Abstract
The Oronogo-Duenweg mining belt is a designated United States Environmental Protection Agency Superfund site due to lead-contaminated soil and groundwater by former mining and smelting operations. Sites that have undergone remediation—in which the O, A, and B horizons have been removed alongside the lead contamination—have an exposed C horizon and are incalcitrant to revegetation efforts. Soils also continue to contain quantifiable Cd and Zn concentrations. To improve soil conditions and encourage successful site revegetation, our study employed three biochars, sourced from different feedstocks (poultry litter, beef cattle manure, and lodgepole pine), at two rates of application (2.5%, and 5%), coupled with compost (0%, 2.5% and 5% application rates). Two plant species—switchgrass (Panicum virgatum) and buffalograss (Bouteloua dactyloides)—were grown in the amended soils. Amendment of soils with poultry litter biochar applied at 5% resulted in the greatest reduction of soil bioavailable Cd and Zn. Above-ground biomass yields were greatest with beef cattle manure biochar applied at 2.5% with 5% compost, or with 5% biochar at 2.5% and 5% compost rates. Maximal microbial biomass was achieved with 5% poultry litter biochar and 5% compost, and microbial communities in soils amended with poultry litter biochar distinctly clustered away from all other soil treatments. Additionally, poultry litter biochar amended soils had the highest enzyme activity rates for β-glucosidase, N-acetyl-β-D-glucosaminidase, and esterase. These results suggest that soil reclamation using biochar and compost can improve mine-impacted soil biogeophysical characteristics, and potentially improve future remediation efforts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mining activity serves as one of the primary anthropogenic agencies for heavy metal deposition into the environment. The resultant heavy metal accumulations in soil, water, and sediments create long-term concerns for the health of impacted ecosystems. These health risks extend not only to aquatic and terrestrial plant and animal species, but also the soil microbial communities that provide vital ecosystem services within these impacted environments (Ali et al. 2019). Given the nondegradable nature of heavy metals, they remain a continual strain on mine-impacted ecosystems until such a time as they are either removed (e.g., excavation coupled with storage in a repository, soil washing) or sequestered (e.g., in situ stabilization, phytoremediation) (Dhaliwal et al. 2020). Some methods, such as excavation, solely address heavy metal remediation, while others, such as phytoremediation, presuppose a soil matrix conducive to plant growth and heavy metal bioavailability (Ali et al. 2013). In contrast, a mounting body of evidence indicates that biochar can significantly reduce heavy metal bioavailability while concomitantly reconditioning the soil to improve its overall health (Ippolito et al. 2019).
The relationship between the health of a soil and its biota is inextricable (Lehman et al. 2015). Soil microbial communities drive nutrient cycling processes, assist in soil formation (Lian et al. 2008), and contribute to soil structure (Amellal et al. 1999; Bossuyt et al. 2001). In addition to the aforementioned roles soil microbial communities play, they also provide an additional number of critical ecosystem services that include, but are not limited to the following: erosion control, reduction of plant pathogens, bioremediation of pollutants, and regulation of atmospheric gases (Barrios 2007; Singh 2015). Regrettably, soil microbial communities and—as a consequence—their functions and ecosystem services, are often deleteriously impacted by mining activities (Liao et al. 2005). It has been well documented that soil heavy metal accumulation leads to suppression of both soil microbial biomass and activity (Chander et al. 1995), over both short- and long-terms (Brookes and Mcgrath 1984). A survey by Zhao et al (2019) of mine-impacted soils revealed significant negative correlations between Cd, Cu, Pb, and Zn and soil microbial community characteristics (e.g., abundance, diversity, uniformity). It should be noted that heavy metal toxicity derived from mining activities, while significant, is not the only stressor on soil microbes. For example, acidity generated from metal sulfides exposed during the mining process, plays an equally impactful role in altering soil microbial community structure and function (Chen et al. 2016). Considering these consequences, careful thought must be taken to create and utilize reclamation materials (e.g., biochar) that can improve soil functionality.
When care is taken in selecting biochars prior to their utilization, they prove more than capable of serving both remediation and reconditioning roles (Beesley et al. 2011; Kavitha et al. 2018). Such success requires a thorough understanding of site conditions, combined with the selection of suitable feedstocks to design a treatment capable of mitigating on-site contamination while avoiding further disruptions to the environment post-application (Ippolito et al. 2019; Novak et al. 2013). A study by Reverchon et al. (2015) demonstrated improvement in soil chemical (e.g., pH, C/N ratio) and plant growth (e.g., photosynthetic N use efficiency) characteristics in a soil/mine rejects mixture after amendment with a jarrah-feedstock biochar, a biochar previously demonstrated to have liming properties, and be capable of improving N dynamics (Reverchon et al. 2014). In a parallel study to our report, Sigua et al. (2019) reported the ability of two manure-based biochars to significantly reduce water-soluble heavy metal (Cd and Zn) concentrations in contaminated soils, and significantly increase maize biomass for phytoremediation purposes. Additionally, Penido et al. (2019) reported that biochar coupled with sewage sludge was able to significantly improve grass germination, height, and biomass while concomitantly reducing bioavailable levels of Cd, Pb, and Zn in soils collected from a former Zn-mining site.
Although an overall significant body of evidence exists documenting the impacts of biochar amendment on mine-impacted soil chemical and physical characteristics, there lacks a commensurate body of knowledge comparing the impact of biochar amendments on the biological characteristics of mine-impacted soils. This paucity of information creates a knowledge gap that threatens the viability of remediation and restoration efforts, particularly as it inhibits projection of soil health improvements both over the short and long term. This is highlighted by the aforementioned study by Reverchon et al. (2015) that called specifically for further investigation into the impact of biochar during mine reclamation on beneficial soil microorganisms. The authors argued that such research could provide insight into the symbiotic relationships between soil microorganisms and plants used for restoration purposes. To address this deficit, we focused on the soils of the Oronogo-Duenweg Mining Belt, one of the most documented, severely mine-impacted areas of the United States (ITRC 2010).
