Quantum yield is used to express the fluorescence intensity. Quantum yield (QY) is defined as the ratio of number of emitted to absorbed photons by CDs [47, 56]. The QY value of CDs without their modifications is less than 10%. To attain high QY of CDs has remained big challenge for the researchers [57, 58]. Kumar et al. [29] used a simple method to find the QY of fluorescence CDs. The fluorescence of CDs was approximated by comparing the intensities of photo-luminescence (PL) intensities and absorbance values at same wavelengths with 0.2 M sulphuric acid (QY = 54%) in quinine sulfate. The absorbance of quinine sulfate, CDs and doped CDs was determined at a wavelength of 360 nm. Different CDs have different decay times in the range of 2–4 ns. The calculated values of QY of sonochemically and chemically prepared CDs along with their biological and physical properties are summarized in Table 3.
Arumugham et al. [60] used economical method to synthesize CDs. The prepared CDs were non-toxic, highly stable and recyclable. These CDs were prepared from aqueous solutions without adding any organic solvents. The external energy is required for the synthesis of CDs. Kong et al. [61] prepared highly biocompatible, water-soluble and fluorescent CDs using ascorbic acid. Kumar et al. [62] used orange juice to prepare CDs, which were better in yield and quality than other methods. Hoan et al. [63] prepared CDs from lemon juice and investigated them for their potential applications in optoelectronics and bio-imaging. A lot of natural raw materials have been used by researchers to prepare CDs, such as chitosan [64], sucrose [65], lotus root [66], carrot roots [67], konjac flour [68], mangosteen peel [69] and curcumin [70]. Dias et al. [71] synthesized CDs from juices of grapes, avocado, lemon and kiwi. They also used milk, willow bark, vegetables in preparation of CDs. These precursors contain lot of phenolic and polyphenolic compounds that show good antioxidant properties. The average size of prepared CDs was 4 nm and quantum yield was 35%. Ring et al. [72] used ultrasonic technique to prepare curcumin quantum dots. The obtained CDs had size of 13.7 nm with zeta potential of 13.3 mV. The quantum yield of these CDs was higher than those prepared by ultrasonic method, as shown in Table 4.
2.2 Mechanism of formation of carbon dots
The formation of CDs follows different mechanisms depending on preparation approaches. The exact mechanism of the formation of CD is not fully understood yet. Many researchers suggested partial or suppositional mechanism of the preparation of CDs [73]. To control the physicochemical properties of CDs, the correct mechanism of production must be determined. In this study, the mechanism of sonochemically synthesized CDs discussed. In this method, the ultrasound radiation produces high temperature spots at pressure above 1000 atm. The temperature may exceed 5000 K and cooling rate may exceed 1010 K/s [74, 75]. These extreme conditions are different from other traditional techniques of preparation of CDs, such as wet chemistry, flame pyrolysis or hydrothermal synthesis and photochemistry. It is hard to find the exact process of sonochemical degradation and carbonization. The formation of nanoparticle in one-pot sonochemical process involves hydrolysis and poly-condensation processes. Large percentage of relatively small gel nuclei is produced and subsequently aggregated to large clusters in the condensation of hydrolytic species during the hydrolyzation of precursors in water. The acoustic cavitation phenomenon can produce a temporary high-pressure and temperature-localized hot zones during the sonosynthesis of nanoparticles. The changes in pressure and temperature can generate \({H}^{.}\) and \({OH}^{.}\) radicals in the sonolysis of water. Such free radicals enable the hydrolysis of precursors and poly-condensation of byproducts to produce nanoparticles. These reactions take place in a ring around the bursting bubbles. The sonochemical reactions occur in the liquid phase outside the collapsing bubbles. The sonochemically produced convection is composed of shock waves and micro-turbulence. The nature of such components impacts the crystallization phases of nanoparticles. The hot bubbles emit high-pressure shock waves while micro-turbulence is produced by the steady oscillatory motion of the liquid due to radial motion of the hot bubbles. In sono-crystallization, nucleation and growth rates are governed by shock waves and microturbulence, respectively. The common fluorescence and optical properties of the product can be studied by considering the functional groups on CDs and carbonization of CDs. First, carbon backbone stores the photo-generated carriers, which leak quickly to other possible state of carbon atom and traps. The crystallinity of graphite acts as a reservoir. From this mechanism, an increase in crystallinity phase of graphite and extent of carbonization will produce higher photostability. This mechanism of formation can be described by considering the example of generation of Sn@CDs, ultrafine CDs and Ga@C-dots [29, 76, 77] through ultrasonic cavitation by considering PEG-400 precursor, as shown in Fig. 3 [78]. The reaction parameters, the ratio of polymer/metal and sonication time were changed to produce high-performance CDs [76].
Schematic set-up of ultra-sonochemical preparation of C-dots, Sn@C-dots, and Sn@C-dots@Sn NPs [78]
3 Chemical structure of CDs
As it is mentioned in the preparation of CDs, there are different synthesis strategies to obtain CDs. According to different preparation methods, there are diverse chemical structures of CDs. For example, GQDs have one or more sheets of graphene and some chemical groups bonded on their edges. Also, they are anisotropic with lateral dimensions larger than their height. GQDs have certain crystallinity with lattice parameter of 0.24 nm due to the presence of carbon core that agree with (100) plane of single graphene, as shown in Fig. 4a [79]. CNDs are spherical and are classified into CQDs with crystal lattice and carbon nanoparticles without crystal lattice [80]. The interlayer space (0.34 nm) of CQDs resembles with (002) d-spacing of graphite, as shown in Fig. 4b. PDs are cross-linked or aggregated polymers which are synthesized from monomers or polymers. Further, the core of carbon and network of polymer chains can form PDs by self-assembly process. All CDs have chemical groups at their surfaces, such as polymer chains, amino-based groups and oxygen-based groups. The Raman spectroscopy, high-resolution TEM and X-ray diffraction (XRD) are the characterization techniques fused to study the core of carbon. The presence of chemical groups is confirmed through X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared (FTIR), matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) and nuclear magnetic resonance (NMR) techniques [9, 81, 82].
4 Effects of sonication process parameters
The physicochemical properties of CDs mainly depend on their morphology and particle size. It has been remained a challenge to prepare CDs with control shape and size for specific applications. In a sonication process, the minimum energy required for sonochemical preparation of CDs comes from cavitation in the solution [29]. The cavitation energy and CDs parameters depend on the type of solvent, sonication amplitude, power of ultrasound, ultrasound frequency, sonication time, temperature of reaction, catalyst type, precursors and doping agents and their concentration [83].
4.1 Effect of solvent
The ultrasound radiations generate the acoustic cavitation in the solution. Hence, different properties of the solvent, such as boiling point, viscosity, vapor pressure, surface tension are the parameters that affect the acoustic cavitation, particle shape and size of CDs [84]. Solvent with less surface tension and viscosity is suitable for the growth of bubbles, which in turn helps in the formation of required particle size with large stability [85]. Wang et al. [86] used sonochemical route and different solvents, such as 1,3-butanediol, ethanol (EN), ethylene glycol (EG) and water to fabricate the fluorine-doped carbon nitride quantum dots. Small and large nanosheets of CDs were obtained using solvents of ethanol and water, respectively. Ethylene glycol and 1,3-butanediol were also used to produce CDs of homogeneous size. Ethylene glycol and 1,3-butanediol are good radical acceptors and have high tendency to reduce as compared to water and ethanol. The addition of solute decreases the surface tension, which is necessary for the synthesis of stable CDs. Stability of nanoparticles or CDs can also be enhanced using surfactants [87]. The absorbed stabilizers on the surface of particles can help to control the growth of stable CDs. When the PEG-400 precursor was used, the formation of CDs followed the processes of condensation, dehydration and dehydrogenation [88]. It is also revealed that the synthesis of 2D and 3D particles need more sonication time as compared to the formation of 0D and 1D carbon dots.
