Abstract
Peat remains the primary constituent of horticultural growing media in professional use. However, use of peat in horticultural growing media results in greenhouse gas emissions and biodiversity loss due to excavation of natural peatlands. Biochar is gaining attention as a sustainable alternative to peat use in horticulture. This study examined the potential of biochar produced from a particular type of sawmill residue, as a partial replacement for peat in horticultural growing media. Five treatments including peat only, biochar only, biochar and peat in 1:1, 1:3, and 3:1 (V/V) ratios were assessed. The addition of biochar into growing media increased the pH and EC of the medium. However, physical properties (air-filled porosity and water holding capacity) were negatively affected with the increase in biochar content in the medium. According to the germination test results, biochar significantly improved germination and the shoot and root length of germinated seeds of cress, lettuce and tomato when compared to peat-only and biochar-only treatments. The inclusion of biochar in 25–50% volume ratio improved plant growth parameters compared to peat-only and biochar-only media. Results obtained from this study suggest that sawmill residue offers great potential as a feedstock for biochar production and inclusion of biochar has positive effects on seed germination and plant growth that might compete with modified peat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A sawmill co-product was used to produce biochar |
Addition of biochar increased the pH, EC and nutrient content in the medium |
The inclusion of biochar in 25 to 50% volume ratio improved plant growth parameters compared to peat-only media |
Higher biochar proportions in the medium diminish the physico-chemical properties and plant growth parameters |
1 Introduction
Peat extracted for horticultural purposes rapidly oxidizes, leading to the emission of the greenhouse gas CO2 from previously stored stocks of carbon in the natural environment (Sohi et al. 2013). Peatlands are wetland ecosystems characterized by the accumulation of organic matter and currently account for 3% of global land area (Robertson 1993; Alexander et al. 2008). Peatlands play an essential role in ecosystem services and contain one-third of soil carbon (C) globally (Page et al. 2011). Depending on human use, peatlands can act as either C sink or C source (Kern et al. 2017). Current use of peat in horticulture involves around 11 million tonnes per year (Clarke and Rieley 2010; Bos et al. 2011). Finding sustainable substitutes for peat used in horticultural media has been recognized as a strategic option by the Dutch government to minimize negative impacts on peatlands resulting from unsustainable peat extraction (Bos et al. 2011).
Materials suitable for peat substitution need to meet several requirements: (i) they must be readily available and secure in supply (ii) have good quality and (iii) their sources and processing have to be sustainable. Above all, the economic viability is a key factor overlying these requirements (Sohi et al. 2013), as the cost of peat without including all externalities is very low. Several candidate materials are already recognized and in use for the replacement of peat in growing media (Barrett et al. 2016) such as compost, coir, bark and wood fiber, etc. However, these materials also have negative environmental impacts due to greenhouse gas emissions associated with processing and transport (Schmilewski 2008). As ingredients to growing media, these materials can be grouped into three categories based on their total carbon footprint associated with processing, handling and transportation: (i) coir (less than 500 kg CO2eq t−1) (ii) peat (from UK and Ireland) and perlite (500–750 CO2eq t−1) and (iii) peat (Finland), green compost, bark, wood fiber and vermiculite (more than 750 CO2eq t−1) (Defra 2009).
Biochar is gaining attention as a partial peat substitute owing to certain attractive physical and chemical properties (Tian et al. 2012; Fornes et al. 2017). These properties include macro-porosity that facilitates the retention and release of water, low bulk density, resistance to compression and shrinkage, the potential for manufacture in different specified particle size ranges and the possibility to improve microbial activity due to its internal pore structure (Shackley et al. 2010; Weber and Quicker 2018). Biochar is produced through slow pyrolysis which is the thermochemical conversion of biomass under an oxygen-limited atmosphere (Lehmann et al. 2006). Biochar itself is highly recalcitrant in the environment compared to raw feedstock material. It will only degrade slowly in the soil environment and help to sequester atmospheric CO2 (Lehmann et al. 2006; Wu et al. 2020). Even though biochar has a lot of favorable properties as a non-peat substitute, the net effect on cost-effectiveness and environmental sustainability can only be positive if the production of biochar is sustainable (Sohi et al. 2013).
When biochar is used as a growing medium component, its characteristics play an important role (Huang and Gu 2019). When biochar is used in relatively larger proportions such as in horticultural growing media (compared to biochar use in, e.g., agricultural soil amendment where its concentration in soil is typically few wt.% or less), biochar characteristics have significant impact on the growing medium’s physico-chemical properties and resulting plant growth (Nieto et al. 2016). Thus, proper characterization of biochar prior to application is essential to identify the optimal characteristics for the growing medium application as well as to maintain consistent quality of biochar over sequential production batches (Kern et al. 2017). Having a high carbon content in biochar increases the stability of the biochar and the growing medium (Kaudal et al. 2018; Rathnayake et al. 2020a, b). Consequently, the biochar’s microbial degradation over time is reduced as well as the greenhouse gas emissions (Lévesque et al. 2018).