The Oronogo-Duenweg Mining Belt is an approximately 700 km2 region comprised of 11 former mining areas located around the City of Joplin, MO, USA. This region is situated within the Tri-State Mining District, which was one of the largest Pb and Zn mining districts in the world before ore production ceased (USEPA 2013). Due to inefficiencies in the smelting process, approximately five percent of all ore was recovered as Pb/Zn concentrates, leaving the remainder as discarded mill waste to be surface applied in what is locally referred to as “chat piles” (Pierzynski and Vaillant 2006). These mining wastes released toxic levels of not only Pb and Zn, but also Cd into the local soils, waterways, and sediments (Gutiérrez et al. 2020). In response to this major environmental threat, the Oronogo-Duenweg Mining Belt was placed onto the US Environmental Protection Agency’s (USEPA) National Priorities List in 1990, to be managed as a Superfund site. Attempted remediation of the Oronogo-Duenweg Mining Belt Superfund site was conducted by disposing the Pb-contaminated waste material (USEPA 2017). During the remediation process, Pb-contamination proved so extensive that soil O, A, and B horizons proved unsalvageable and therefore were removed, leaving the parent material (C horizon) exposed. This parent material is characterized by poor water infiltration, large rock fragments (up to 15 cm in size), high residual Cd and Zn concentrations, and generally poor soil fertility characteristics, leaving a barren landscape. Such undesirable landscapes dissuade landowners against necessary remediation efforts. A holistic approach to remediation would include restoration approaches, whereby solutions to restore soil fertility and return vegetation to the landscape would be incorporated into the overall cleanup effort. Such restoration approaches would ameliorate the biological, chemical, and physical issues of the parent material.
Therefore, the objective of this preliminary reclamation study was to understand the impact of three biochars, designed from different feedstocks, on the chemical and biological characteristics of this mine-impacted, post-remediation soil after amendment. We combined differing rates (2.5% and 5%) of biochar with differing rates (0%, 2.5%, and 5%) of a manure-based compost, and examined mine soil microbial biomass, function, and structure. Since revegetation of mine-impacted lands is often considered a desired end goal, we looked at the growth of two grasses, buffalograss and switchgrass in mining impacted soils with biochar and compost amendments. Buffalograss was chosen for the study as an example of a native grass that could provide ground cover for animal species endemic to southwest Missouri. Switchgrass was chosen for its known tolerance to poor soil conditions (Skeel and Gibson 1996). The ultimate aim of this study was to utilize this data to inform on-site restoration efforts at sites within the Oronogo-Duenweg Mining Belt.
2 Materials and methods
2.1 Site description, soil characterization and preparation, and biochar preparation
The site is located outside of Webb City, MO, USA, within the Oronogo-Duenweg Mining Belt of the Tri-State Mining District. Typical for mine waste sites of this area, soil from the site was contaminated with Pb, Zn, and Cd. These heavy metals leached into the soil from the overlying chat piles, consisting of non-ore waste rock and tailings, deposited as waste after Pb and Zn extraction. During EPA-directed remediation efforts, the chat piles and any Pb-contaminated soil were removed until Pb levels reflected background concentrations (Johnson et al. 2016). At the site in question, cleanup efforts subsequently left the orange-colored subsoil layer exposed, a result typical for remediation in the area.
Prior to mining, the soil was mapped to the Reuter series (USDA Taxonomic Classification: Loamy, mixed, superactive, mesic, shallow Vitritorrandic Haploxerolls), while the exposed subsoil is an amalgamation of gravelly silt loam, cobbly clay, and 2–15-cm sized rock fragments. For the experiment, the exposed subsoil layer was collected by backhoe, deposited into 50-gallon, plastic-lined drums, and shipped to the Agricultural Research Service’s (US Department of Agriculture) Coastal Plains Soil, Water, and Plant Research Center, located in Florence, SC, USA. Upon arrival, the subsoil material was air-dried and sieved through a 12.7 mm screen to remove the larger coarse fragments. The remaining material (henceforth referred to as mining impacted soils) was stored in 50-gallon plastic-lined drums in anticipation of greenhouse experiments.
Three feedstocks were used to produce biochar in this study: first, a beef cattle manure (BC) taken from a local feedlot in Webb City, MO, USA, mixed 1:1 with locally sourced green waste, and weathered for two years prior to pyrolysis; second, lodgepole pine (Pinus contorta; LPP); and third, poultry litter (PL). The BC was sieved (6 mm), and pyrolyzed at 500 °C with a residence time of approximately 4 h (Novak et al. 2013). Both the LPP and PL biochars were commercially sourced, with the PL gasified using proprietary methodology. The LPP was produced using a two-stage process (Ippolito et al. 2017), with the first stage performed at 500–700 °C in a low O2 environment, with a residence time of < 1 min, and the second stage performed at 300–550 °C in an anaerobic environment, with a residence time of 15 min. For a complete analysis of feedstock and biochar properties, please refer to Novak et al. (2019) and Sigua et al. (2019).
2.2 Experimental setup
The experiment was designed to be a 50-day greenhouse study conducted in pots (15 cm top diameter × 17 cm depth). Experimental treatments consisted of single biochar additions to mining impacted soils at rates of 2.5, and 5% (w/w) in combination with compost (e.g., unpyrolyzed BC feedstock) at rates of 0, 2.5, and 5% (w/w). Control treatments consisted of unamended, mine-impacted soils, as well as a pair (2.5% and 5.0%) of compost-only treatments. In addition to the biochar and compost treatments, two grasses—switchgrass (Panicum virgatum) and buffalograss (Bouteloua dactyloides)—were included to assess their potential as ground cover candidates in future field restoration efforts. Each treatment combination was performed in triplicate, resulting in a total of 126 pots arranged in a completely randomized experimental design.
For each treatment, biochar and compost were added to 1500 g of mine soil at their respective rates, and hand incorporated before being placed into pots and tamped down to achieve a bulk density of 1.5 g/cm3 (Novak et al. 2018). Seeds (20 per pot) were planted to a depth of 1 cm, and deionized water was added to bring the soil gravimetric moisture content to 15% (w/w) on an air-dry basis. Greenhouse conditions for the 50-day study were as follows: mean air temperature of 29.1 ± 3.3 °C and mean relative humidity 81 ± 9.4%. Pots were fertilized on Day 16, delivering an equivalent of 3 kg N/ha (in the form of NH4NO3), to combat N-deficiency symptoms. All P and K were supplied by the amendments. Soil moisture was monitored daily, and pots were irrigated by hand using tap water several times per week.
2.3 Soil and plant analysis
At the experimental end point, each pot was destructively sampled. Plant above-ground biomass, and soil subsamples were harvested and oven-dried overnight at 60 °C and 105 °C, respectively. Soil subsamples were extracted using 0.01 M CaCl2 for the determination of bioavailable Cd and Zn by inductively coupled plasma-optical emission spectroscopy (ICP-OES), using a PerkinElmer Optima 7300 DV ICP-OES (Waltham, MA, USA) according to Ippolito et al. (2017). Plant samples were digested using a hot block acid digestion method using concentrated HNO3 at 60 °C for 30 min, immediately followed with the addition of 30% H2O2 at 90 °C for 90 min (Huang and Schulte 1985). After digestion, Cd and Zn were determined by ICP-OES. Soil pH was measured using a 2:1 deionized H2O: soil suspension (Novak and Watts 2005).