4.2 Effect of power of ultrasound
The power of sonication is a very important parameter in a way to find the shape and size of CDs. High value of ultrasonic power can cause disturbance in the cavitation. The disturbed cavitation results in uncontrolled bubbling in the solution and formation of larger CDs [89]. The use of power in the range of 20–30 W is better to form fine sized nanomaterials, metallic nanoparticles, pure CDs and doped CDs.
4.3 Effect of ultrasound frequency
The frequency of sonication controls the rates of growth and collapsing of bubbles. The collapsing of bubbles increases with the ultrasonic frequency [90]. The growth of collapsing bubbles can be restricted with higher frequencies. The small size particles can be prepared by the small size bubbles [89]. However, the energy of bubbles, frequency and size of bubbles has no clear impact on the particle size. Small-sized nanoparticles form at optimum frequency, whereas below and above the optimum frequency, larger nanoparticles are produced [89]. The most acceptable range of frequency is 100–400 kHz for the formation of nanoparticles and CDs of small sizes. There is no report on the effect of ultrasonic frequency on the synthesis of CDs. This avenue should be explored in the future research undertakings. The most of sonochemical studies, documented in the literature, were executed at 20 kHz.
4.4 Effect of sonication time
The size of both pure and doped CDs also depends on the time of sonication. The prolonged treatment (large than 6 h) formed the heterogeneous nanoparticles [91, 92]. In this case, the initially synthesized nanoparticles behave as seeds and support in the synthesis of bigger-sized nanoparticles. The coating of small-sized particles at the surface of CDs will produce bigger particles. The size of bigger particles depends on the amount of small-sized particles and the concentration of precursor [76]. The sonication time of 90–180 min is generally needed for the formation of CDs. Recently, Wu et al. [57] produced CDs at different sonication times (1, 2, 2.5 and 3 h) by setting the starting temperature of 55ºC and 70% amplitude. The time of sonication affects the intensity of fluorescence of CDs. The maximum intensity of fluorescence was obtained at 3 h of sonication and minimum for half an hour.
4.5 Effect of precursors and catalysts
The most commonly used precursors for CD synthesis are classified into molecular compounds, natural sources and organic compounds. The molecular compounds include sodium 11-aminoundecanoate, octa-decyl ammonium citrate and ammonium citrate [93, 94]. The natural compounds include banana juice, orange juice, potato, egg, beer, coffee, meat, beverage, soya milk, sugar, Punica granatum fruit, bread, sucrose, jiggery, lysozyme, grass, silk Bombyx mori and starch [31]. Similarly, organic compounds include boric acid, benzene, citric acid, polyethylene glycol, proteins, amino acids, ethylene glycol, glycerol, glucose, diamine and N-acetylcysteine [29]. The selection of precursor is made by checking the compatibility with the method of synthesis of CDs. Each precursor imparts unique physical and chemical properties to CDs. For example, the best precursor for the formation of CDs is PEG-400 [30]. The organic compounds are preferred when high CDs yield is required. Large-sized chemical compounds needed large energy for their complete conversion to CDs. Hence, less yield of CDs is obtained at similar experimental conditions. Amino acids/proteins are utilized to dope nitrogen into CDs. Similarly, the boric acid is the best choice for the doping of boron to fabricate doped CDs (B@CDs) through the sonochemical method [95]. The advantage of using boric acid is that it contains oxygen and hydrogen along with boron. So, boric acid produces B@CDs without generating any byproducts. The correct concentration of boric acid yields better doped CDs. There are many catalysts that may be used to synthesize CDs, but the problem is that they may reduce the purity of CDs. The catalysts many also increase the cytotoxicity of CDs. It is often suggested to perform sonochemical method of CDs without using catalysts.
5 Advantages of ultrasonic synthesis of CDs
There are a very few publications on the synthesis of CDs through ultrasonic treatment approach. Li et al. [96] prepared water-soluble CDs of ultra-small size (5 nm) using acid and glucose through ultrasonic treatment. The particles showed high quantum yield (7%) and exhibited stability for 6 months. Dias et al. [26] synthesized CDs from peels of fruits and juices of grapes, avocado, lemon and kiwi. They also used milk, willow bark and vegetables that contain lot of phenolic and polyphenolic compounds. These compounds exhibit good antioxidant properties. The average size of prepared CDs was measured about 4 nm. Wang et al. [97] used ultrasonic treatment to prepare CDs using ammonia and ascorbic acid. The average size of CDs was 3.36 nm. Dang et al. [98] used ultrasonic treatment to fabricate CDs using oligomer-polyamide as a source of carbon. The as-fabricated CDs had high crystallinity, functional groups and were well dispersed. The average size of CDs was 2–4 nm. Li et al. [96] prepared CDs from H2O2 and activated carbon through ultrasonic treatment method. The TEM results verified that the average size of CDs was 5 nm and there were lot of hydroxyl groups on the surface of CDs. Zhu et al. [99] prepared CDs of size 3.7 nm through the microwave irradiation method. They used microwave oven (500 W) for 180 s to heat the solution of polyethylene glycol and saccharides. Liu et al. [100] used 4,7,10-trioxa-1,13-tridecanediamine (TTDDA) as the passivating agent and glycerol as a source of carbon to prepare CDs with size of 6 nm. Cao et al. [101] used aqueous solution of arginine and glucose to prepare CDs. The solution was heated in microwave oven (700 W) for 10 min. The average size of as-prepared CDs was measured about 7 nm. Wei et al. [102] prepared CDs within 2 min using ethylenediamine and glucose as a source of nitrogen and carbon, respectively, through carbonization method. The size of the prepared CDs was 7 nm and 48% of quantum yield. Wang et al. [103] prepared CDs with average size of 9 nm using citric acid. The quantum yield increased using thermogravimetric analyzer for thermal reduction of CDs. The quantum yield increased by five times as compared to non-reduced CDs. Feng et al. [104] used pyrolysis method to synthesize CDs from citric acid. The passivation agent was di-ethylene-tri-amine and TEM results showed that the size of as-prepared CDs was 8 nm. Qian et al. [105] used solvothermal method to prepare CDs from hydroquinone and SiCl4. They used autoclave of steel at 200 °C for 2 h to heat the mixture of hydroquinone and SiCl4 in acetone. The average size was observed to be 7 nm. Shan et al. [106] prepared CDs using solvothermal method. Hydroquinone was used as the source of carbon and the average size was found to be 16 nm. Most ultrasonic-based methods give CDs with average size less than those prepared with other methods. Conclusively, the ultrasonic approach is better than other approaches in producing fine CDs. Table 5 compares different methods of preparation of CDs and their effect on the CD size.
