Abstract
The carbon anode material for lithium-ion battery was prepared by pyrolysis fuel oil and waste polyethylene terephthalate (PET) additive. The pitch was synthesized as a medium material for carbon anode by heat treatment. The waste PET additive improved the softening point and thermal stability of the pitch. La and Lc of the anode material (heat-treated pitch) increased at higher treatment temperature but decreased by waste PET additive. The electric capacity was evaluated based on effects of defective cavity and developed graphite interlayer, respectively. When the La and Lc of the anode material decreased, the electric capacity by cavity increased based on defective graphite structure. Therefore, the addition of waste PET causes the improved capacity by the cavity. The anode material which has a high efficiency (over 95%) and C-rate (95%, 2 C/0.1 C) was obtained by controlling the process of heat treatment and PET addition. The mechanism of lithium-ion insertion was discussed based on effects of defective cavity and developed graphite interlayer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lithium-ion batteries (LIBs) are being used extensively in energy-intensive portable devices such as smart phones, notebooks, and electric vehicles. Important factors in the commercialization of lithium-ion batteries are long cycle characteristics, energy density, and light-weight design [1, 2]. The long cycle characteristics indicate the structural stability of the anode/cathode active material and the solid–fluid interface. Also, higher energy densities represent greater capacity per unit mass. Graphite is the most widely used anode materials for lithium-ion batteries, which has excellent performance such as structural stability, low operating potential and long cycle life. Graphite is generally classified as natural graphite and artificial graphite. Artificial graphite is produced by heat treatment using pitch and coke as precursors at temperatures above the graphitization temperature (about 3000 °C). Natural graphite is buried globally. It not only has an advantage such as low cost and high capacity, but also has a disadvantage that structural collapse occurs relatively easily during charging and discharging [3]. On the other hand, artificial graphite is used in many cases, because it has structural stability even during charging and discharging.
The artificial graphite has been produced using pitch and coke made from petroleum residues or coal tar [4,5,6,7]. The petroleum residues and coal tar are mainly composed of aromatic hydrocarbons, the pitch and coke are synthesized by thermal condensation polymerization of them. Pitch has been applied in various application industries (such as impregnation, binding, coating or feed for coke). The key process parameters that determine the properties of pitch include the reaction temperature, time, and pressure. The artificial graphite is synthesized by hot pressing of pitch/coke blender, heat treatment and impregnation. Many researchers focus on anode materials of lithium-ion batteries using petroleum-based byproducts as raw materials for carbon and graphite materials [8,9,10,11,12,13,14].
The investment for carbon anode material has been increasing, because the demand is increasing more rapidly in the field of automobile and portable devices. As a result, the price of pitch, coke and artificial graphite is gradually increasing, causing a problem in battery cost. Therefore, it is important not only to improve the energy density of graphite-based battery anode materials, but also to reduce the cost of material process cost.
In recent years, Asian nations prohibit the importing waste plastic, and it is becoming a global environmental issue [15,16,17]. Polyethylene terephthalate (PET) is a polymer obtained by polymerizing ethylene glycol and terephthalic acid, and it can be converted to a carbon material by heat treatment [18,19,20]. It also has an oxygen atom. Therefore, it is expected that the cross-linking can be driven during pitch synthesis by oxygen radicals in PET additive [21, 22]. The crystallinity is generally determined by softening point of the pitch and the heat treatment temperature, and the softening point of the pitch is increased by the cross-linking effect. In addition, the pitch with high softening point results in developed crystallinity during the heat treatment [23, 24]. Oxygen radicals will thus provide defects in the crystals as impurities. When cavities occur, additional space is provided for lithium ions and the capacitance is increased.
In this study, the capacity improvement of lithium-ion battery was investigated by petroleum-based anode material based on effect of PET waste addition. The pitch was synthesized using petroleum residue and PET by heat treatment. The crystal and electrochemical properties of prepared anode were studied based on heat treatment process and waste PET additive. The mechanism was discussed to investigate the relationship among the battery performance, synthesis process parameters and waste PET addition.