2.4 Phospholipid fatty acid (PLFA) analysis
Upon termination of the study, 15 g of fresh soil was also collected from each pot, lyophilized to maintain PLFA integrity (Veum et al. 2019), and shipped on dry ice to MIDI Inc. (Newark, DE, USA) for high-throughput PLFA analysis as described by Buyer and Sasser (2012). PLFA’s with retention times lower than C14:0 and greater than C22:0 were removed prior to data analysis (Ducey et al. 2015).
2.5 Soil enzyme assays
Enzymatic activity levels for three extracellular enzymes: β-glucosidase (BG), N-acetyl-β-D-glucosaminidase (NAG), and acid phosphatase (AP); and one intracellular enzyme: esterase (EST), were determined by methods developed (Deng et al. 2011) and modified by Deng et al. (2013). A total of 2 g of soil was removed from each sample, split into 1 g subsamples and slurried in 150 mL deionized water for 30 min using a magnetic stir bar at 600 rpm. An additional 1 g subsample was used to determine soil moisture content by drying for 12 h at 105 °C. In black, flat-bottom, 96-well plates (ThermoFisher, Waltham, MA, USA), 50 μL of Modified Universal Buffer (pH 5.5 in wells used to test NAG, pH 6.0 for all others), 50 μL of methylumbelliferyl-labeled enzyme substrate (MilliporeSigma, Burlington, MA, USA), and 100 μL aliquots of soil slurry were added to their respective wells and incubated at 37 °C for 1 h. After incubation, 50 μL of 0.1 M THAM (pH 12) was added to each well to terminate the enzymatic reactions. Fluorescence was measured on a Biotek FLx800 Plate Reader (Biotek, Winooski, VT, USA) at 360-nm excitation and 460-nm emission. Methylumbelliferone standards (MilliporeSigma, Burlington, MA, USA) were prepared in concentrations of 0–50 μM to develop standard curves for each soil suspension. Autohydrolysis was measured by incubating substrate in deionized water and used to correct final enzymatic activity rates (Deng et al. 2011), with any resulting negative assay values eliminated from analysis.
2.6 Statistical analysis
Analysis of variance and regression analyses were performed using Minitab 17 (Minitab Incorporated, State College, PA, USA). Analysis of variance (ANOVA) was conducted using the general linear model, with pairwise comparisons using Fisher’s Least Square Difference Method (LSD); differences between any two means were considered significant at a p < 0.05 and all usage of the word “significant” further in the text carries this connotation. Additionally, four-way ANOVA was performed in SAS using PROC GLM (SAS, 2000). The model included biochar type (BC), biochar application rate (BC%), plant species (P), and compost amendment rate (C%). To account for extraction efficiencies of PLFA from biochar-amended soils (Gomez et al. 2014), PLFAs were normalized as a ratio to C16:0 (Drijber et al. 2000). Additionally, PLFAs occurring at ratios below 0.02, in greater than 90% of samples in both grass treatment groups with no apparent treatment pattern, were omitted from the data set. This resulted in removal of 10 PLFA’s (32 remaining, excluding C16:0) from the analysis. Non-metric multidimensional scaling (NMS) of microbial community population data was performed in PC-Ord v.6 (MJM Software Design, Gleneden Beach, OR, USA), with a secondary matrix containing pH, plant above-ground biomass, soil Cd and Zn concentrations, and plant tissue Cd and Zn concentrations. PC-Ord v.6 was also used to determine effects of plant species and biochar type, as well as compost and biochar amendment rates on soil microbial communities via permutational analyses of variance (PERMANOVA).
3 Results and discussion
3.1 Soil heavy metal concentrations post-treatment
Concentrations (mg/kg) of Cd and Zn in soils, post-treatment, are listed in Table 1. For the buffalograss portion of the study, except for the LPP 2.5% biochar/0% compost treatment, all other treatments significantly reduced bioavailable Cd and Zn concentrations as compared to the control. Compost-only treatments in buffalograss pots (amendment rates of 2.5% and 5%) resulted in significant (i.e., p < 0.05) reductions of bioavailable Cd and Zn. Addition of BC and PL biochars further reduced Cd and Zn concentrations with concentrations significantly lower than their respective compost alone treatment. The most significant reductions for both heavy metals, when compared to unamended soil, occurred at the PL 5% biochar amendment rate (Table 1). Amendment of mining impacted soils with LPP biochar showed mixed results; the greatest reductions in Cd and Zn concentrations occurred at the rate of LPP 2.5% biochar/5% compost (Table 1), and increasing the application rate of biochar did not result in a further reduction of heavy metal concentrations (Table 1).
A complete discussion of bioavailable Cd and Zn in switchgrass pots can be found in Novak et al. (2019). Briefly, for compost-only treatments, only compost added at a rate of 5% to the mining impacted soils demonstrated a significant reduction in Cd and Zn concentrations. All BC and PL biochar treatments—with the exception of BC 2.5%/0% compost—significantly reduced concentrations of Cd and Zn as compared to the control soil (Table 1), and addition of BC and PL biochar—but not LPP biochar—to compost consistently resulted in significantly greater reductions in heavy metal concentrations than a similar rate of compost alone. As with the buffalograss portion of the study, PL 5% biochar treatments resulted in the greatest reductions in heavy metal concentrations. LPP biochar alone did not result in a significant reduction in Cd or Zn concentration as compared to the control, and only LPP 2.5% biochar/2.5% compost resulted in a significant reduction in Zn over compost-only at a similar rate (Table 1).
Decreases in soil bioavailable Cd and Zn concentrations after biochar amendment are attributable, in large part, to changes in soil pH (Table 2). For buffalograss, the influence of pH on soil bioavailable Cd (R2 = 0.89) and Zn (R2 = 0.94) concentrations was significant. Similar results were observed for switchgrass, with significant influence of pH on soil bioavailable Cd (R2 = 0.82) and Zn (R2 = 0.86) concentrations. Certain biochars, especially those derived from poultry litter (Revell et al. 2012), have been previously demonstrated to have liming potential (Singh et al. 2017). The ability to increase soil pH, especially in mining impacted soils which are often highly acidic, has been demonstrated to be a critical factor during revegetation efforts (Goecke et al. 2011; Phillips et al. 2016). The benefits of liming are many, such as: increasing soil pH decreases heavy metal solubility (Chuan et al. 1996); major plant nutrients are most plant-accessible in the near neutral pH range (Alam et al. 1999); and roots can suffer low pH injury in highly acidic soils (Arnon and Johnson 1942). In addition to its liming potential, biochar has been demonstrated to sequester heavy metals by binding them to oxygen-containing surface functional groups (Uchimiya et al. 2011), or causing oxide, hydroxide, and carbonate phase heavy metal precipitation (Ippolito et al. 2019). Also, manure-derived biochars such as PL typically contain relatively high P concentrations (Ippolito et al. 2020), and thus may have the potential to chemically immobilize Cd and Zn as phosphate mineral precipitates (Andrunik et al. 2020). All three biochars chosen for inclusion in this study were selected because they demonstrated excellent heavy metal removal qualities (Ippolito et al. 2016).