6 Applications of CDs
Carbon dots find their applications in cellular imaging, electronics, catalysis, bio-sensing, power, small molecules detection, drug delivery, photo-thermal therapy, photodynamic therapy and other biomedical activities. Some of the key applications are discussed in this review.
6.1 Antimicrobial and anti-parasitic
Many bacteria species show high resistance against the available antibiotics. The limited production of antibiotics is a matter of concern. Therefore, it is necessary to develop new antibiotics to meet the global demand. The nanoscale materials have shown reasonably good antibacterial properties [107]. Many investigations have been conducted on antibacterial activity of nanoparticles of zinc, iron, gallium, silver and metal oxide (TiO2, ZnO) [108, 109]. The carbon-based materials also gained importance due to their exceptional antibacterial activity. CDs are more important due to their fluorescence properties, high biocompatibility, biodegradability, water solubility, small particle size and large cellular uptake [110]. Kumar et al. [108] determined the antimicrobial performance of gallium-doped CDs (Ga@CDs) against the Pseudomonas aeruginosa. Sonochemically prepared Ga@CDs revealed outstanding stability over 60 days and minimum inhibitory concentration (MIC) values ranging from 0.34 to 1.36 ppm after doping CDs (1 g) with gallium in the range of 70–340 μg [108]. The diseases of parasites are prevailing around the globe. World Health Organization (WHO) revealed that over 14 million human beings are infected by skin diseases and more than 2 million cases are registered annually [111]. The lengthy treatment is required for skin diseases and diagnostic tests are extremely limited for skin diseases. Further, these are not only selective and sensitive, but also time-consuming. Hence, sensitive, reliable and inexpensive procedure with the ability of early detection should be developed [112, 113]. Another vector-borne disease is Leishmaniasis, which is caused by the transmission of intracellular protozoa of Leishmania genus [114]. Sonochemically prepared CDs are preferred materials for the treatment of skin diseases due to their inexpensive preparation, excellent biocompatibility and better optical properties. Kumar et al. [115] revealed this application of sonochemically synthesized Ga@CDs. They explored the application of CDs and Ga@CDs in CD-based ointment. The existing ointments have limited optical properties, so it is very difficult to trace them in tissues and cells. However, the incorporation of CDs in ointment provides them optical fluorescent property that enables them to easily track CDs in parasitic cells. The optically active and detectable ointment is regarded more effective against the parasites without harming the host cells.
6.2 Use in catalysis
CDs have considerable quantities of oxygen, carbon and hydrogen. These are much important in catalytic applications of CDs due to their fascinating structures. Functionalized CDs and bare CDs can be utilized in polymerization reaction as a catalyst [116]. Sun et al. [117] described the behavior of various CDs as electron acceptor and donors under photoexcitation. Kang et al. [118] used photo-enhanced hydrogen bond catalytic activity of CDs in Aldol condensation reactions for the organic transformation. Zhao et al. [119] used CDs in polymerization through free radicals, where CDs worked as free-radical scavengers. Gedanken et al. [120] examined the catalytic uses of sonochemically synthesized CDs in polymerization and photo-catalysis reaction. Nanocomposites of CDs also have demonstrated the outstanding catalytic efficiency. They synthesized Sn@C-dots/TiO2 nanocomposites through a sonochemical process completed in two steps. The precursor PEG at temperature of 260 °C and molten tin were exposed to sonication to synthesize Sn@C-dots. TiO2 was added during sonication process to enhance the adhesion of Sn@C-dots on the surface of TiO2. The formulated nanocomposites were tested to assess the photocatalytic performance under solar radiation. Sn@C-dots/TiO2 showed excellent results in photo-degradation of crystal violet and methylene blue under the solar radiation than C-dot/TiO2 and TiO2. The mechanism of photo-degradation, photo-catalysis, and charge separation in the presence of Sn@C-dots/TiO2 photo-catalyst under the sunlight is presented in Fig. 5.
Schematic description of the mechanism of photo-degradation over Sn@C-dot/TiO2 and charge separation under sunlight [120]
6.3 Polymerization
Polymers are prepared from monomers through polymerization. Different catalysts and initiators are used in the process of polymerization. There are many kinds of polymerization process based on different procedures of polymerization. One of such kind is the radical polymerization, which is initiated by the free radicals [121]. Among the different kinds of polymers, more interesting polymers are conducting polymers (CPs) due to their use in different applications including electronic devices, optical devices and sensors [122, 123]. The formation of CPs, such as polypyrrole (PPY) and polyaniline (PANI), needs powerful oxidizing agents. Molecules are widely polymerized by the process of chemical oxidation such as monomer (aniline). There is a key role of initiators in polymerization, for instance, ammonium persulfate and potassium persulfate are used to polymerize pyrrole and aniline. Recently, it has been reported that CDs have an interesting property of producing free radicals in aqueous solutions. Therefore, CDs are useful products that could be used in polymerization reactions as initiators [124]. Moorthy et al. [116] investigated the sonochemically prepared CDs as initiators in polymerization of different polymers including pyrrole and aniline. Micro- and nano-constituents of polymers of PPY (polypyrrole) and PANI (polyaniline) were prepared using CDs as catalyst through one-step polymerization reaction [125]. For polymerization process, CDs were employed with UV light [126]. Moorthy et al. [116] determined the absorption of methylene blue by PPY and PANI to investigate the performance of CDs. The results of SEM of fabricated PPY and PANI are reported in Fig. 6. The absorption capacities of PPY and PANI were about 19.31 and 19.2 mg/g, respectively [127]. It was recently stated that CDs were used successfully in the formation of poly(4, 4′-oxybisbenzenamine) (POBBA) and copolymers made of POBBA [126]. CDs are required only for the formation of POBBA, whereas the formation of PPY and PANI require UV light and CDs. Sonochemical prepared CDs were used to prepare copolymers and POBBA. SEM images of CDs based POBBA and copolymers are presented in the Fig. 6c, d. Results showed that the mechanism of polymerization is affected by reaction time, reaction temperature and performance of CDs as initiators [126]. Furthermore, CD-based polymer poly(4,4′-diaminodiphenyl-methane) (PDDM) revealed outstanding dye adsorption capacity with removal efficiency of 92% in 2 h [55].
6.4 Use in bioimaging
The use of fluorophores in bioimaging has been reduced due to their limited fluorescence and cytotoxicity. There is a need of pairing fluorophores with antibodies for uptake by the cells. CDs are becoming as an alternative of inorganic and organic fluorophores for the bioimaging due to their excellent biocompatibility, optical properties and cellular uptake [45]. The biological use of sonochemical prepared CDs revealed pledging bioimaging outcomes as compared to other methods [108]. There are high chances of induction of cytotoxicity while using chemically prepared CDs. The chemical synthesis of CDs involves robust conditions and toxic chemicals. There is a common use of CDs in intracellular imaging of different kinds of cells including neuronal cells like SH-SY5Y, PC12 and other HepG2 cells, HeLa cells, etc. [95, 108, 128]. Kumar et al. [10] used metals (Sn, Ga, Ag, Au and Zn) to sonochemically prepare the metal-doped CDs (M@CDs). The interaction between neuron cells and (M@CDs) was investigated for neurological interactions and imaging. The M@CDs nanocomposites showed promising results due to their selective affinity, biocompatibility and photo-stability (Fig. 7).