Biochars with high pH could alleviate the complications associated with acidic pH in soil substrates and can substitute liming requirements (Margenot et al. 2018). Biochars produced from different feedstock materials at the same production temperature as well as biochars produced from the same feedstock material but under different pyrolysis temperatures could have considerably different pH values (Ronsse et al. 2013; Weber and Quicker 2018). For example, the pH of biochars produced from pruning waste at 300 ºC and 500 ºC were 7.53 and 10.30, respectively (Nieto et al. 2016). On the other hand, the pH of biochars produced of mixed soft wood, greenhouse waste and poultry litter at 550 °C were 8.45, 9.65, and 9.51, respectively (Singh et al. 2017). Generally, biochars produced from high ash containing feedstocks and biochars produced at higher pyrolysis temperatures have higher pH (Blok et al. 2017). Moreover, depending on the biochar content in the growing medium, its effect on the resulting growing medium’s pH could be varied (Kern et al. 2017; Huang and Gu 2019). For instance, the higher the biochar content in the medium, the higher the pH of the medium (Vaughn et al. 2013). Also, the magnitude of the changes in pH of the medium with different biochar rates is highly dependent on the biochar type, which is ultimately dependent on the feedstock composition and production temperature (Nieto et al. 2016). On the other hand, having a high ash content and high alkalinity could lead to phytotoxicity as well as nutrient unavailability and antagonistic effects on nutrient utilization (Rogovska et al. 2012; Reza et al. 2020). Finally, the presence of potentially toxic elements and organic compounds (i.e., volatile organic compounds and polyaromatic hydrocarbons) could adversely impact seed germination and plant growth (Munzuroglu and Geckil 2002; Buss et al. 2016).
In terms of physical biochar properties, the biochar particle size distribution affects structural properties (i.e., bulk density, water holding capacity, porosity, etc.) and nutrient availability in the medium (Ferlito et al. 2020; Prasad et al. 2020). All these physical properties of the growing medium are heavily dependent on the production conditions (i.e., pyrolysis temperature and heating rate), the type of feedstock material used in biochar production and the biochar content in the medium (Ronsse et al. 2013; Antonangelo et al. 2019; Keskinen et al. 2019). For instance, water holding capacities of the growing media with 75% (v/v) and 50% (v/v) of pruning waste biochar produced at 300 ºC were 38.63% and 40.54%, respectively (Nieto et al. 2016). On the other hand, for the same pruning waste feedstock material, biochar produced at 500 ºC resulted 51.73% and 52.74% of water holding capacities when those biochars were incorporated in 75% (v/v) and 50% (v/v) in the growing medium, respectively (Nieto et al. 2016). Air-filled porosity could be changed with the biochar type and its concentration used in the growing medium. For instance, Tian et al. (2012) reported an increase of air-filled porosity (i.e., 15.28–21.55%) when green waste biochar content in the medium increased from 50% (v/v) to 100% (v/v). Moreover, that green waste biochar had higher quantity of particles over 2 mm compared to peat material used in their study and the increase in air-filled porosity was linked to the improvement of the macro-porosity of the medium due to these larger biochar particles (Tian et al. 2012). Thus, particle size distribution plays an important role in determining hydro-physical properties of the growing medium (Huang and Gu 2019).
In recent literature, biochar has been produced under various pyrolysis conditions from various waste feedstock sources and their potential to replace peat and peat-based commercial growing media has been evaluated. According to those studies, paper sludge biochar was able to replace 50% of brown peat (Méndez et al. 2015), pruning waste biochar was able to replace 50–75% of brown peat (Nieto et al. 2016), green waste biochar was able to replace 50% of peat substrate (Tian et al. 2012), sugarcane biochar could replace 25–50% of peat-based commercial growing media (Webber et al. 2018b), pine wood biochar could substitute 25–75% of peat-based commercial growing media (Webber et al. 2018a), and wood biochar was able to substitute 20% of peat (Blok et al. 2017) without plant growth inhibition. Moreover, Tian et al., (2012) reported 20% of plant biomass gain after addition of 50% of biochar into the medium. Altland et al., (2017) reported that 15–20% of rice hull biochar addition could increase the tomato shoot growth significantly due to favorable physico-chemical properties in the medium. In previous work, Blok et al. (2017) reported increased plant growth using different types of biochar at 20% (v/v) content in replacing peat in potting soil.
The wood processing industry, especially sawmills produce a range of co-products, including a fraction that arises from ring debarking. This fraction is produced from the outer 1 cm of the harvested tree that is removed in the absence of dead bark (Arets et al. 2011). Referred to in this work as the vascular cambial zone (VCZ), this material has been mainly used as a low-cost mulching material in agriculture and horticulture, or locally burned for bioenergy generation. Due to high production volumes of VCZ material and its relatively high mineral nutrient composition, it has the potential to more usefully create biochar useful to plant growth and crop production (Forest Research 2019). In the context of finding alternative uses for VCZ materials and finding a sustainable peat replacement, we examined biochar produced by slow pyrolysis of VCZ from Sitka spruce (Picea sitchensis). In addition to assessing the conversion of Sitka spruce VCZ to biochar, we also assessed the resulting biochar for physico-chemical properties relevant to its potential use in partial substitution of peat in horticultural growing media, and validated partial peat substitution through germination and plant growth assays.