3.2 Above-ground plant biomass and tissue heavy metal concentrations
ANOVA results indicated that plant species did influence above-ground biomass (AGB; Supplemental Tables 1 and 2), with results graphically displayed in Fig. 1. For buffalograss (Fig. 1a), only 4 treatments out of 20 resulted in AGB significantly greater than the control. Three of these treatments were soils amended with BC biochar: 2.5% biochar/5% compost; 5% biochar/2.5% compost; and 5% biochar/5% compost, which resulted in buffalograss AGB levels (0.51 ± 0.10 g) significantly greater than all other treatments (Fig. 1a). The fourth significant treatment over the control soil was PL 2.5% biochar/0% compost.
For switchgrass, 13 of 20 treatments showed significant increases in switchgrass AGB over the control (Fig. 1b), and the influence on AGB by both biochar type and compost application rate were significant (Supplemental Tables 1 and 2). This included five of six BC treatments, all PL treatments, and one LPP treatment. For BC biochar, the only treatment not have a significant increase in switchgrass AGB was BC 2.5% biochar/0% compost; conversely, for LPP biochar, the only treatment resulting in a significant increase in switchgrass AGB was 2.5% biochar/5% compost. Maximal AGB values were achieved only when biochar treatments incorporated compost amendment. The three highest amounts of switchgrass AGB were attained with BC biochar under the following treatments: 2.5% biochar/5% compost at 1.64 ± 0.16 g; 5% biochar/2.5% compost at 1.79 ± 0.12 g; and 5% biochar/5% compost at 1.71 ± 0.38 g. Interestingly, while two of the three highest AGB values were achieved with 5% BC biochar amendment rates, AGB values for PL and LPP biochar were highest with a 2.5% biochar amendment. These results may be explained by Novak et al. (2019), who hypothesized on the possibility of increased pH levels leading to lower accessibility to P and other micronutrients for switchgrass at the highest PL amendment rate (i.e., 5%). For LPP biochar, higher pH levels were not a cause of concern, with a soil pH range of 4.54 to 5.06 for amended soils.
Plant tissue heavy metal concentrations are shown in Table 3. In buffalograss, BC and PL biochar amendments both significantly reduced Cd and Zn tissue concentrations as compared to the control. For LPP biochar, only LPP 2.5%/5% compost, and the two LPP 5% biochar with compost treatments resulted in significantly lower Cd and Zn concentrations as compared to the control. For switchgrass, the BC and PL results were similar; five of six BC biochar treatments and all PL biochar treatments resulted in significantly lower Cd and Zn tissue concentrations. Similarly, LPP biochar also fared better, with Cd being significantly reduced as compared to the control in compost-containing treatments, while all treatments containing LPP biochar led to a significant reduction in Zn tissue concentration when compared to the control soil.
Overall, for both grasses, BC biochar provided the greatest AGB yields, followed by PL biochar. Likewise, these two biochars resulted in lower Cd and Zn tissue concentrations in more treatment combinations than LPP biochar. It should be noted that AGB yields with buffalograss were generally less than half of those achieved with switchgrass, with many of the biochar/compost treatments failing to yield numbers significantly greater than the control. These results potentially indicate that buffalograss, at least in comparison to switchgrass, may not be the most suitable candidate for revegetation efforts in this disturbed soil. In a study by Nelson et al. (2015) looking at the phytoremediation of iron mine tailings, they hypothesized that quailbush might be a more resilient plant species over buffalograss in extended adverse soil conditions. However, if soil conditions can be significantly improved, buffalograss could be a viable candidate for revegetation efforts. A study by Robins, performed at high-elevation and semi-arid conditions, demonstrated that switchgrass, a warm-season grass, was more productive during the summer, but cold-season grasses such as buffalograss were able to generate more biomass across the entire growing season (Robins 2010). Regarding biochar, 11 of 12 treatments (across both grass studies) containing LPP biochar failed to increase AGB as compared to the controls, indicating that LPP biochar may not be as suitable a candidate for assisting in revegetation efforts in this study soil regardless of plant species used.
3.3 Microbial biomass
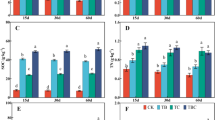
Total PLFA concentrations were used to determine microbial biomass for all treatments (Fig. 2). For treatments planted with buffalograss (Fig. 2a), microbial biomass in biochar treatments sans compost trended upward, but only PL was significant compared to the control soil. Increasing compost from 2.5% to 5% led to significant increases in microbial biomass for all treatments (Fig. 2a) as compared to the control. However, when compared to the compost-only treatments, only BC 5% biochar/5% compost, and all PL biochar treatments resulted in significantly greater microbial biomass values (Fig. 2a). Microbial biomass concentrations for BC and LPP were similar across treatments except for the 5% biochar/5% compost treatment, where BC biochar resulted in greater microbial biomass. PL biochar on the other hand resulted in significantly greater microbial biomass under all treatments when compared to BC and LPP (Fig. 2a), with maximal microbial biomass achieved with PL 5% biochar/5% compost (mean: 52.2 nmol g−1 dry soil). This may be attributable to the higher nutrient content of PL biochar—particularly N, P, and S—as compared to both the BC and LPP biochars (Sigua et al. 2019).
In treatments planted with switchgrass, patterns for microbial biomass across treatments were similar to those for buffalograss. One exception was that, in addition to PL biochar with no compost, BC biochar with no compost also resulted in significantly greater microbial biomass values as compared to the control soil. When compared to compost-only treatments, all BC 5% biochar treatments, and all PL biochar treatments were significantly greater than their compost-only counterparts (Fig. 2b). Microbial biomass concentrations for the 2.5% application rate of BC and LPP biochars were similar, though the 5% treatments for these two biochars diverged, with LPP treated soils having significantly lower microbial biomass values. When compared to BC and LPP, PL biochar led to significantly greater microbial biomass values in 11 of 12 treatments (5 of 6 BC; 6 of 6 LPP; Fig. 2b). Interestingly, as determined by Novak et al. (2019) the LPP used in this study had a higher C content, that—if not purely in recalcitrant form—may potentially encourage greater overall microbial growth. However, the PL biochar had the greater N content, and therefore a lower C:N ratio. Eiland et al. (2001) demonstrated lower C:N ratio composts, early in the composting process, resulted in greater microbial biomass gains over their higher C:N ratio compost counterparts; as such, microbes favored non-nitrogen limiting conditions early in the process. Later in the composting process however, those microbial biomass patterns inverted, and the higher C:N ratio composts had greater microbial biomass values as compared to the lower C:N ratio composts. It is possible that the use of PL biochar when reclaiming soils may have an important role in boosting early microbial biomass numbers, which will allow soils to increase microbial numbers early in the reclamation process. Likewise, addition of LPP biochar in mine-impacted soils, while not potentially beneficial for initial plant or microbial biomass, may net long-term gains as reclaimed soils mature and the microbial communities are able to process higher C:N materials.