Confocal microscopic images of neuronal cells (SH-SY5Y) after incubation with formulated carbon dot samples. Top: bright field imaging, middle: fluorescent imaging and bottom: overlap image. Scale bar = 10 μm [30]
Song et al. [129] developed a detection technique to detect the cancer cells through the design of folic acid and CDs that are endo-cytosible by the folate receptor (FR) molecules. The results were confirmed through comparative analysis with FR-negative MCF-7 cells. The proposed method was used to analyze the HeLa cells. Lai et al. [130] used glycerol to prepare CDs through pyrolysis process. The improvement in biocompatibility and stabilization was made by the process of encapsulation of PEG. They studied the release of anti-cancer drug through ratiometric changes for CDs. Pan et al. [131] fabricated CDs for the imaging of HeLa cell. The thermal reduction method was used to fabricate CDs by the reduction of single-layer graphene oxide in the presence of HNO3 and H2SO4. Hu et al. [132] prepared blue nitrogen-doped CDs through the hydrothermal method for the imaging of HeLa cells. The prepared CDs were non-toxic for cells and mostly remained in the region of cytoplasm. Polythiophene phenyl propionic acid-derived red-emissive CDs were reported by Ge et al. [133]. They employed these CDs for vivo and vitro imaging. The treatment of HeLa cells with the prepared CDs demonstrated the red fluorescence when excited with light of wavelength 543 nm. Dehvari et al. [134] used waste shells of crab to fabricate CDs through green, fast and efficient sonochemical method. The prepared CDs exhibited excellent fluorescent and biocompatibility intensity. The CDs showed excellent solubility of water due to the presence of carboxyl and hydroxyl functional groups on their surface. The microscopic study showed that the conjugated CD–folic acid structure could work as an excellent fluorescent imaging agent, especially for cancer cells. Li et al. [135] used green, fast and facile strategy to fabricate CDs from gelatin. In this sonochemical treatment, CDs were prepared by the irradiation of ultrasonic waves for 30 min. The prepared CDs showed remarkable water dispersibility and fluorescent properties, negligible cytotoxicity and high photo-stability.
6.5 Neural tissue engineering
The regeneration of damaged nerves in neural disorders is a big challenge of present times. The regeneration capacity of nerves is very slow. Many approaches have been used to increase regeneration, such as the controlled use of biomaterials or scaffold materials to carry the nerve growth factors at the area of action [136]. Biocompatible nanoparticles are highly demanded for neurological tissue and engineering uses because a lot of nanoparticles are toxic, hence their use is limited. Kumar et al. [30] used CD-based nanoparticles for the engineering of neural tissues. They conducted studies on different types of sonochemically formulated CDs. These studies involved the biocompatibility of CDs with the neuronal cells, impact of CDs on the process of differentiation of neural cell and neural network outgrowth. The sonochemically formulated CDs showed outstanding biocompatibility even at small concentration (0.1 mg/mL) of CDs. Also, metal-doped CDs were prepared to increase the CD quantum yield. The CDs were successfully doped with five metals (tin, gold, zinc, silver and gallium). Even very small concentration (0.1 mg/mL) of doped CDs showed very good biocompatibility for neuronal cells (PC12). SEM images of nanocomposites (CDs, M@CDs) treated SH-SY5Y neuronal cells are given in Fig. 7. The differentiation processes of PC12 neuronal cells were not affected by M@CDs. Nissan et al. [137] manufactured Ga@CDs@Ga nanoparticles through sonochemical irradiation of molten Ga and precursor PEG 400 with particle size of 50–200 nm. The substrate glass, coated with Ga@CDs@Ga, worked as reinforce material for the outgrowth and differentiation of neuronal cells as compared to the bare substrate. Results showed that the number of initiated branches increased up to 97% from the neuronal soma in neural growth due to favorable association between the neuronal cells and surface nanoparticles. Also, it was reported that the differentiation processes of neural cells were affected by the topographic cues, polymer fibers and substrates of nano-pattern [138,139,140]. This interesting investigation on the applications of CDs for neural tissue engineering opens a new field, but further study is needed to consider the interactions between CDs and biomolecules.
6.6 Superconductivity
The phenomenon whereby the electrical resistivity of some substances suddenly decreases to zero at their transition temperature (Tc) is known as superconductivity. It has been remained hot topic in research for decades. Superconductivity affects the transportation and energy storage without resistance at room temperature [141]. The oxidation and agglomeration of the nanoparticles are the major issues in superconducting behavior of nanoparticles. Coating carbon on the surface of nanoparticles is seen to be an encouraging method for overcoming these difficulties [142]. Shani et al. [143] fabricated tin coated carbon layer through a sonochemical technique. The substrate was used for the measurement of magnetic moments of product having superconductivity of type-1. The coherence length decreased at lower size of carbon-coated tin nanosphere. However, the measurement of room temperature superconductivity remained a challenge. Shani et al. [143] also used sonication process to fabricate the carbon-coated Pb and Sn spheres. Among the prepared samples, the nano-spheres of carbon-coated Pb demonstrated high critical field (Hc) due to the modification in their coherence length.
6.7 Preparation of biofuels
The demand of renewable energy resources is increasing rapidly due to growing population and dwindling fossil fuels. The consumption of petrol in transportation accounts for over 30 percent of global energy use. Biodiesel is one of widely accepted green energy options. The algal oils and cooking oil can be used to produce biodiesel [144]. Microalgae can transform CO2, wastewater and sunlight into energy storage molecules, such as triglycerols and fatty acids [145]. The most commonly used catalysts for the synthesis of biodiesel through transesterification process are H2SO4 and NaOH. The SrO is the solid catalyst for the transesterification process. This is the strongest alkali metal oxide to recycle leftover cooking oil fatty acid methyl esters (FAME). Using SrO as a catalyst in a microwave oven, it takes 10 s to entirely convert waste oil into biodiesel [146]. The biodiesel can also be prepared using CDs. Tangy et al. [147] prepared SrO nanoparticles and functionalized them with CDs. They also analyzed the catalytic efficiency of composites and nanoparticles to produce FAME through microalgae. Ultrasonic waves, PEG-400 and Sr (NO3)2 were used to prepare the composites. The obtained catalyst showed 45.5% FAME yield and 97% lipid conversion in less than 3 min [147]. These catalysts also showed 2.4-fold increment in the performance as compared to conventional catalyst (SrO) due to the presence of functional groups, such as carboxylic, phenolic and oxygen, on the surface of CDs that promotes the process of esterification of fatty acids.
6.8 Use in supercapacitors
The carbon dot-based composites show high conductivity with relatively lower electrolyte diffusion length in the process of charging and discharging of capacitors. Many researchers combined CDs with metal chalcogenides to prepare supercapacitors. Kumar et al. [148] manufactured the composite of CDs by modifying the activated carbon. The CDs were prepared using PEG 400 in a sonochemical method. The sonochemical method was used to deposit CDs on the carbon. The incorporated activated carbon induced pores in the composite. The manufactured composite was used in supercapacitors as an electrode. The energy efficiency of the composite was more than 96% as compared to the pristine AC. The supercapacitor with electrode of composite had capacitance of 134 F/g as compared to pristine AC (100 F/g).