2 Materials and methods
2.1 VCZ biochar and peat
Production of biochar was carried out using the stage III rotary kiln pilot-scale pyrolysis unit at the University of Edinburgh (Mašek et al. 2018). The feedstock was sourced from the Petersmuir sawmill, BSW Ltd, Scotland. The VCZ material was found to comprise 40% wood and 60% bark by volume, incorporating the vascular cambium. Whole feedstock material was derived from the sawmill residue produced from Sitka spruce (Picea sitchensis) logs during the process of ring debarking. Feedstock material was pyrolyzed at nominal highest treatment temperature (HTT) of 550 °C. Feedstock material was heated at a rate of 78 °C/min and the residence time at HTT was 3.9 min. The peat used in this study was a commercial peat intended for use in vegetable seedling production which was obtained from Peltracom (Belgium) and which consisted of 70% neutralized white peat and 30% neutralized black peat of Latvian origin.
2.2 Biochar and peat characterization
2.2.1 C, H, N and S analysis
The C, H, N, S analysis of biochar and peat was performed in triplicates in a Flash 2000 Elemental Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The 2,5-bis(5-tert-butyl-benzoxazol-2-yl) thiophene (BBOT) was used as standard reference material during CHNS analysis.
2.2.2 Ash content, volatile matter content and fixed carbon content
Proximate analysis was carried out according to the method described in Singh et al. (2017). Briefly, the moisture content was determined by keeping the sample at 105 °C for 18 h in a conventional oven. Then, oven-dried weights of sample containing crucibles were recorded. Volatile matter content was determined by holding the sample containing crucibles (with the lid on) at 950 °C for 10 min in a muffle furnace. Ash content was determined after heating the sample containing, open crucibles at 750 °C for 6 h in the muffle furnace.
2.3 Growing medium formulation
Five formulations were tested: biochar, peat and three biochar–peat mixtures (Table 1). All the biochar–peat mixtures were defined on a volume basis and implemented from homogeneous ingredients.
2.4 Physical properties of the growing media
2.4.1 Particle size distribution
Particle size distribution of each medium was analyzed using 50 g of dry sample. An AS200 sieve analyzer (Retsch, AS 2000, Germany) was used to separate different particle size fractions by shaking for 15 min. The sieve sizes ranged from 0.075 mm to 4 mm. Duplicates were carried out for each treatment.
2.4.2 Structural properties
Dry bulk density, total pore space, air space and water holding capacity were determined using the method described in Nieto et al. (2016). Briefly, each substrate was filled into a container with a known volume which has sealed drainage holes at the bottom. Then, water was added until the medium got saturated while being put on a watertight pan. Then after saturating, the seal which covered the bottom hole was removed and the sample was allowed to freely drain overnight. The released water quantity was measured to calculate the air space percentage inside the medium. The medium and the container were subsequently weighed. After that, the medium inside the container was put into a pre-weighed pan and put into a drying oven at 105 °C for 24 h. Then, the oven-dried weight of the medium was used to calculate the water holding capacity of the medium. The total porosity of the medium was calculated by summing up the air space and water holding capacity. Dry bulk density was calculated using oven-dried mass of the medium divided by the container volume.
2.5 Chemical properties of the growing medium formulations
2.5.1 pH and EC and organic matter content
The pH and EC of biochar, peat and biochar and peat mixtures were measured in 1:10 (m/v) ratio with deionized water after shaking for 90 min and using an electrical conductivity electrode (WTW-LF537, Germany) and a pH meter (Model 520, Orion, Boston, MA, USA) according to the method described in Singh et al. (2017). Triplicates were analyzed for each mixture. To measure the organic matter content, 0.5 g of the oven-dried (105 °C) material was weighed into a crucible of a known mass. The sub-sample was kept in the muffle furnace at 550 °C for 4 h. The mass loss on reweighing was taken as an estimate for organic matter content.
2.5.2 Elemental composition
The total nutrient content and potentially toxic elements in the biochar, peat and, biochar–peat mixtures were analyzed using the modified dry ashing method described in Singh et al. (2017). Briefly, finely ground peat and biochar samples were oven dried at 105 °C for 24 h. Approximately 200 mg of sample was weighed into a digestion tube, and subsequently heated at 500 °C for 8 h. After the samples cooled down to ambient air temperature, the mass of the sample contained in the tube was recorded. The samples were then digested with 5 ml of concentrated (70%) HNO3 (Chem-Lab, Zedelgem, Belgium) and evaporated until dry. After cooling, 1 ml of HNO3 and 4 ml of H2O2 (30% VWR chemicals, Belgium) were added and evaporated to dryness. Finally, 2 ml of HNO3 was added to dissolve the solids. The resulting solution was filtered using Whatman No. 41 filter paper and the filtrate was diluted to 50 ml with deionized water. The resultant solutions were analyzed using ICP-OES (Varian Vista MPX, Varian Palo Alto, California, USA) and ICP-MS (Varian Vista-MPX CCD Simultaneous, Varian Inc., Victoria, Australia) for K, Ca, Mg, Fe, Al, Mn, P, Cr, Co, Ni, Cu, Zn, As, Mo, Cd and Pb. Triplicate sub-samples were analyzed from each material.