Comparing microbial biomass between grass species revealed that microbial biomass values were greater in the switchgrass portion for 13 of the 21 treatments studied. This included all BC biochar treatments, five of six PL treatments, one LPP treatment, and the highest compost-only treatment. Given the similarity in pH values between the soils in both portions of the grass study (Table 2), differences in microbial biomass are most likely due to other environmental factors. The differences in microbial biomass between the plant species may, in part, be attributable to the two plant species themselves. First, a study conducted by Innes et al. (2004) demonstrated that certain plant species influenced microbial biomass in a soil type-dependent manner. Second, differences in the below-ground biomass of the two plants species may have influenced their respective microbial biomass. While not reported in this study, buffalograss below-ground biomass was significantly lower than the switchgrass below-ground biomass values reported by Novak et al. (2019). Prior studies have demonstrated that increases in root biomass have been associated with corresponding increases in microbial biomass (Spehn et al. 2000). Overall, significant treatment effects on microbial biomass, as determined by ANOVA, were determined for plant species, biochar type, and compost amendment rates for both buffalograss and switchgrass portions of the experiment; only buffalograss was significantly influenced by biochar amendment rate (Supplemental Tables 1 and 2).
3.4 Soil microbial community structure and function in response to biochar treatment
Non-metric multidimensional scaling (NMS) analysis was used to visualize the response of microbial communities to biochar treatments (Fig. 3). Centroids (with standard error bars) based on triplicate samples for each treatment were derived from a total of 32 PLFAs (expressed as the ratio to C16:0). Microbial communities with similar structures cluster closer together. For buffalograss, there was considerable overlap between BC and LPP clusters, both of which overlapped with the control soil. LPP also overlapped with the compost-only cluster. PL biochar-treated mining-impacted soils did not overlap with any other soils, indicating a considerable, significant shift (PERMANOVA; p = 0.0002) in the microbial communities of soils treated with PL biochar. For switchgrass samples, microbial community structure followed a pattern like buffalograss. Considerable overlap between BC and LPP clusters was evident, with both biochar groups overlapping with the compost-only samples, and BC biochar overlapping with the control soil. As with buffalograss, the PL cluster was distinct from all other samples, indicating a significant shift (PERMANOVA; p = 0.0002) in microbial community structure. Ducey et al. (2015) previously demonstrated the ability of PL-derived biochar to shift microbial communities, and these results appear to be at least partially explained by changes in soil pH (R2 = 0.352). In the current study, PERMANOVA analysis revealed that, in addition to biochar type, soil microbial community structure was significantly affected by plant species (p = 0.0002), and compost amendment rate (p = 0.0002), but not by biochar amendment rate (p = 0.07). These results may be attributable to differences in root exudates between plant species, or in the case of compost, the availability of organic carbon or other micronutrients, supplied in greater abundances with increasing amendment rates (Wu et al. 2016).
Nonmetric multidimensional scaling plot showing mine soil microbial community structure in response to treatment. Symbols represent the centroid of the triplicate samples of each treatment. Horizontal and vertical bars represent standard error in relation to their centroids along axes 1 and 2, respectively. Plant species is indicated by symbol color (red = buffalograss; blue = switchgrass). Biochar amendment rate is indicated by symbol size (smaller = 2.5%; larger = 5%). Biochar type is indicated by symbol type (circle = BC, beef cattle manure; triangle = PL, poultry litter; square = LPP, lodgepole pine; compost only = + ; control = X). Compost amendment rate is indicated by the symbol border color (no color = 0%; grey = 2.5%; black = 5%). Joint plot vectors (red lines) were selected for display based on a combined R2 cutoff of 0.35 or greater
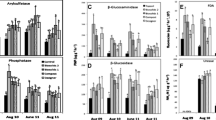
To determine where shifts in the microbial community structure were greatest, PLFAs (expressed as a percentage of total microbial-associated PLFA) were sorted into the following microbial-related groups: total fungi; Gram-negative bacteria; Gram-positive bacteria; and actinomycetes (Fig. 4). In both the buffalograss and switchgrass portions of the experiment, PL biochar treatment resulted in significantly increased relative abundances of total fungi, with a concomitant significant decrease in Gram-negative bacteria as compared to all other treatments (Fig. 4). It has been previously reported that Gram-negative bacteria are favored in heavy metal-contaminated soils (Frostegard et al. 1993), so these results may be partially explained by the ability of PL biochar to significantly reduce heavy metal bioavailability in soil (Table 1). Similar results were seen by Xu et al., (2018) where Gram-negative abundances were increased in unamended, metal-contaminated soils. The authors also demonstrated an increase in fungal abundances upon application of biochar, potentially linked to a reduction in metal-related stresses (Xu et al. 2018). Overall, while trends in the relative abundances of all microbial groups, in response to treatment, looked very similar between both grass species (Fig. 4), Gram-negative, Gram-positive, and actinomycetes were all significantly influenced by plant species (Supplemental Table 1). Additionally, all microbial groups, as determined by ANOVA, were significantly influenced by biochar type (Supplemental Table 1).
Relative abundances (%) of microbial groups based on total PLFA. The upper boxes represent Q3 (75th percentile), while lower boxes represent Q1 (25th percentile), both from the midline (median). Whiskers represent maximum and minimum values, while outliers (> 1.5 times the corresponding quartile) are represented by points beyond each whisker. Box and whisker plots that do not share a letter, for that grass species and microbial group, are significantly different (p < 0.05)
Microbial function was assessed using fluorometric measurement of soil enzyme assays (Fig. 5). BG, NAG, and EST were all significantly influenced by plant species, and all four enzymes assayed were significantly influenced by biochar type (Supplemental Tables 1 and 2). In a previous report that examined soil microbial activity under a variety of grass species, Haney et al. (2010), demonstrated that soil microbial communities under buffalograss had significantly greater soil microbial activities as compared to those grown under switchgrass; a result likewise reflected in this study. Likewise, compost application rate significantly influenced BG and EST activities for both grasses, while biochar amendment rate significantly influenced the switchgrass NAG and EST activities (Supplemental Table 1). Since biochar type was the only consistent influencing factor for all four enzymes (like the microbial groups), Fig. 5 is presented according to biochar type. For BG, NAG, and EST, PL resulted in significantly greater enzymatic activity than any other biochar treatment. The only other treatment with enzyme activity rates significantly greater than the control was LPP biochar for NAG in buffalograss. For AP, in buffalograss, no biochar treatment resulted in enzyme activities greater than the control, though both PL and LPP did trend upward over the control, similar to the compost-only treatment. Similarly, in switchgrass, while only BC biochar amended soil had enzyme activity rates significantly greater than the control soil, all other treatments did have means greater than the control.