7 Conclusion
Being highly biocompatible, photo-stable, nontoxic, water-soluble, photosensitive and easy to functionalize, the carbon dots have many novel applications in live cell imaging, bio-sensing, catalysis, electronics, power, biomedicine and targeted drug delivery. However, the success of each application highly depends on physical, chemical, thermal and electrical properties of the carbon dots. The particle size is of significant importance in assessing the suitability of the carbon dots for specific applications. Multiple synthesis techniques have been reported in the literature. Each method has known merits and demerits. The green sonochemical is the best-known method to produce fine and homogeneously distributed carbon dots. It is concluded as an eco-friendly and low-cost method of synthesis of nanomaterials and carbon dots in particular. Despite the fact of using 90% less energy than other methods, this method has been overlooked in the published literature. The quantum yield of carbon dots can be improved by compositing with metals and metal oxides. The use of CDs in optical active textiles, wearable electronics, products for space applications and drug carry devices need to be examined.
References
Himaja AL, Karthik PS, Singh SP (2015) Carbon dots: the newest member of the carbon nanomaterials family. Chem Rec 15:595–615
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11:1620–1636
Luo PG, Yang F, Yang ST, Sonkar SK, Yang L, Broglie JJ, Liu Y, Sun YP (2014) Carbon-based quantum dots for fluorescence imaging of cells and tissues. Royal Soc Chem Adv 4:10791–10807
Kumar VB, Sahu AK, Mohsin ASM, Li X, Gedanken A (2017) Refractive-index tuning of highly fluorescent carbon dots. ACS Appl Mater Interfaces 9:28930–28938
Zhu S, Song S, Zhao Y, Shao X, Zhang J, Yang J (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8(2):355–381
Feng XL, Wu JS, Ai M, Pisula W, Zhi LJ, Rabe JP, Mullen K (2007) Triangle-shaped polycyclic aromatic hydrocarbons. Angew Chem Int Ed 46:3033–3036
R. Kumar (2019) Lipid-based nanoparticles for drug-delivery systems, Nanocarriers Drug Delivery, Elsevier. 249–284
Namdari P, Negahdari B, Eatemadi A (2017) Synthesis, properties and biomedical applications of carbon-based quantum dots: an updated review. Biomed Pharmacother 87:209–222
Kumar R, Singh A, Garg N, Siril PF (2018) Solid lipid nanoparticles for the controlled delivery of poorly water soluble non-steroidal anti-inflammatory drugs. Ultrason Sonochem 40:686–696
Kumar R, Singh A, Garg N (2019) Acoustic cavitation assisted hot melt mixing technique for solid lipid nanoparticles formulation, characterization, and controlled delivery of poorly water soluble drugs. J Drug Deliv Sci Technol 54:101277
Dong QY, Chen CQ, Zheng XT, Gao LL, Cui ZM, Yang HB, Guo CX (2012) One step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J Mater Chem 22:8764–8766
Wang L, Zhu SJ, Wang HY, Qu SN, Zhang YL, Zhang JH, Chen QD, Xu HL, Han W, Yang B (2014) Common origin of green luminescence in carbon nanodots and graphene quantum dots. Am Chem Soci Nano 8:2541–2547
Chan KK, Yap SHK, Yong KT (2018) Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications. Nano Micro Lett 10:72
Wang X, Qu K, Xu B, Ren J, Qu X (2011) Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem 21:2445–2450
Niu F, Xu Y, Liu M, Sun J, Guo P, Liu J (2016) Bottom-up electrochemical preparation of solid-state carbon nanodots directly from nitriles/ionic liquids using carbon-free electrodes and the applications in specific ferric ion detection and cell imaging. Nanoscale 8:5470–5477
Arsalani N, Nezhad-Mokhtari P, Jabbari E (2019) Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif Cells Nanomed Biotechnol 47:540–547
Farshbaf M, Davaran S, Rahimi F, Annabi N, Salehi R, Akbarzadeh A (2017) Carbon quantum dots: recent progresses on synthesis, surface modification and applications. Artif Cells Nanomed Biotechnol 46:1–18
Nguyen V, Yan L, Xu H, Yue M (2018) One-step synthesis of multi-emission carbon nanodots for ratiometric temperature sensing. Appli Surf Sci 427:1118–1123
Zeng H, Li L, Ding Y, Zhuang Q (2018) Simple and selective determination of 6-thioguanine by using polyethylenimine (PEI) functionalized carbon dots. Talanta 178:879–885
Liu R, Wu D, Liu S, Koynov K, Knoll W, Li Q (2009) An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers. Angewandte Chemie Int Edit 121:4668–4671
Wang Y, Zhu Y, Yu S, Jiang C (2017) Fluorescent carbon dots: rational synthesis, tunable optical properties and analytical applications. RSC Adv 7:40973–40989
Sim LC, Tai JY, Khor JM, Wong JL, Lee JY, Leong KH, Saravanan P, Aziz AA (2019) Carbon dots synthesized from green precursors with an amplified photoluminescence: synthesis, characterization, and its application. Springer, Plant Nanobionics, pp 1–33
Hou Y, Lu Q, Deng J, Li H, Zhang Y (2015) One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal Chim Acta 866:69–74
de Medeiros TV, Manioudakis J, Noun F, Macairan JR, Victoria F, Naccache R (2019) Microwave-assisted synthesis of carbon dots and their applications. J Mater Chem C 7:7175–7195
Kumar R, Soni P, Siril PF (2019) Engineering the morphology and particle size of high energetic compounds using drop-by-drop and drop-to-drop solvent-antisolvent interaction methods. Am Chem Soc Omega 4:5424–5433
Dias C, Vasimalai N, Sarria MP, Pinheiro I, Vilas-Boas V, Peixoto J, Espina B (2019) Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 9:199
Li H, He X, Liu Y, Huang H, Lian S, Lee ST, Kang Z (2011) One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 49:605–609
Wei K, Li J, Ge Z, You Y, Xu H (2014) Sonochemical synthesis of highly photoluminescent carbon nanodots. RSC Adv 4:52230–52234
Kumar VB, Porat Z, Gedanken A (2016) Facile one-step sonochemical synthesis of ultrafine and stable fluorescent C-dots. Ultrason Sonochem 28:367–375
Kumar VB, Kumar R, Gedanken A, Shefi O (2019) Fluorescent metal-doped carbon dots for neuronal manipulations. Ultrason Sonochem 52:205–213
Yang Z, Xu M, Liu Y, He F, Gao F, Su Y, Wei H, Zhang Y (2014) Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 6:1890–1895
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2017) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 184:343–368
Bottini M, Balasubramanian C, Dawson MI, Bergamaschi A, Bellucci S, Mustelin T (2006) Isolation and characterization of fluorescent nanoparticles from pristine and oxidized electric arc-produced single-walled carbon nanotubes. J Phys Chem B 110(2):831–836
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126(40):12736–12737