2.6 Germination assay
To assess the phytotoxicity of the formulated growing media, a germination assay was conducted. Briefly, 10 seeds of three species were used: cress (Lepidium sativum) variety Common, lettuce (Lactuca sativa) variety Appia, and tomato (Solanum lycopersicum) variety St. Pierre. Seeds and media containing petri dishes were maintained at 25 °C for 3 days in an incubation chamber. The number of germinated seeds and shoot and root length of the germinated seeds were counted and recorded. The assay was applied with 10 replicates (10 seeds per replicate). Ratio of shoot length to root length of germinated seeds was expressed as germination index. Germination rate was calculated using following Eq. 1.
2.7 Plant growth assay
A plant growth assay was conducted using tomato (Solanum lycopersicum) variety St. Pierre inside a laboratory growing chamber with controlled daylight for 12 h. The experiment was carried out up to four weeks, to assess the suitability of the growing medium formulations to support early seedling growth. Five replicates per treatment were carried out in which 200 cm3 of each pot with a volume of 275 cm3 were filled. One seedling per pot was maintained throughout the plant growth assay. At the end of the four weeks after seeding, the number of leaves per seedling was recorded. After uprooting the seedlings, the fresh weight and lengths of both shoots and roots were measured. Fresh seedling samples were dried at 70 °C for 24 h to determine the shoot and root dry weights of each seedling.
2.8 Statistical analysis
Both germination assay and preliminary plant growth assay were arranged in a completely randomized design. Statistical analyses were performed using Microsoft Excel 2016 and IBM’s SPSS 22 software packages. Differences between the treatments were assessed using Tukey’s post hoc test (at a significance level of 0.05) performed after a one-way ANOVA (analysis of variance).
3 Results and discussion
3.1 Properties of biochar and peat
The results from the basic chemical analysis of biochar and peat used in the experiments are shown in Table 2.
The bulk elemental ratio (ultimate analysis) results show higher total C content and lower total H and N content in biochar compared to peat. Pyrolysis involves the progressive elimination of H and O and relative enrichment in C. As the relative enrichment in C and depletion in hetero-elements like H and O is linked to biochar stability, the H/C ratio is widely used as an indicator of biochar stability (Crombie et al. 2013). Although the O/C ratio can fulfill a similar function, the O content is rarely determined directly, and H/C has been found a more sensitive indicator. Based on the molar H/C ratio (0.4 ± 0.02), the stability of biochar used in this study was considerably higher than the requirement (< 0.70) proposed by the international research community (Budai et al. 2013; Schmidt et al. 2016). In contrast, the recalcitrance of peat is low relative to the H/C benchmark (1.4 ± 0.02). Fixed carbon measured in proximate analysis is considered to reflect the content of aromatic moieties in a substrate that are not readily mineralized (Leng et al. 2019). It is also considered to provide an indication of relative stability (Crombie et al. 2013). The biochar used in this study had a fixed C content of 70 ± 2.43%, indicating higher intrinsic stability. The stability of peat is comparatively low. Having material with higher stability in growing media could increase their resistance towards microbial degradation and lower the CO2 emissions upon degradation (Blok et al. 2017; Lévesque et al. 2018).
Volatile matter shows the opposite pattern, considered to comprise the labile, readily mineralizable fraction of a substrate. The ash reflects the inorganic component with variable elemental composition (Crombie et al. 2013; Antonangelo et al. 2019). The ash includes some nutrient elements that can improve plant nutrition, but also potentially phytotoxic elements that inhibit plant growth (Singh et al. 2017). The higher ash content in biochar is indicative of a potentially higher nutrient content compared to peat (Weber and Quicker 2018). Proximate composition of biochar is, therefore, a feedstock-dependent property as well as a reflection of pyrolysis conditions (Rathnayake et al. 2020b; Reza et al. 2020). Biochar in this study had a comparatively lower ash content (9.0 ± 0.31%) compared to biochars that are derived from grass or crop residues (20–30%) (Vassilev et al. 2010; Weber and Quicker 2018). The biochar has a low N content owing to the volatility of N under pyrolysis conditions. Consequently, biochar had a higher C/N ratio compared to fresh plant biomass and also the peat used in this study. Degradable plant residues with high C/N ratio can immobilize accessible nitrogen in soil, owing to the minimum requirement for N in microbial growth (Carter et al. 2013). Non-degradable C cannot result in this effect, but volatile C and chemical N sorption by biochar may impact plant N availability (Bhatta et al. 2016).