Soil enzyme activities. Note that vertical axes are not shown at the same scale between grass species or enzyme. The upper boxes represent Q3 (75th percentile), while lower boxes represent Q1 (25th percentile), both from the midline (median). Whiskers represent maximum and minimum values, while outliers (> 1.5 times the corresponding quartile) are represented by points beyond each whisker. Box and whisker plots that do not share a letter, for that grass species and enzyme, are significantly different (p < 0.05)
The influence of biochar type on NAG activity can be viewed in the context of PL’s influence on fungi (Fig. 4). NAG, also known as chitinase, catalyzes the hydrolysis of N-acetyl-β-D-glucosamine residues from the non-reducing ends of chitooligosaccharides. These oligomers are abundant in fungal cell walls, and the enzyme is highly correlated with fungal biomass (Miller et al. 1998). Additionally, the influence of increasing compost amendment rates on the enzymatic activity for both BG and EST can be explained by the potential of increased available C (de Almeida et al. 2015). Likewise, the influence of pH on microbial activity has been well documented (Xu et al. 2017), and may explain increases in microbial activity in association with PL biochar amendment. Overall results, however, compared to soils not impacted by mining, demonstrate that microbial activity for these soils should be considered low. For example, a study by Kim et al. (2019) using a similar fluorometric enzyme assay (as the one employed in this study) reported the activity rates of the three extracellular enzymes BG, NAG, and AP as two orders of magnitude greater in forest soils as compared to what was reported in this study. Additionally, for this study, we employed the intracellular enzyme esterase as an indicator of general microbial activity. Esterases are a class of hydrolase enzyme that are responsible for splitting esters/lipids (lipases), and according to a metagenomic study conducted by Souza et al. (2018) are the most abundant of the hydrolases found in soil. In a study by Lagomarsino et al. (2021), likewise conducted in a forest soil, esterase enzyme activities were two to three orders of magnitude greater than we have reported.
Previous studies, in conjunction with this current study, have demonstrated the ability of biochar amendment to boost microbial biomass and influence microbial composition. Studies have also shown that these amended soils can support those changes for extended periods of time (Ducey et al. 2013). These studies, however, often look at biochar amendment of soils that—while potentially degraded due to erosion or other natural or man-made processes—have not experienced decimation of their entire O, A, and B soil horizons. The results compiled in this study pose a few questions as to the state of the parent material. For example, what is the diversity of the microbial community of this mine-impacted soil? And what biochemical processes—if any—does the existing microbial community encode for that will support reclamation efforts? While results from this study indicate that biochar amendment was able to boost microbial biomass, there appeared to be a disconnect between microbial biomass gains and increases in microbial activity. Given the poor microbial activity of the parent material, it is possible that these soils do not possess the capacity for cycling nutrients, stimulating soil formation, or performing other required ecosystem services that would be expected of a healthy soil. Therefore, it would prove beneficial to perform metagenomic analyses of mine-impacted soils post-remediation but pre-reclamation, to determine if additional strategies are necessary to stimulate microbial diversity and “fill in the gaps” for any necessary soil biochemical pathways that lack representation (and even functional redundancy) within the microbial community. In addition, it should be noted that this was a short-term study, with a duration of only 50 days. This may potentially be an insufficient length of time to allow the microbial communities to acclimate and establish themselves. Longer duration experiments will be required to address this issue.
In reclamation scenarios where the existing soils lack the means to provide the necessary biological activity required for successful restoration efforts, methods such as bioaugmentation hold promise. In such a case, effective microorganisms would be applied to mine-impacted soils, providing the necessary biological potential required for a functional soil, while additional amendments—e.g., biochar and compost—provide elements necessary to restore the physical and chemical characteristics required to support those functions. The use of effective microorganisms has been demonstrated to hold promise in this regard. For example, Zornoza et al. (2017) demonstrated the ability of bioaugmentation to improve microbial biomass and activity in Cd and Zn contaminated soils, which in turn resulted in increased plant richness and density and C sequestration. Similarly, plant growth-promoting rhizobacteria (PGPR) have been used successfully to promote plant growth in coal mine-impacted soils with low microbial activity (Grobelak et al. 2018). It should be noted that, while such reclamation efforts in mine-impacted soils may successfully enhance soil fertility, soil microbial community structure and function may be altered in ways that do not approximate their adjacent, pristine neighboring ecosystems (Dimitriu et al. 2010). This issue of course, when compared to zero attempts at reclamation, does not seem to be a major hurdle if biochemical processes can be successfully restored.
4 Conclusion
Overall results indicate that—to varying degrees—biochar feedstock, as well as biochar and compost application rates, influenced soil physicochemical factors, plant above-ground biomass, plant Cd and Zn tissue concentrations, microbial abundance and composition, and enzyme activity. Analysis of soil bioavailable Cd and Zn concentrations demonstrated that the two manure-based biochars, PL and BC, resulted in the greatest reductions, and that application rates of 5% for both biochars coupled with either 2.5% or 5% compost achieved best results. The effect of biochar amendment on above-ground biomass revealed that BC biochar provided the greatest yields. Additionally, above-ground biomass was significantly influenced by plant species, with switchgrass yields generally double those of buffalograss. Both BC and PL biochars resulted in lower Cd and Zn plant tissue concentrations in more treatment combinations than LPP biochar. PL biochar resulted in significantly greater microbial biomass under all treatments compared to BC and LPP biochars, with greatest microbial biomass in soils amended with PL biochar 5% coupled with 5% compost. Poultry litter amendment resulted in a significant, observable shift in soil microbial community composition for both grasses. Furthermore, soil microbial communities were significantly influenced by plant species and compost amendment rate, but not biochar amendment rate. Parsing microbial community structure data, we observed that poultry litter significantly increased the relative abundances of fungi, and concomitantly decreased Gram-negative bacterial relative abundances. Microbial activity was greatest in soils grown with buffalograss, and biochar type was a significant influencing factor for all four enzymes studied. Poultry litter resulted in the greatest enzyme activity rates for the enzymes BG, NAG, and EST, while BC biochar-amended soils grown with switchgrass demonstrated significantly elevated AP enzyme activity. Overall, while biochar type, biochar amendment rate, composition amendment rate, and plant species all significantly influenced most variables, only biochar type had a significant effect on all variables measured in this study. These results indicate that mine-impacted soils amended with biochar—particularly those derived from manure-based feedstocks—see significant impacts to their physicochemical and biological characteristics, and potentially indicate long-term positive outcomes for soil reclamation efforts at these sites.