2 Experimental methods and analysis
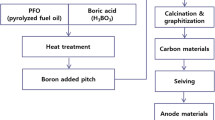
2.1 The synthesis of pitch from PFO and waste PET
PFO (Pyrolysis fuel oil, petroleum residue, supplied from LG Chem., Korea) and waste PET (supplied from Lotte Chem., Korea) were used as precursors for synthesis of pitch. The pitch was synthesized thermally using a 5 L autoclave at 410 °C for 3 h. To analyze the effect of waste PET addition on the characteristics of the anode materials, waste PET was added at contents of 5 wt%. The specific reaction conditions are shown in Table 1. The softening point of the synthesized pitch was determined using DP 70 (made by Mettler Toledo), in accordance with ASTM 3461. The pitch was preheated to 50 °C and subsequently heated at a rate of 2.0 °C/min to perform the softening point analysis. The thermal characteristics of the pitch were evaluated using a Thermo plus EVO II TG8120, a thermogravimetric analyzer (TGA) made by Rigaku. Also, elemental analysis and Fourier-transform infrared spectroscopy (FT-IR) were employed using a Thermo Scientific Flash 2000 analyzer and Bruker ALPHA-P and ALPHA-T analyzer to examine the increase in oxygen content in the pitch with respect to the waste PET addition.
2.2 Preparation and analysis of anode materials according to thermal treatment temperature
The synthesized pitch were heat treated for 1 h at different reaction temperatures (1000, 1500, 2000, and 2400 °C) to produce anode materials. The applied experimental conditions are shown in Table 2. The heat treatment conditions were determined based on previous research results conducted by the present research team on thermal treatment process conditions, as follows: nitrogen gas was injected so that an inert atmosphere was maintained. In this atmosphere, the samples were subject to the first heat treatment for increasing carbonization yield, during which the temperature was raised at a rate of 5 °C/min to 500 °C for 1 h. The first heat treatment induced bond formation due to condensation/cross-linking reactions of low molecular weight materials and thus a carbon yield would increase. Subsequently, the temperature was raised to the target heat treatment at the same heating rate [25]. The heat-treated samples were ground by ball milling and sifted through a 500-mesh sieve before testing. X-ray diffraction analysis (XRD) was conducted using the Rigaku diffractometer Ultima IV to analyze the crystallization of the prepared anode materials. This test was performed using Cu-Kα (λ = 1.5418 Å) at a current of 40 mA and a voltage of 40 kV. The scanning rate was set at 3°/min over a scanning angle (2θ) range of 0° to 90°. Collected diffraction data were subject to data reduction using PDXL software. The d-spacing (D) and crystal size (Lc) of the specimens were measured using the Bragg–Scherrer formula [26].
The interlayer spacing (d00x) was calculated from the Bragg’s equation formula:
where λ is the radiation wavelength, and θ is the (00x) reflection angle [(001) is (002) or (004)].
The Scherrer equation was used to determine the crystalline height (La, Lc) formula:
where β00x is the half-height width of the (00x) reflection.
2.3 Assembly and electrochemical analysis of lithium-ion battery
To determine the electrochemical characteristics of the prepared anode materials, each anode material (carbon) was mixed with a water-soluble binder [CMC (carboxymethyl cellulose): SBR (styrene-butadiene rubber) = 1:1 wt%] at a ratio of 8:2 wt% to produce a slurry solution. A doctor blade was used to coat this slurry solution on copper foil, which was subsequently dried in a vacuum oven at 120 °C for 12 h. The coated electrodes were rolled using a roll press machine and, in an argon atmosphere glove box, made into coin cells that use Li metal as a reference electrode. To examine the electrochemical characteristics of the prepared batteries, a charge and discharge meter was used for cycle and rate controlling tests at a driving voltage of 0–2.5 V (WBCS 3000 Battery Cycler, Won A Tech).
3 Results and discussion
3.1 Pitch synthesis and analysis according to PET addition
The softening point and elemental content of synthesized pitch are shown in the Table 3. The softening point in the synthesized pitch was increased by addition of waste PET. In general, PET was degraded at 400 °C or higher, and it was decomposed into substances that have various oxygen functional groups that participate in the pitch synthesis reaction. The decomposed substances were led the cross-linking and polymerization to increase the softening point in synthesized pitch [27]. The softening point of pitch without added waste PET was 125.6 °C, and the softening point of pitch with waste PET was increased doubled to 237.7 °C. During pitch synthesis reaction, the oxygen functional group was caused cross-linking for polymerization of high molecular weight. As a result of EA analysis of the synthesized pitch, C and O content increased and H content decreased as waste PET was added. The increase of oxygen content can be confirmed by the introduction of oxygen content by the addition of waste PET. When waste PET was added, a large amount of alkyl side chain contained in PFO was decomposed by oxygen functional groups to increase aromatization and decrease H/C ratio [28]. Therefore, it was considered that the amount of aromatic structure increases in the low molecular chain structure [29].