3.2 Growing medium physical properties
Particle size distribution of each substrate formulation used in this study is reported in Table 3. Particle size distribution affects the bulk density of a growing medium and in turn, the particle size of its constituents is relevant. The target bulk density for a growing medium depends on the plants to be grown, their containers (type and size), growing conditions (i.e., outdoor or indoor), type of irrigation, handling requirements, etc. (Barrett et al. 2016). Bulk density, air-filled porosity, water holding capacity and total porosity are shown in Table 4. Reflecting the smaller-sized particles provided by biochar, dry bulk density was much higher for BC100 (0.28 ± 0.02 g/cm3) than for P100 (0.15 ± 0.02 g/cm3). The differences in total porosity and air-filled porosity were proportionally similar, but inversely related to those of bulk density (total porosity of 49.18 ± 0.18% for BC100 compared to 84.42 ± 0.59% for P100; air-filled porosity 4.81 ± 0.43% for BC100 compared to 13.71 ± 0.15% for P100). The difference in water holding capacity was proportionally greater: 70.72 ± 0.45% for P100 compared to 44.38 ± 0.25% for BC100. Particle size distribution (PSD) affects bulk density, water holding capacity and porosity of the growing medium (Nemati et al. 2015). Coarse particles provide air voids, while fine particles are associated with moisture retention (Landis et al. 2009). The particle size distribution has a huge impact on drainage and penetrability of a porous medium and makes up its texture (Blok et al. 2008). The texture of the medium determines the solute and gas flows inside the medium (Blok et al. 2017). The most desirable particle size fraction for a containerized growing medium is between 0.25 mm and 2 mm (Méndez et al. 2015). In the range of 0.25–2 mm, BC100 and P100 had the lowest and the highest amount of particles, respectively. Addition of biochar reduced the amount of particles in the desirable size fraction. Although the biochar used in this study diminished the textural quality of the growing media, PSD is an adjustable property of biochar and can be tuned to requirements (Sohi et al. 2013).
The effect of increasing the biochar content on mixed formulations was not linear with respect to the physical parameters. This is probably because large pores in which smaller-sized particles of denser biochar can reside depend on the abundance of the coarser peat ingredient (Wallach 2008). Biochar increased dry bulk density by 47% in BC50P50 compared to P100, but bulk density of BC75P25 was only 53% higher than the P100. Small-sized biochar particles located in other pores diminish porosity less than replacing peat with biochar. Nieto et al. (2016) also observed increase in bulk density after incorporation of pruning waste biochar into the growing medium. Air-filled porosity was 10% lower for BC50P50 compared to P100, but 51% lower for BC75P25 because of lower porosity in the basic ingredients. Dumroese et al. (2018) also reported a decrease of air-filled porosity with the increase of wood biochar content in the medium. However, Nieto et al. (2016) observed increase and decrease of air-filled porosity in the medium when biochars produced at high temperature (500 °C) and produced at low temperature (300 °C), respectively. Moreover, that effect was noted when biochar content in the medium increased from 50% to 75% (v/v).
Effects on water holding capacity depend on pore-size distribution and pore connectivity rather than directly on particle size (Edeh et al. 2020). Larger pores filled with porous small particles still exhibit porosity, but lower total pore volume and smaller pore size. Consequently, water holding capacity of peat–biochar mixtures decreased with higher biochar content, being 3% lower for BC25P75 than P100, 23% lower for BC50P50, and 31% lower for BC75P25. Nieto et al. (2016) observed a decrease of total porosity in the medium when biochar incorporated, compared to peat-only control. Also, they observed a slight increase of water holding capacity with the increase of biochar content in the medium from 50% to 75%. However, addition of biochar in both 50% and 75% levels decreased the water holding capacity in the medium compared to peat-only control.
Méndez et al. (2015) reported an increase of bulk density, air-filled porosity, water holding capacity and total porosity by 88%, 30%, 20% and 21%, respectively, after addition of 50% (volume basis) deinking sludge biochar into a peat-based growing medium. On the other hand, Tian et al. (2012) observed an increase in bulk density and water holding capacity by 23% and 1% and a decrease of total porosity and air-filled porosity by 15% and 41%, respectively, after addition of 50% of green waste biochar into peat-based growing media. According to Méndez et al. (2015), the most desirable values for water holding capacity, air-filled porosity and total porosity are 60–100%, 10–30% and 50–80%, respectively. BC25P75, BC50P50 and P100 treatments in our study are in the optimum range for air-filled porosity. Only P100 and BC25P75 are in the optimum range of the water holding capacity. Except BC100, all the other treatments are in the optimum range for the total porosity. BC25P75 and P100 fall within the optimum range for all these parameters.