References
Alam SM, Naqvi SSM, Ansari R (1999) Impact of soil pH on nutrient uptake by crop plants. Handbook Plant Crop Stress 2:51–60
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals–concepts and applications. Chemosphere 91:869–881
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J Chem-Ny 2019
Amellal N, Bartoli F, Villemin G, Talouizte A, Heulin T (1999) Effects of inoculation of EPS-producing Pantoea agglomerans on wheat rhizosphere aggregation. Plant Soil 211:93–101
Andrunik M, Wolowiec M, Wojnarski D, Zelek-Pogudz S, Bajda T (2020) Transformation of Pb, Cd, and Zn minerals using phosphates. Minerals 10:342
Arnon DI, Johnson CM (1942) Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiol 17:525–539
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Bossuyt H, Denef K, Six J, Frey SD, Merckx R, Paustian K (2001) Influence of microbial populations and residue quality on aggregate stability. Appl Soil Ecol 16:195–208
Brookes PC, Mcgrath SP (1984) Effects of metal toxicity on the size of the soil microbial biomass. J Soil Sci 35:341–346
Buyer JS, Sasser M (2012) High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol 61:127–130
Chander K, Brookes PC, Harding SA (1995) Microbial biomass dynamics following addition of metal-enriched sewage sludges to a sandy loam. Soil Biol Biochem 27:1409–1421
Chen LX, Huang LN, Mendez-Garcia C, Kuang JL, Hua ZS, Liu J, Shu WS (2016) Microbial communities, processes and functions in acid mine drainage ecosystems. Curr Opin Biotech 38:150–158
Chuan MC, Shu GY, Liu JC (1996) Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Poll 90:543–556
de Almeida RF, Naves ER, da Mota RP (2015) Soil quality: Enzymatic activity of soil β-glucosidase. Glob J Agric Res Rev 3:146–150
Deng SP, Kang H, Freeman C (2011) Microplate fluorimetric assay of soil enzymes. In: Dick RP (ed) Methods of Soil Enzymology, vol 9. SSSA Book Series. Soil Science Society of America, Madison, WI, pp 311–318
Deng SP, Popova IE, Dick L, Dick R (2013) Bench scale and microplate format assay of soil enzyme activities using spectroscopic and fluorometric approaches. Appl Soil Ecol 64:84–90
Dhaliwal SS, Singh J, Taneja PK, Mandal A (2020) Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ Sci Pollut R 27:1319–1333
Dimitriu PA, Prescott CE, Quideau SA, Grayston SJ (2010) Impact of reclamation of surface-mined boreal forest soils on microbial community composition and function. Soil Biol Biochem 42:2289–2297
Drijber RA, Doran JW, Parkhurst AM, Lyon DJ (2000) Changes in soil microbial community structure with tillage under long-term wheat-fallow management. Soil Biol Biochem 32:1419–1430
Ducey TF, Ippolito JA, Cantrell KB, Novak JM, Lentz RD (2013) Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl Soil Ecol 65:65–72
Ducey TF, Novak JM, Johnson MG (2015) Effects of biochar blends on microbial community composition in two coastal plain soils. Agriculture 5:1060–1075
Eiland F, Klamer M, Lind AM, Leth M, Baath E (2001) Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb Ecol 41:272–280
Frostegard A, Tunlid A, Baath E (1993) Phospholipid fatty-acid composition, biomass, and activity of microbial communities from 2 soil types experimentally exposed to different heavy-metals. Appl Environ Microb 59:3605–3617
Goecke P, Ginocchio R, Mench M, Neaman A (2011) Amendments promote the development of Lolium perenne in soils affected by historical copper smelting operations. Int J Phytoremediat 13:552–566
Gomez JD, Denef K, Stewart CE, Zheng J, Cotrufo MF (2014) Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur J Soil Sci 65:28–39
Grobelak A, Kokot P, Hutchison D, Grosser A, Kacprzak M (2018) Plant growth-promoting rhizobacteria as an alternative to mineral fertilizers in assisted bioremediation - sustainable land and waste management. J Environ Manage 227:1–9
Gutiérrez M, Qiu X, Collette ZJ, Lurvey ZT (2020) Metal content of stream sediments as a tool to assess remediation in an area recovering from historic mining contamination. Minerals 10
Haney RL, Kiniry JR, Johnson MVV (2010) Soil microbial activity under different grass species: Underground impacts of biofuel cropping. Agr Ecosyst Environ 139:754–758
Huang CYL, Schulte EE (1985) Digestion of plant-tissue for analysis by ICP emission-spectroscopy. Commun Soil Sci Plan 16:943–958
Innes L, Hobbs PJ, Bardgett RD (2004) The impacts of individual plant species on rhizosphere microbial communities in soils of different fertility. Biol Fert Soils 40:7–13
Ippolito JA, Olszyk D, Ducey TF, Sigua GC, Trippe K, Phillips CL, Spokas KA, Novak JM, Johnson MG (2016) Biochar selection for reducing mine soil metal availability. ASA-CSSA-SSSA International Annual Meeting. Phoenix, AZ. https://scisoc.confex.com/scisoc/2016am/videogateway.cgi/id/27455?recordingid=27455
Ippolito JA, Berry CM, Strawn DG, Novak JM, Levine J, Harley A (2017) Biochars reduce mine land soil bioavailable metals. J Environ Qual 46:411–419
Ippolito JA, Cui L, Novak JM, Johnson MG (2019) Biochar for mine-land reclamation. In: Ok YS, Tsang DCW, Bolan N, Novak JM (eds) Biochar from Biomass and Waste: Fundamentals and Applications, 1st edn. Elsevier, Amsterdam, pp 75–90
Ippolito JA, Cui L, Kammann C, Wrage-Monnig N, Estavillo JM, Feurtes-Mendizabal T, Cayuela ML, Sigua G, Novak J, Spokas K, Borchard N (2020) Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2:421–438
ITRC (2010) Case study: Oronogo-Duenweg mining site, Jasper County, Missouri. https://www.itrcweb.org/miningwaste-guidance/cs34_oronogo_duenweg.pdf
Johnson AW, Gutierrez M, Gouzie D, McAliley LR (2016) State of remediation and metal toxicity in the Tri-state Mining District, USA. Chemosphere 144:1132–1141
Kavitha B, Reddy PVL, Kim B, Lee SS, Pandey SK, Kim KH (2018) Benefits and limitations of biochar amendment in agricultural soils: A review. J Environ Manage 227:146–154
Kim S, Li G, Han SH, Kim C, Lee ST, Son Y (2019) Microbial biomass and enzymatic responses to temperate oak and larch forest thinning: Influential factors for the site-specific changes. Sci Total Environ 651:2068–2079