The thermal behavior of the synthesized pitch is shown in Fig. 1. The synthesized pitch begins to decompose at 200 °C, but decomposition begins at 380 °C with the addition of waste PET. The samples were prepared into a coke at the pitch of 500 °C and no decomposition occurs. It can be seen that the amount of residual residues increased from 35 to 53 wt% when waste PET was added. The thermal properties were improved by the addition of waste PET, and the improved thermal properties were attributed to the polymerization phenomenon due to cross-linking of oxygen functional groups. The DTG analysis results showed that the petroleum-based pitch had two peaks.
In general, the softening point and thermal stability of the pitch increase by the increasing of content in the high molecular weight materials [7]. And the high molecular weight material is produced by polymerizing the low molecular weight materials during the pitch synthesis process [30]. The addition of waste PET increased the softening point and thermal stability of the pitch. Therefore, it was considered that the waste PET was induced the polymerization of low molecular weight materials during pitch modification process.
To analyze the effect of the waste PET addition on the surface chemistry of the pitch, FT-IR analysis is shown in Fig. 2. There was no significant difference in peak height between samples when waste PET was added. It can be seen that the waste PET content contained a small amount of oxygen functional groups as small as 5 wt% of the total, and had a surface chemistry similar to the pitch. However, the addition of waste PET slightly increased the peak corresponding to the C=O structure at 1650–1720 cm−1 due to the effect of oxygen functional groups [31].
3.2 Crystallinity analysis with respect to heat treatment temperature
To analyze the crystallinity of the anode materials with respect to the heat treatment temperature, their XRD patterns were examined, as shown in Fig. 3 and summarized in Table 4. As the heat treatment temperature increased (1000 → 1500 → 2000 → 2400 °C), the Lc value of the anode materials was increased (15 → 42 → 214 → 283). The Lc value at 2400 °C was found to be nearly 19 times the value at 1000 °C. Based on the d-spacing D (002) and La values, the prepared anode materials were compared with regards to the crystallinity and the degree of graphitization. When the heat treatment temperature was raised from 1000 to 2400 °C, the d-spacing D (002) decreased from 3.50 to 3.38 Å. This observation can be interpreted as showing that, as the heat treatment temperature increases, the d-spacing D (002) approaches 3.354 Å of the theoretical d-spacing of graphite. In this regard, the synthesized pitch is considered to be soft carbon materials which, driven by thermal energy, can be easily converted into a graphite structure. It was also observed that, as the heat treatment temperature increased (1000 → 1500 → 2000 → 2400 °C), the La values increased (45 → 53 → 86 → 283), and when the temperature reached 2400 °C of the graphitization temperature, the La value was found to increase significantly [32]. Overall, the increasing heat treatment temperature is considered to improve the crystallinity of the anode materials, thereby allowing them to have a structure similar to that of graphite. La, Lc and number of layers in anode material were decreased at the same heat treatment temperature by addition of waste PET; therefore, the addition of waste PET was inhibited the development of graphite structure during graphitization process [33].
3.3 Electrochemical analysis
The Galvanostatic charge/discharge curves of the anode material are shown in Fig. 4. In the case of the anode material treated at relatively low temperature, the protruded part appeared about ~ 0.5 V (red arrow), and the protruded part decreased at higher heat treatment temperature. Generally, when the graphite structure is not developed, an unstable SEI layer is formed, and thus a protruding portion appears in the discharge curve. On the other hand, when the graphite structure develops and a stable SEI layer is produced, the protruding part of the discharge curve decreases [34]. Therefore, it was noted that the anode material treated over 2000 °C had a developed graphite structure and formed a stable SEI layer. Kasuh reported that lithium ion is inserted between graphite layers at a discharge voltage lower than 0.2 V vs Li+/Li in the galvanostatic charge/discharge curve but in cavity by crystal structure defects at a discharge voltage more than 0.2 V vs Li+/Li [35]. The prepared anode material showed the lithium-ion insertion in both graphite layer structure and defective cavity.