Sun YP, Zhou B, Lin Y, Wang W, Fernando KS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128(24):7756–7757
Zhao QL, Zhang ZL, Huang BH, Peng J, Zhang M, Pang DW (2008) Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem Commun 2008:5116
Liu H, Ye T, Mao C (2007) Fluorescent carbon nanoparticles derived from candle. Angewandte Chemie Int Edit 46:6473–6475
Wang F, Pang S, Wang L, Li Q, Kreiter M, Liu C (2010) One-Step synthesis of highly luminescent carbon dots in noncoordinating solvents. J Mater Chem 22:4528–4530
Zheng M, Xie Z, Qu D, Li D, Du P, Jing X, Sun Z (2013) On–Off–On fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect. Am Chem Soc Appl Mater Interfaces 5:13242–13247
Krysmann MJ, Kelarakis A, Dallas P, Giannelis EP (2012) Formation mechanism of carbogenic nanoparticles with dual photoluminescence emission. J Am Chem Soc 134:747–750
Yao S, Hu Y, Li G (2014) A one-step sonoelectrochemical preparation method of pure blue fluorescent carbon nanoparticles under a high intensity electric field. Carbon 66:77–83
Xue M, Guan W, Gu W, Guo C, Su S, Xu P, Ye L (2014) Microwave-assisted polyol synthesis of carbon nitride dots from folic acid for cell imaging. Int J Nanomed 9:5071
Zhu H, Wang X, Li Y, Wang Z, Yang F, Yang X (2009) Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem Commun 34:5118
Wei W, Xu C, Wu L, Wang J, Ren J, Qu X (2015) Non-enzymatic-browning-reaction: a versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci Rep 4:3564
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for Multicolor patterning, sensors, and bioimaging. Angewandte Chemie Int Ed 52:3953–3957
Guo Y, Wang Z, Shao H, Jiang X (2013) Hydrothermal synthesis of highly fluorescent carbon nanoparticles from sodium citrate and their use for the detection of mercury ions. Carbon 52:583–589
Dong Y, Pang H, Bin Yang H, Guo C, Shao J, Chi Y, Li CM, Yu T (2013) Carbon based dots co-doped with nitrogen and sulfur for High quantum yield and excitation- independent emission. Angewandte Chemie Int Ed 52:7800–7804
Qu D, Zheng M, Zhang L, Zhao H, Xie Z, Jing X, Haddad RE, Fan H, Sun Z (2015) Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci Rep 4:5294
Fang Y, Guo S, Li D, Zhu C, Ren W, Dong S, Wang E (2012) Easy synthesis and imaging applications of cross-linked green fluorescent hollow carbon nanoparticles. Am Chem Soc Nano 6:400–409
Bhunia SK, Saha A, Maity AR, Ray SC, Jana NR (2013) Carbon nanoparticle-based fluorescent bioimaging probes. Sci Rep 3:1473
Zhuo Y, Miao H, Zhong D, Zhu S, Yang X (2015) One-step synthesis of high quantum yield and excitation independent emission carbon dots for cell imaging. Mater Lett 139:197–200
Jiang H, Chen F, Lagally MG, Denes FS (2010) New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir 26:1991–1995
Kwon W, Rhee SW (2012) Facile synthesis of graphitic carbon quantum dots with size tunability and uniformity using reverse micelles. Chem Commun 48:5256
Lai CW, Hsiao YH, Peng YK, Chou PT (2012) Facile synthesis of highly emissive carbon dots from pyrolysis of glycerol; gram scale production of carbon dots/ mSiO2 for cell imaging and drug release. J Mater Chem 22:14403
Maruthapandi M, Kumar VB, Gedanken A (2018) Carbon dot initiated synthesis of poly (4,4′-diaminodiphenylmethane) and its methylene blue adsorption. Am Chem Soc Omega 3:7061–7068
Wang Y, Kalytchuk S, Wang L, Zhovtiuk O, Cepe K, Zboril R, Rogach AL (2015) Carbon dot hybrids with oligomeric silsesquioxane: solid-state luminophores with high photoluminescence quantum yield and applicability in white light emitting devices. Chem Commun 51:2950–2953
Wu ZL, Gao MX, Wang TT, Wan XY, Zheng LL, Huang CZ (2014) A general quantitative pH sensor developed with dicyandiamide N-doped high quantum yield graphene quantum dots. Nanoscale 6:3868–3874
Shi L, Yang JH, Zeng HB, Chen YM, Yang SC, Wu C, Zeng H, Yoshihito O, Zhang Q (2016) Carbon dots with high fluorescence quantum yield: the fluorescence originates from organic fluorophores. Nanoscale 8:14374–14378
Kumar R, Kumar VB, Marcus M, Gedanken A, Shefi O (2019) Element (B, N, P) doped carbon dots interaction with neural cells: promising results and future prospective, Proc. SPIE 10892, Colloidal Nanoparticles for Biomedical Applications XIV,1089214
Arumugham T, Alagumuthu M, Amimodu RG, Munusamy S, Iyer SK (2020) A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain Adv Mater Technol 23:00138
Kong W, Wu D, Li G, Chen X, Gong P, Sun Z, Chen G, Xia L, You J, Wu Y (2017) A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta 165:677–684
Kumar D, Singh K, Verma V, Bhatti HS (2014) Synthesis and characterization of carbon quantum dots from orange juice. J Bionanosci 8:274–279
Hoan BT, Tam PD, Pham VH (2019) Green synthesis of highly luminescent carbon quantum dots from lemon juice. J Nanotechnol: 2852816
Xiao D, Yuan D, Hea H, Lu J (2013) Microwave-assisted one-step green synthesis of amino-functionalized fluorescent carbon nitride dots from chitosan. Luminescence 28:612–615
Liu Y, Xiao N, Gong N, Wang H, Shi X, Gu W, Ye L (2014) One-step microwaveassisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 68:258–264
Gu D, Shang S, Yu Q, Shen J (2016) Green synthesis of nitrogen-doped carbon dots from lotus root for Hg(II) ions detection and cell imaging. Appl Surf Sci 390:38–42
S.L. D’souza, S.S. Chettiar, J.R. Koduru, S.K. Kailasa, (2018) Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik 158:893–900
Teng X, Ma C, Ge C, Yan M, Yang J, Zhang Y, Moraiscd PC, Bi H (2014) Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and l-lysine for bioimaging. J Mater Chem B 2:4631–4639
Aji M, Susanto PA (2017) Wiguna, Sulhadi, Facile synthesis of luminescent carbon dots from mangosteen peel by pyrolysis method. J Theor Appl Physics 11:119–126
Shi Y, Li C, Liu S, Liu Z, Zhu J, Yang J, Hu X (2015) Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Adv 5:64790–64796
Dias C, Vasimalai N, Sarria MP, ´ I. Pinheiro, V. Vilas-Boas, J. Peixoto, B. Espina, (2019) Biocompatibility and bioimaging potential of fruit-based carbon dots. Nanomaterials 9:199
Ring L, Yenn TW, Wen-Nee T, Tumin ND, Yusof FAM, Yacob LS, Rosli MIHB, Taher MA (2020) Synthesis of curcumin quantum dots and their antimicrobial activity on necrotizing fasciitis causing bacteria. Mater Today Proceed 31:31–35
Nalesso S, Bussemaker MJ, Sear RP, Hodnett M, Lee J (2019) A review on possible mechanisms of sonocrystallisation in solution. Ultrason Sonochem 57:125–138
Suslick KS, McNamara WB, Didenko Y (1999) Hot spot conditions during multi-bubble cavitation, Sonochemistry and Sonoluminescence 191–204