3.3 Growing medium chemical properties
The initial pH, EC and organic matter content of the substrate formulations are listed in Table 5. P100 had relatively higher organic matter (OM) content than the BC100. Thus, OM content in growing medium increases with the peat content. As peat is made of accumulated organic materials, peat has higher organic matter content (94–99%) (Girkin et al. 2019). Even though biochar had a higher total carbon content and fixed carbon content than peat used in this study, lower organic matter content compared to peat was observed. Additionally, the biochar used in this study had a higher ash content than the peat as well. The apparent contradiction in organic matter content and carbon content may be due to the difference in chemical composition of the organic matter in peat and biochar. During pyrolysis, most of the organic materials present in the feedstock convert into chemically more aromatic forms (thus more C rich, while lower in H and O content compared to the feedstock). Thus, biochar could have higher carbon content and fixed carbon contents. Having higher organic matter content in a growing medium is essential for improving water holding capacity and nutrient retention (van der Wal and de Boer 2017).
Typical growing media exhibit pH in the range of 5.5–6.5, and exhibit EC less than of 1 mS/cm (Blok et al. 2008; Barrett et al. 2016). Addition of biochar to the medium increased the alkalinity of the medium and only P100 and BC25P75 fell within the optimum pH range for growing media. The alkalinity of biochar depends largely on the amount and composition of its ash, which is in turn is a function of the feedstock material and the extent of mass reduction during pyrolysis, particularly affected by temperature (Dumroese et al. 2011). The presence of excessive salts is liable to damage seed germination and early stage growth through an increase in osmotic pressure (Mumme et al. 2018). On the other hand, plant uptake of key nutrients is pH sensitive and liming agents are typically required to raise the pH of peat-based growing media to optimal levels. Alkaline biochar has a potential to mitigate the requirement for liming materials and mitigate the typical downward drift of pH that occurs in the growing media over time (Kern et al. 2017). In short, the balance in content of nutrient elements versus nutrient- and non-nutrient saline and/or alkaline elements will determine the benefits of including biochar in growing media. Optima may be hard to achieve without flexibility in feedstock and processing options (Margenot et al. 2018).
In previous work, Blok et al. (2017) advised that biochar with high nutrient content could result in salinity and alkalinity issues in growing media and feedstock with lower nutrient content should be preferred and used for pH adjustment in growing media containing acidic peat. Other studies have already reported the potential for biochar to increase the pH and EC of the medium (Steiner and Harttung 2014; Nieto et al. 2016). Biochar had significantly higher macro- and micronutrient content compared to peat (Table 6). The macro- and micronutrient content of the growing medium increased with biochar content. Proper plant growth depends on the correct balance of available plant nutrients in the growing medium to avoid deficiencies while avoiding toxicity or antagonistic effects. The ratio of macro- and micronutrients in biochar is entirely dependent on the ash composition of the biomass feedstock, their concentration and biochar alkalinity (relative to peat) affects their availability to plants (Blok et al. 2017; Antonangelo et al. 2019). The results in Table 6 show the initial nutrient content of the growing medium formulations, which is relevant to early stage plant growth. Such potentially positive effects on macro- and micronutrient content resulting from mixing biochar and peat were also previously reported by Gaskin et al., (2008).
Elemental analysis does not confirm increased nutrient availability, however, since this is affected by pH and the effects of physical properties on the availability of water in the medium. Although BC100 had the highest initial nutrient content of the tested media, a combination of sub-optimal EC, pH and PSD were liable to affect its potential benefits to plant growth as shown in previous studies (Nieto et al. 2016; Margenot et al. 2018). Since the elemental ratios in ash are fixed and the concentration of ash increases during pyrolysis, the possibility for toxicity to disturb or restrict metabolic functions must be considered (Hoover 2018). In this study, neither peat nor biochar displayed concentrations of potentially toxic elements (PTEs) in excess of those proposed by IBI or EBC (Budai et al. 2013; Schmidt et al. 2016). The biochar had higher concentrations of Cr and Zn compared to peat (Table 7).
3.4 Seed germination
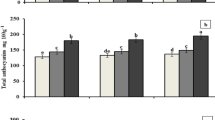
Results of the germination assay are shown in Fig. 1. Germination assays can be used to evaluate the phytotoxicity of the growing medium formulations. Cress, lettuce and tomato seeds are used in most of the past studies due to their higher growth response and sensitivity to phytotoxic substances. Both promotion and inhibition of seed germination after mixing with biochar were observed by other studies (Margenot et al. 2018; Mumme et al. 2018; Rathnayake et al. 2021). In our study, for lettuce, germination rate was 6% higher in BC75P25 and BC25P75 treatments compared to P100. BC50P50 and BC100 had the highest germination rate (19% and 13% increase, respectively) for lettuce compared to other treatments. For tomato, BC50P50 exhibited 17% higher germination rate compared to peat while B100 revealed 7% reduction of germination rate. However, cress, tomato and lettuce seeds did not show any significant difference in germination rate among the treatments. Higher volatile matter content, higher ash content, and higher alkalinity in biochar could adversely impact seed germination due to the salt stress and disruption of cell metabolic pathways (Torbaghan 2012; Pavel et al. 2013; Dalias et al. 2018; Intani et al. 2019). Furthermore, phytotoxicity of biochar could arise from volatile organic compounds associated with the biochar arising from post-handling, etc. (Buss and Mašek 2014). Finally, metal stress imposed by potentially toxic elements could contribute to the inhibition of seed germination which depends on the seed structure (seed coating, etc.) and plant species (Munzuroglu and Geckil 2002).