Figure 5 shows the discharge capacity of prepared anode material based on effects of graphite layer structure and defective cavity. As the heat treatment temperature increased, the discharge capacity by defective cavity and the total discharge capacity decreased. Over 2000 °C, the graphite structure developed and the discharge capacity by graphite layer structure and the total discharge capacity increased. As seen from the XRD results in Table 4, La and Lc increased as the heat treatment temperature increased. From these results, it can be seen that as the La and Lc decreased, the discharge capacity due to the cavity increased. On the contrary, as the La and Lc increased, the discharge capacity due to the graphite structure increased. Waste PET addition more decreased La and Lc than them of samples treated at same temperature (see Table 4) and increased the discharge capacity by developed cavity. Eventually, waste PET addition increased the total discharge capacity.
The rate and electrochemical properties of the anode material are shown in Figs. 6, 7, and Table 5. At the heat treatment temperatures of 1000, 1500, 2000 and 2400 °C, the discharge capacities of first cycle at 0.1 C was 313, 205, 149 and 219 mAh/g, respectively. The results of Table 4 showed that the crystal structure of heat-treated samples became similar to that (3.354 Å) of graphite based on D (002) decrease (3.5 Å → 3.43 Å). As a result, the defective cavity decreased and the capacity decreased accordingly. The interlayer capacity increased with the crystal structure similar to that of graphite. Similarly, the initial efficiency was 83% lower at 1000 °C, but the initial efficiencies was 90% (1500 °C), 86% (2000 °C), and 89% (2400 °C), respectively, as the heat treatment temperature increased. This suggested that the SEI layer is over-formed on the exposed surface of PET0_1000 (treated at 1000 °C) due to the large number of defective cavities in the carbon crystal structure. Also, the rate characteristic of all samples showed that the 2 C/0.1 C value was higher than 90%. Furthermore, the electrochemical properties were investigated based on effects of waste PET additive. The addition of waste PET interrupted growth of pitch crystal structure, it formed the defects in heat-treated pitch (in the Sect. 3.2). The formed defect increased the storage capacity of lithium ions by forming a cavity for lithium-ion storage during intercalation/deintercalation of lithium ions [36]. Therefore, the addition of waste PET improved the cavity capacity of pitch-based anode electrode material, it increased the discharge capacity.
4 Conclusions
The advantage of waste PET additive was discussed based on PFO-based carbon anode material. PET additive developed the capacity and with keeping an initial efficiency (95%) and C-rate (95%, 2 C/0.1 C). The defective cavity of anode which caused by PET additive increased capacity and C-rate. It might be resulted by developed lithium-ion storage and pass way. It is noted that treat process of PET additive was well addressed based on high-performance anode material.
References
Kim JS, Pfleging W, Kohler R, Seifert HJ, Kim TY, Byun DJ, Jung HG, Choi WC, Lee JK (2015) Three-dimensional silicon/carbon core-shell electrode as an anode material for lithium-ion batteries. J Power Sources 279:13–20. https://doi.org/10.1016/j.jpowsour.2014.12.041
Luo J, Liu J, Zeng Z, Ng CF, Ma L, Zhang H, Lin J, Shen Z, Fan HJ (2013) Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett 13:6136–6143. https://doi.org/10.1021/nl403461n
Ohta N, Nagaoka K, Hoshi K, Bitoh S, Inagaki M (2009) Carbon-coated graphite for anode of lithium ion rechargeable batteries: graphite substrates for carbon coating. J Power Sources 194:985–990. https://doi.org/10.1016/j.jpowsour.2009.06.013