Vončina DB, Majcen-Le-Marechal A (2003) Reactive dye decolorization using combined ultrasound/H2O2. Dyes Pigment 59:173–179
Kumar VB, Tang J, Lee KJ, Pol VG, Gedanken A (2016) In situ sonochemical synthesis of luminescent Sn@C-dots and a hybrid Sn@C-dots@Sn anode for lithium- ion batteries. RSC Adv 6:66256–66265
Kumar VB, Perelshtein I, Lipovsky A, Porat Z, Gedanken A (2015) The sonochemical synthesis of Ga@C-dots particles. RSC Adv 5:25533–25540
Kumar VB, Tang J, Lee KJ, Pol VG, Gedanken A (2016) In situ sonochemical synthesis of luminescent Sn@ C-dots and a hybrid Sn@ C-dots@ Sn anode for lithium-ion batteries. RSC Adv 6(70):66256–66265
Lin LX, Zhang SW (2012) Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem Commun 48:10177–10179
Nie H, Li M, Li QS, Liang SJ, Tan YY, Sheng L, Shi W, Zhang S (2014) Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem Mater 26:3104–3112
Tetsuka H, Asahi R, Nagoya A, Okamoto K, Tajima I, Ohta R, Okamoto A (2012) Optically tunable amino-functionalized graphene quantum dots. Adv Mater 24:5333–5338
Mason TJ (2007) Sonochemistry and the environment—providing a “green” link between chemistry, physics and engineering. Ultrason Sonochem 14:476–483
Xu H, Zeiger BW, Suslick KS (2013) Sonochemical synthesis of nanomaterials. Chem Soc Rev 42:2555–2567
Mohandes F, Salavati-Niasari M (2013) Sonochemical synthesis of silver vanadium oxide micro/nanorods: solvent and surfactant effects. Ultrason Sonochem 20:354–365
Ashokkumar M, Lee J, Kentish S, Grieser F (2007) Bubbles in an acoustic field: an overview. Ultrason Sonochem 14:470–475
Wang N, Fan H, Sun J, Han Z, Dong J, Ai S (2016) Fluorine-doped carbon nitride quantum dots: ethylene glycol-assisted synthesis, fluorescent properties, and their application for bacterial imaging. Carbon 109:141–148
Cho HH, Yang H, Kang DJ, Kim BJ (2015) Surface engineering of graphene quantum dots and their applications as efficient surfactants. ACS Appl Mater Interfaces 7:8615–8621
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914
Brotchie A, Grieser F, Ashokkumar M (2009) Effect of power and frequency on bubblesize distributions in acoustic cavitation. Phys Rev Lett 102:084302
Ashokkumar M (2011) The characterization of acoustic cavitation bubbles—an overview. Ultrason Sonochem 18:864–872
Morel MH, Dehlon P, Autran JC, Leygue JP, Bar-L Helgouach C (2000) Effects of temperature, sonication time, and power settings on size distribution and extractability of total wheat flour proteins as determined by size-exclusion high performance liquid chromatography. Cereal Chem 77:685–691
Joyce E, Phull SS, Lorimer JP, Mason TJ (2003) The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason Sonochem 10:315–318
Das R, Bandyopadhyay R, Pramanik P (2018) Carbon quantum dots from natural resource: a review. Mater Today Chem 8:96–109
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun 48:8835
Kumar VB, Kumar R, Friedman O, Golan Y, Gedanken A, Shefi O (2019) One-pot hydrothermal synthesis of elements (B, N, P)-doped fluorescent carbon dots for cell labelling, differentiation and outgrowth of neuronal cells. Chem Select 4:4222–4232
Li H, He X, Liu Y, Huang H, Lian S, Lee ST, Kang Z (2011) One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 49(2):605–609
Wang F, Wang S, Sun Z, Zhu H (2015) Study on the ultrasonic single-step synthesis and optical properties of nitrogen-doped carbon fuorescent quantum dots. Fuller Nanotub Carbon Nanostruct 23(9):769–776
Dang H, Huang LK, Zhang Y, Wang CF, Chen S (2016) Large-scale ultrasonic fabrication of white fluorescent carbon dots. Ind Eng Chem Res 55(18):5335–5341
Zhu H, Wang X, Li Y, Wang Z, Yang F, Yang X (2009) Microwave synthesis of fluorescent carbon nanoparticles with electro-chemiluminescence properties. Chem Commun
Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J, Liu Y, Wang H, Wang W, Liu W (2012) Nanocarrier for gene delivery and bioimaging based on carbon dots with PEI passivation enhanced fluorescence. Biomaterials 33(13):3604–3613
Cao X, Wang J, Deng W, Chen J, Wang Y, Zhou J, Du P, Xu W, Wang Q, Wang Q, Yu Q, Spector M, Yu J, Xu X (2018) Photoluminescent cationic carbon dots as efficient non-viral delivery of plasmid SOX9 and chondrogenesis of fibroblasts. Sci Rep 8:7057
Wei X, Xu Y, Li Y, Yin X, He X (2014) Ultrafast synthesis of nitrogen-doped carbon dots via neutralization heat for bioimaging and sensing. Royal Soc Chem Adv 4(84):44504–44508
Wang J, Wei J, Su S, Qiu J (2014) Novel fluorescence resonance energy transfer optical sensors for vitamin B12 detection using thermally reduced carbon dots. New J Chem 39(1):501–507
Feng T, Ai X, An G, Yang P, Zhao Y (2016) Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. Am Chem Soc Nanotechnol 10(4):4410–4420
Qian Z, Shan X, Chai L, Ma J, Chen J, Feng H (2014) Si-doped carbon quantum dots: a facile and general preparation strategy, bioimaging application, and multifunctional sensor. ACS Appl Mater Interfaces 6(9):6797–6805
Wang F, Wang S, Sun Z, Zhu H (2015) Study on the ultrasonic single-step synthesis and optical properties of nitrogen-doped carbon fluorescent quantum dots. Fuller Nanotub Carbon Nanostruct 23(9):769–776
Ji H, Sun H, Qu X (2016) Antibacterial applications of graphene-based nanomaterials: recent achievements and challenges. Adv Drug Deliv Rev 105:176–189
Kumar VB, Natan M, Jacobi G, Porat Z, Banin E, Gedanken A (2017) Ga@C-dots as an antibacterial agent for the eradication of Pseudomonas aeruginosa. Int J Nanomed 12:725–730
Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M (2009) Antibacterial activities of zinc oxide nanoparticles against escherichia coli O157:H7. J Appl Microbiol 107:1193–2120
Yang J, Zhang X, Ma Y-H, Gao G, Chen X, Jia H-R, Li Y-H, Chen Z, Wu F-G (2016) Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl Mater Interfaces 8:32170–32181
Raviglione MC, Snider DE, Kochi A (1995) Global epidemiology of tuberculosis. JAMA 273:220
Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, Wurm EMT, Yoong C, Robertson TA, Soyer HP, Roberts MS (2011) Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev 63:470–491
Baroli B (2010) Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J Pharm Sci 99:21–50
Gubler DJ (1998) Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis 4:442
Kumar VB, Dolitzky A, Michaeli S, Gedanken A (2018) Antiparasitic ointment based on a biocompatibile carbon dot nanocomposite. ACS Appl Nano Mater 1:1784–1791