Shoot and root lengths of the germinated seeds were significantly different among plant species and treatments. For cress, BC50P50 and BC75P25 showed 19% and 10% increment in shoot length compared to P100. However, BC100 and BC25P75 showed 40% and 23% reduction in shoot length compared to P100. Lettuce shoot length was 7% lower for BC25P75 compared to P100. On the other hand, all the other treatments exhibited an increase of the shoot length in germinated lettuce seeds (71%, 58% and 67% in BC50P50, BC75P25 and BC100, respectively). For tomato, BC75P25 and BC50P50 treatments had highest shoot lengths in germinated seeds compared to P100 (240% and 258% increase in BC75P25 and BC50P50, respectively). Root length is a key indicator for the initial establishment phase of seedlings in growing media (Landis et al. 2009; Ferlito et al. 2020). Root lengths of the germinated seeds were significantly higher in BC50P50 treatment compared to P100. This was 69%, 121% and 146% increase in cress, lettuce and tomato seeds, respectively. This may be due to the combined effect of P content and physico-chemical properties in BC75P25 and BC50P50 treatments compared to P100 and B25P75 treatments. Even though, BC100 had the highest P content, the overall effect on root elongation could be negative from the poor physical properties in the medium due to the low porosity (Table 4). The germination index was also higher for BC100 than for P100 or any of the formulated mixes. There is a variation in germination index across the media, with greater shoot length in BC75P25 and BC50P50. Shoot length increased for lettuce seeds when biochar content exceeded 25%. Several studies have reported the positive effects of biochar on seed germination (Gravel et al. 2013; Hoover 2018). However, soluble phytotoxic compounds have previously been considered implicated in inhibited germination and root and shoot growth of germinated seeds (Buss and Mašek 2014).
3.5 Preliminary plant growth
Growth of tomato plants over 4 weeks was greater for the peat–biochar formulations than for P100, but P100 performed better than BC100 (Table 8). Number of leaves was 25% higher in BC25P75 and 25% lower in B100 compared to P100. In terms of shoot length, only BC25P75 and BC50P50 showed an increase of shoot length (19% and 25% in BC25P75 and BC50P50, respectively). Shoot length was 14% and 43% lower compared to P100 in BC75P25 and BC100, respectively. BC25P75, BC50P50 and BC75P25 showed 46%, 47% and 26% gain in root length compared to P100. Tomato can resist higher proportions of biochar in the medium due to its resistivity towards salinity (Dumroese et al. 2018). However, BC100 showed 33% lower root length compared to P100. This may be due to the poor physico-chemical properties in BC100 (Table 4). Shoot dry weight was increased by 44% in BC25P75 and BC50P50 compared to P100. On the other hand, BC75P25 and B100 showed 18% and 61% reduction in root dry weight compared to P100. Based on shoot and root lengths, BC50P50 and BC25P75 showed the best performance. This result was reflected in shoot and root dry weight which was higher for BC50P50 and BC25P75 compared to all other media formulations. This may be due to the favorable physical properties such as air-filled porosity and water holding capacities in BC25P75 and BC50P50 compared to other treatments.
The adverse effect of using only biochar has been previously reported (Nieto et al. 2016). The harvested seedlings were not chemically analyzed to assess nutrient uptake and the pH and EC of the media at the end of the assay. Based on the other analyses conducted (pH, EC, elemental composition and germination assay), it may be inferred that the nutrient status, pH and EC were positively affected by biochar in the formulated mixes. Although Table 4 highlights potential for negative effects arising from the physical/structural properties of biochar, these may not have been accentuated under the controlled conditions of the study. In formulations where biochar was in a high proportion of a growing medium, these negative effects may have become more apparent (Dumroese et al. 2011).
4 Conclusions
This study assessed the potential of VCZ biochar produced from sawmill residue to replace peat use in horticulture. The partial replacement of biochar in peat increased tomato plant growth compared to pure peat and pure biochar as growing media, as well as germination of tomato, lettuce, and cress seeds. At higher biochar contents in biochar–peat media, air-filled porosity and water holding capacity of the medium decreased though not beyond the optimum range for the growing media. The effects of biochar at contents of 25% and 50% by volume were positive in terms of macronutrients and not negative concerning pH or EC when compared to peat. These effects outweighed potentially adverse effects on physical properties, at least under the controlled moisture conditions used in this study. The foundation laid by this study can help in consecutive investigation of the use of VCZ biochar in horticultural growing media for optimizing plant growth under different agronomic conditions.
Data availability
All data generated or analyzed during this study are included in this article.