Kim JG, Kim JH, Song BJ, Lee CW, Im JS (2016) Synthesis and its characterization of pitch from pyrolyzed fuel oil (PFO). J Ind Eng Chem 36:293–297. https://doi.org/10.1016/j.jiec.2016.02.014
Kim JG, Liu F, Lee CW, Lee YS, Im JS (2014) Boron-doped carbon prepared from PFO as a lithium-ion battery anode. Solid State Sci 34:38–42. https://doi.org/10.1016/j.solidstatesciences.2014.05.005
Kim BH, Kim JH, Kim JG, Im JS, Lee CW, Kim S (2017) Controlling the electrochemical properties of an anode prepared from pitch-based soft carbon for Li-ion batteries. J Ind Eng Chem 45:99–104. https://doi.org/10.1016/j.jiec.2016.09.008
Kim JG, Kim JH, Song BJ, Jeon YP, Lee CW, Lee YS, Im JS (2016) Characterization of pitch derived from pyrolyzed fuel oil using TLC-FID and MALDI-TOF. Fuel 167:25–30. https://doi.org/10.1016/j.fuel.2015.11.050
Dauche FM, Bolanos G, Blasig A, Thies MC (1998) Control of mesophase pitch properites by supercritical fluid extraction. Carbon 36:953–961. https://doi.org/10.1016/S0008-6223(97)00212-1
Greinke RA, Singer LS (1988) Constitution of coexisting phase in mesophase pitch during heat treatment: mechanism of mesophase formation. Carbon 26:665–670. https://doi.org/10.1016/0008-6223(88)90069-3
Takami N, Satoh A, Hara M, Ohsaki T (1995) Structural and kinetic characterization of lithium intercalation into carbon anodes for secondary lithium batteries. J Electrochem Soc 142:371–379. https://doi.org/10.1149/1.2044017
Han YJ, Kim JD, Yeo JS, An JC, Hong IP, Nakavayashi K, Miyawaki J, Jung JD, Yoon SH (2015) Coating of graphite anode with coal tar pitch as an effective precursor for enhancing the rate performance in Li-ion batteries: effects of composition and softening points of coal tar pitch. Carbon 94:432–438. https://doi.org/10.1016/j.carbon.2015.07.030
Huang S, Guo H, Li X, Wang Z, Gan L, Wang J, Xiao W (2013) Carbonization and graphitization of pitch applied for anode materials of high power lithium ion batteries. J Solid State Electrochem 17:1401–1408. https://doi.org/10.1007/s10008-013-2003-9
Wu YP, Rahm E, Holze R (2003) Carbon anode materials for lithium ion batteries. J Power Sources 114:228–236. https://doi.org/10.1016/S0378-7753(02)00596-7
Endo M, Kim C, Karaki T, Nishimura Y, Matthews MJ, Brown SDM, Dresselhaus MS (1999) Anode performance of a li ion battery based on graphitized and B-doped milled mesophase pitch based carbon fibers. Carbon 37:561–568. https://doi.org/10.1016/S0008-6223(98)00222-X
Sharma R, Bansal PP (2016) Use of different forms of waste plastic in concrete—a review. J Clean Prod 112:473–482. https://doi.org/10.1016/j.jclepro.2015.08.042
Henningsson S, Hyde K, Smith A, Campbell M (2004) The value of resource efficiency in the food industry: a waste minimization project in East Anglia, UK. J Clean Prod 12:505–512. https://doi.org/10.1016/S0959-6526(03)00104-5
Xiad S, Dong H, Geng Y, Brander M (2018) An overview of China’s recyclable waste recycling and recommendations for integrated solutions. Resour Conserv Recycl 134:112–120. https://doi.org/10.1016/j.resconrec.2018.02.032
Amy LB, Shunli W, Jenna RJ (2018) The chinese import ban and its impact on global plastic waste trade. Sci Adv 4:1–7. https://doi.org/10.1126/sciadv.aat0131
Achillas DS, Karayannidis GP (2004) The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut 34:987–998. https://doi.org/10.1016/j.wasman.2013.07.015
Choudhary R, Kumar A, Murkute K (2018) Properties of waste polyethylene terephthalate (PET) modified asphalt mixes: dependence on PET size, PET content, and mixing process. Period Polytech Civ Eng 68:62. https://doi.org/10.3311/PPci.10797
Chiu SJ, Cheng WH (1999) Thermal degradation and catalytic cracking of poly(ethylene terephthalate). Polym Degrad Stab 63:407–412. https://doi.org/10.1016/S0141-3910(98)00121-9