Moorthy M, Kumar VB, Porat Z, Gedanken A (2018) Novel polymerization of aniline and pyrrole by carbon dots. New J Chem 42:535–540
Wang X, Cao L, Lu F, Meziani MJ, Li H, Qi G, Zhou B, Harruff BA, Kermarrec F, Sun YP (2009) Photoinduced electron transfers with carbon dots. Chem Commun 25:3774
Han Y, Huang H, Zhang H, Liu Y, Han X, Liu R, Li H, Kang Z (2014) Carbon quantum dots with photoenhanced hydrogen-bond catalytic activity in aldol condensations. Am Chem Soc Catalysis 4:781–787
Zhao S, Lan M, Zhu X, Xue H, Ng TW, Meng X, Lee CS, Wang P, Zhang W (2015) Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl Mater Interfaces 7:17054–17060
Kumar VB, Perkas N, Porat Z, Gedanken A (2017) Solar-light-driven photocatalytic activity of novel Sn@C-Dots-Modified TiO2 catalyst. Chem Select 2:6683–6688
Matyjaszewski K, Davis TP (2002) Handbook of radical polymerization. Wiley, Hoboken
Dutt S, Siril PF, Sharma V, Periasamy S (2015) Gold core –polyaniline shell composite nanowires as a substrate for surface enhanced Raman scattering and catalyst for dye reduction. New J Chem 39:902–908
Dutt S, Siril PF (2014) Morphology controlled synthesis of polyaniline nanostructures using swollen liquid crystal templates. J Appl Polymer Sci 131
Ipe BI, Lehnig M, Niemeyer CM (2005) On the generation of free radical species from quantum dots. Small 1:706–709
Maruthapandi M, Nagvenkar AP, Perelshtein I, Gedanken A (2019) Carbon-dot initiated synthesis of polypyrrole and polypyrrole@CuO micro/nanoparticles with enhanced antibacterial activity. ACS Appl Polymer Mater 1:1181–1186
Maruthapandi M, Kumar VB, Levine M, Gedanken A (2018) Fabrication of poly (4,4′- oxybisbenzenamine) and its conjugated copolymers initiated by easily accessible carbon dots. Eur Polymer J 109:153–161
Maruthapandi M, Kumar VB, Luong JHT, Gedanken A (2018) Kinetics, isotherm, and thermodynamic studies of methylene blue adsorption on polyaniline and polypyrrole macro-nanoparticles synthesized by C-Dot-initiated polymerization. Am Chem Soc Omega 3:7196–7203
Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY (2007) Carbon dots for multiphoton bioimaging. J Am Chem Soc. https://doi.org/10.1021/ja073527l
Song Y, Shi W, Chen W, Li X, Ma H (2020) Fluorescent carbon nanodots conjugated with folic acid for distinguishing folate-receptor-positive cancer cells from normal cells. J Mater Chem 22(25):12568–12573
Lai CW, Hsiao Y, Peng Y, Chou PT (2012) Facile synthesis of highly emissive carbon dots from pyrolysis of glycerol; gram scale production of carbon dots/mSiO2 for cell imaging and drug release. J Mater Chem 22(29):14403–14409
Pan D, Guo L, Zhang J, Xi C, Xue Q, Huang H, Wu M (2012) Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J Mater Chem 22(8):3314–3318
Hu C, Liu Y, Yang Y, Cui J, Huang Z, Wang Y, Rong J (2013) One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J Mater Chem B 1(1):39–42
Ge J, Jia Q, Liu W, Guo L, Liu Q, Lan M, Zhang H, Meng X, Wang P (2015) Red emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv Mater 27(28):4169–4177
Dehvari K, Liu KY, Tseng PJ, Gedda G, Girma WM, Chang JY (2019) Sonochemical-assisted green synthesis of nitrogen-doped carbon dots from crab shell as targeted nanoprobes for cell imaging. J Taiwan Inst Chem Eng 95:495–503
Li C, Sun X, Li Y, Liu H, Long B, Xie D, Wang K (2021) Rapid and green fabrication of carbon dots for cellular imaging and anti-counterfeiting applications. ACS Omega 6(4):3232–3237
Zilony N, Rosenberg M, Holtzman L, Schori H, Shefi O, Segal E (2017) Prolonged controlled delivery of nerve growth factor using porous silicon nanostructures. J Control Release 257:51–59
Nissan I, Kumar VB, Porat Z, Makovec D, Shefi O, Gedanken A (2017) Sonochemically-fabricated Ga@C-dots@Ga nanoparticle-aided neural growth. J Mater Chem B 5:1371–1379
Baranes K, Chejanovsky N, Alon N, Sharoni A, Shefi O (2012) Topographic cues of nano-scale height direct neuronal growth pattern. Biotechnol Bioeng 109:1791–1797
Baranes K, Hibsh D, Cohen S, Yamin T, Efroni S, Sharoni A, Shefi O (2019) Comparing transcriptome profiles of neurons interfacing adjacent cells and nanopatterned substrates reveals fundamental neuronal interactions. Nano Lett 19:1451–1459
Antman-Passig M, Shefi O (2016) Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration. Nano Lett 16:2567–2573
Buzea C, Robbie K (2005) Assembling the puzzle of superconducting elements: a review. Supercond Sci Technol 18:R1–R8
S.J. Ye, A. Matsumoto, Y.C. Zhang, H. Kumakura, Strong enhancement of high field critical current properties and irreversibility field of MgB2 superconducting wires by coronene active carbon source addition via the new B powder carbon coating method, Superconductor Science and Technology, 27 (2014) 085012.
Shani L, Kumar VB, Gedanken A, Shapiro I, Shapiro BY, Shaulov A, Yeshurun Y (2018) Type-I superconductivity in carbon-coated Sn nano-spheres. Physica C (Amsterdam, Neth) 546:6–10
Demirbaş A (2008) Production of biodiesel from algae oils. Energy Sour Part A Recover, Util Environ Effects 31:163–168
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: from CO2 fixation to biofuel production. Renew Sustain Energy Rev 15:3252–3260
Koberg M, Abu-Much R, Gedanken A (2011) Optimization of bio-diesel production from soybean and wastes of cooked oil: combining dielectric microwave irradiation and a SrO catalyst. Biores Technol 102:1073–1078
Tangy A, Kumar VB, Pulidindi IN, Kinel-Tahan Y, Yehoshua Y, Gedanken A (2016) In-Situ transesterification of Chlorella vulgaris using carbon-dot functionalized strontium oxide as a heterogeneous catalyst under microwave irradiation. Energy Fuels 30:10602–10610
Kumar VB, Borenstein A, Markovsky B, Aurbach D, Gedanken A, Talianker M, Porat Z (2016) Activated carbon modified with carbon nanodots as novel electrode material for supercapacitors. J Phys Chem C 120:13406–13413
Acknowledgements
The authors would also like to acknowledge the efforts of King Khalid University, Saudi Arabia (Deanship of Scientific Research) for support through the Research Groups Project under the Grant Number (R.G.P.1/73/42).
Funding
This work is completed under the Research Groups Project under the Grant Number (R.G.P.1/73/42).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest regarding the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, M., Naz, M.Y., Shukrullah, S. et al. One-pot sonochemical preparation of carbon dots, influence of process parameters and potential applications: a review. Carbon Lett. 32, 39–55 (2022). https://doi.org/10.1007/s42823-021-00273-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-021-00273-y