Lagomarsino A, De Meo I, Agnelli AE, Paletto A, Mazza G, Bianchetto E, Pastorelli R (2021) Decomposition of black pine (Pinus nigra j. F. Arnold) deadwood and its impact on forest soil components. Sci Total Environ 754
Lehman RM et al (2015) Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability (Basel) 7:988–1027
Lian B, Chen Y, Zhu L, Yang R (2008) Effect of microbial weathering on carbonate rocks. Earth Sci Front 15:90–99
Liao M, Chen CL, Huang CY (2005) Effect of heavy metals on soil microbial activity and diversity in a reclaimed mining wasteland of red soil area. J Environ Sci China 17:832–837
Miller M, Palojarvi A, Rangger A, Reeslev M, Kjoller A (1998) The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl Environ Microb 64:613–617
Nelson KN, Neilson JW, Root RA, Chorover J, Maier RM (2015) Abundance and activity of 16s rRNA, amoA and nifH bacterial genes during assisted phytostabilization of mine tailings. Int J Phytoremediat 17:493–502
Novak JM, Watts DW (2005) An alum-based water treatment residual can reduce extractable phosphorus concentrations in three phosphorus-enriched coastal plain soils. J Environ Qual 34:1820–1827
Novak JM, Cantrell KB, Watts DW, Busscher WJ, Johnson MG (2013) Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J Soil Sediment 14:330–343
Novak JM et al (2018) Remediation of an acidic mine spoil: Miscanthus biochar and lime amendment affects metal availability, plant growth, and soil enzyme activity. Chemosphere 205:709–718
Novak JM, Ippolito JA, Watts DW, Sigua GC, Ducey TF, Johnson MG (2019) Biochar compost blends facilitate switchgrass growth in mine soils by reducing Cd and Zn bioavailability. Biochar 1:97–114
Penido ES, Martins GC, Mendes TBM, Melo LCA, Guimaraes ID, Guilherme LRG (2019) Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotox Environ Safe 172:326–333
Phillips CL, Trippe KM, Whittaker G, Griffith SM, Johnson MG, Banowetz GM (2016) Gasified grass and wood biochars facilitate plant establishment in acid mine soils. J Environ Qual 45:1013–1020
Pierzynski GM, Vaillant GC (2006) Remediation to reduce ecological risk from trace element contamination: A decision case study. J Nat Resour Life Sci Educ 35:85–94
Revell KT, Maguire RO, Agblevor FA (2012) Field trials with poultry litter biochar and its effect on forages, green peppers, and soil properties. Soil Sci 177:573–579
Reverchon F et al (2014) Changes in δ15N in a soil-plant system under different biochar feedstocks and application rates. Biol Fert Soils 50:275–283
Reverchon F et al (2015) A preliminary assessment of the potential of using an acacia-biochar system for spent mine site rehabilitation. Environ Sci Pollut R 22:2138–2144
Robins JG (2010) Cool-season grasses produce more total biomass across the growing season than do warm-season grasses when managed with an applied irrigation gradient. Biomass Bioenerg 34:500–505
Sigua GC, Novak JM, Watts DW, Ippolito JA, Ducey TF, Johnson MG, Spokas KA (2019) Phytostabilization of Zn and Cd in mine soil using corn in combination with biochars and manure-based compost. Environments 6
Singh JS (2015) Microbes play major roles in the ecosystem services. Clim Change Environ Sustain 3
Singh B, Dolk MM, Shen Q, Camps-Arbestain M (2017) Biochar pH, electrical conductivity and liming potential. In: Singh B, Camps-Arbestian M, Lehmann J (eds) Biochar: A Guide to Analytical Methods. CSIRO Publishing, Australia, pp 23–38
Skeel VA, Gibson DJ (1996) Physiological performance of Andropogon gerardii, Panicum virgatum, and Sorghastrum nutans on reclaimed mine spoil. Restor Ecol 4:355–367
Souza RC, Cantao ME, Nogueira MA, Vasconcelos ATR, Hungria M (2018) Outstanding impact of soil tillage on the abundance of soil hydrolases revealed by a metagenomic approach. Braz J Microbiol 49:723–730
Spehn EM, Joshi J, Schmid B, Alphei J, Korner C (2000) Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant Soil 224:217–230
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J Hazard Mater 190:432–441
USEPA (2013) Record of decision amendment plan, Oronogo-Duenweg mining belt superfund site, Jasper County, Missouri, mine and mill waste Operable Unit 1. Lenexa, KS. https://archive.epa.gov/region07/cleanup/npl-archive/web/pdf/000030285041.pdf
USEPA (2017) Fourth five-year review report for Oronogo-Duenweg mining belt superfund site, Jasper County, Missouri. https://semspub.epa.gov/work/07/30323583.pdf
Veum KS, Lorenz T, Kremer RJ (2019) Phospholipid fatty acid profiles of soils under variable handling and storage conditions. Agron J 111:1090–1096
Wu HP et al (2016) Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl Microbiol Biot 100:8583–8591
Xu ZW et al (2017) Soil enzyme activity and stoichiometry in forest ecosystems along the north-south transect in eastern China. Soil Biol Biochem 104:152–163
Xu YL et al (2018) Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ 621:148–159
Zhao XQ, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotox Environ Safe 170:218–226
Zornoza R, Gomez-Garrido M, Martinez-Martinez S, Gomez-Lopez MD, Faz A (2017) Bioaugmentaton in technosols created in abandoned pyritic tailings can contribute to enhance soil C sequestration and plant colonization. Sci Total Environ 593–594:357–367
Acknowledgements
The information in this document has been funded in part by the U.S. Environmental Protection Agency. It has been subjected to internal review according to Agricultural Research Service policy and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. We thank Kandis Diaz, within the Department of Soil and Crop Sciences at Colorado State University, for laboratory assistance. We would also like to thank the reviewers of this manuscript for their invaluable suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ducey, T.F., Novak, J.M., Sigua, G.C. et al. Microbial response to designer biochar and compost treatments for mining impacted soils. Biochar 3, 299–314 (2021). https://doi.org/10.1007/s42773-021-00093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-021-00093-3