Holland BJ, Hay JN (2002) The thermal degradation of PET and analogous polyesters measured by thermal analysis—Fourier transform infrared spectroscopy. Polymer 43:1835–1847. https://doi.org/10.1016/S0032-3861(01)00775-3
Fernandez JJ, Figueiras A, Granda M, Bermejo J, Menendez R (1995) Modifiacation of coal-tar pitch by air-blowing—I. Variation of pitch composition and properties. Carbon 33:295–307. https://doi.org/10.1016/0008-6223(94)00130-R
Metzinger T, Huttinger KJ (1997) Investigations on the cross-linking of binder pitch matrix of carbon bodies with molecular oxygen—Part I. Chemistry of reactions between pitch and oxygen. Carbon 35:885–892. https://doi.org/10.1016/S0008-6223(97)00038-9
Han YJ, Hwang JU, Kim KS, Kim JH, Lee JD, Im JS (2019) Optimization of the preparation conditions for pitch based anode to enhance the electrochemical properties of LIBs. J Ind Eng Chem 73:241–247. https://doi.org/10.1016/j.jiec.2019.01.031
Tai FC, Wei C, Chang SH, Chen WS (2010) Raman and X-ray diffraction analysis on unburned carbon powder refined from fly ash. J Raman Spectrosc 41:933–937. https://doi.org/10.1002/jrs.2532
Bernard S, Beyssac O, Benzerara K, Finding N, Tzvekov G, Brown GE (2010) XANES, Raman and XRD study of anthracene-based cokes and saccharose-based chars submitted to high-temperature pyrolysis. Carbon 48:2506–2516. https://doi.org/10.1016/j.carbon.2010.03.024
Jung MJ, Jung JY, Lee DY, Lee YS (2015) A new pitch reforming from pyrolysis fuel oil by UV irradiation. J Ind Eng Chem 22:70–74. https://doi.org/10.1016/j.jiec.2014.06.026
Lee SI, Mitani S, Park CW, Yoon SH, Korai Y, Mochida I (2005) Electric double-layer capacitance of microporous carbon nano spheres prepared through precipitation of aromatic resin pitch. J Power Sources 139:379–383. https://doi.org/10.1016/j.jpowsour.2004.07.004
Kim JG, Kim JH, Song BJ, Lee CW, Lee YS, Im JS (2016) Empirical approach to determine molecular weight distribution using MALDI-TOF analysis of petroleum-based heavy oil. Fuel 186:20–23. https://doi.org/10.1016/j.fuel.2016.08.052
Torregrosa-Roriguez P, Martinez-Escandell M, Rodriguez-Reinoso F, Marsh H, Gomez de Salazar C, Romero Palazon E (2000) Pyrolysis of petroleum residues: II. Chemistry of pyrolysis. Carbon 38:535–546. https://doi.org/10.1016/S0008-6223(99)00133-5
Cheng X, Zha Q, Li X, Yang X (2008) Modified characteristics of mesophase pitch prepared from coal tar pitch by adding waste polystyrene. Fuel Process Technol 89:1436–1441. https://doi.org/10.1016/j.fuproc.2008.07.003
Kim JD, Roh JS, Kim MS (2017) Effect of carbonization temperature on crystalline structure and properties of isotropic pitch-based carbon fiber. Carbon Lett 21:51–60. https://doi.org/10.5714/CL.2017.21.051
Wang C, Appleby AJ, Little FE (2001) Charge-discharge stability of graphite anodes for lithium-ion batteries. J Electroanal Chem 497:33–46. https://doi.org/10.1016/S0022-0728(00)00447-2
Mabuchi A, Tokumitsu K, Fujimoto H, Kasuh T (1995) Charge-discharge characteristics of the mesocarbon microbeads heat-treated at different temperatures. J Electrochem Soc 142:1041–1046. https://doi.org/10.1149/1.2044128
Tokumitsu K, Fujimoto H, Mabuchi A, Kasuh T (1999) High capacity carbon anode for Li-ion battery A theoretical explanation. Carbon 37:1599–1605. https://doi.org/10.1016/S0008-6223(99)00031-7
Acknowledgements
This work was supported by Korea Evaluation institute of Industrial Technology (KEIT) through the Carbon Cluster Construction project [10083621, Development of preparation technology in petroleum-based artificial graphite anode] funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, K.S., Hwang, J.U., Im, J.S. et al. The effect of waste PET addition on PFO-based anode materials for improving the electric capacity in lithium-ion battery . Carbon Lett. 30, 545–553 (2020). https://doi.org/10.1007/s42823-020-00124-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-020-00124-2