References
Alexander PD, Bragg NC, Meade R, et al (2008) Peat in horticulture and conservation: the UK response to a changing world. Mires Peat 3:
Altland JE, Locke JC, Altland JE, Locke JC (2017) High rates of gasified rice hull biochar affect geranium and tomato growth in a soilless substrate. J Plant Nutr 40:1816–1828. https://doi.org/10.1080/01904167.2016.1249800
Antonangelo JA, Zhang H, Sun X, Kumar A (2019) Physicochemical properties and morphology of biochars as affected by feedstock sources and pyrolysis temperatures. Biochar 1:325–336. https://doi.org/10.1007/s42773-019-00028-z
Arets EJMM, Van der Meer PJ, Verwer CC, et al (2011) Global Wood Production. Assessment of industrial round wood supply from forest management systems in different global regions. Alterra Report- Wageningen UR 1808:79
Barrett GE, Alexander PD, Robinson JS, Bragg NC (2016) Achieving environmentally sustainable growing media for soilless plant cultivation systems—a review. Sci Hortic (Amsterdam) 212:220–234. https://doi.org/10.1016/j.scienta.2016.09.030
Bhatta B, Chen D, Babu D et al (2016) Biomass and bioenergy an examination of physical and chemical properties of urban biochar for use as growing media substrate. Biomass Bioenerg 84:49–58. https://doi.org/10.1016/j.biombioe.2015.11.012
Blok C, De Kreij C, Baas R, Wever G (2008) Analytical methods used in soilless cultivation, 1st edn. Elsevier Ltd.
Blok C, Van der Salm C, Hofland-Zijlstra J, et al (2017) Biochar for horticultural rooting media improvement: evaluation of biochar from gasification and slow pyrolysis. Agronomy 7:. https://doi.org/https://doi.org/10.3390/agronomy7010006
Bos MG, Diemont WH, Verhagen A (2011) Sustainable peat supply chain : report of the ad hoc working group enhancing the sustainability of the peat supply chain for the Dutch horticulture. Alterra Report- Wageningen UR 2167:
Budai A, Zimmerman AR, Cowie AL, et al (2013) Biochar carbon stability test method : an assessment of methods to determine biochar carbon stability. Int Biochar Initiave 1–10
Buss W, Graham MC, Shepherd JG, Mašek O (2016) Risks and benefits of marginal biomass-derived biochars for plant growth. Sci Total Environ 569–570:496–506. https://doi.org/10.1016/j.scitotenv.2016.06.129
Buss W, Mašek O (2014) Mobile organic compounds in biochar e A potential source of contamination e Phytotoxic effects on cress seed (Lepidium sativum) germination. J Environ Manag 137:111–119. https://doi.org/10.1016/j.jenvman.2014.01.045
Carter S, Shackley S, Sohi S et al (2013) The impact of biochar application on soil properties and plant growth of pot grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 3:404–418. https://doi.org/10.3390/agronomy3020404
Clarke D, Rieley J (2010) Strategy for Responsible Peatland Management, 6th Editio. International Peatland Society
Crombie K, Mašek O, Sohi SP et al (2013) The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 5:122–131. https://doi.org/10.1111/gcbb.12030
Dalias P, Prasad M, Mumme J et al (2018) Journal of environmental chemical engineering low-cost post-treatments improve the e ffi cacy of hydrochar as peat replacement in growing media. J Environ Chem Eng 6:6647–6652. https://doi.org/10.1016/j.jece.2018.10.042
Defra (2009) A preliminary assessment of the greenhouse gases associated with growing media materials. 1–30. http://randd.defra.gov.uk/Document.aspx?Document=IF0154_9283_FRP.pdf
Dumroese RK, Heiskanen J, Englund K, Tervahauta A (2011) Pelleted biochar : chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenerg 35:2018–2027. https://doi.org/10.1016/j.biombioe.2011.01.053
Dumroese RK, Pinto JR, Heiskanen J, et al (2018) Biochar can be a suitable replacement for Sphagnum peat in nursery production of Pinus ponderosa seedlings. Forests 9:. https://doi.org/https://doi.org/10.3390/f9050232
Edeh IG, Mašek O, Buss W (2020) A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci Total Environ 714:. https://doi.org/https://doi.org/10.1016/j.scitotenv.2020.136857
Ferlito F, Torrisi B, Allegra M, et al (2020) Evaluation of conifer wood biochar as growing media component for citrus nursery. Appl Sci 10:. https://doi.org/https://doi.org/10.3390/app10051618
Forest Research (2019) Tree bark biochar: a green bullet for Scotland’s carbon store. https://www.forestresearch.gov.uk/news/tree-bark-biochar-a-green-bullet-for-scotlands-carbon-store/
Fornes F, Belda RM, De CF, Cebolla-cornejo J (2017) Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J Sci Food Agric 97:3675–3684. https://doi.org/10.1002/jsfa.8227
Gaskin J., Steiner C, Harris K, et al (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51:2061–2069. https://doi.org/https://doi.org/10.13031/2013.25409
Girkin NT, Vane CH, Cooper HV et al (2019) Spatial variability of organic matter properties determines methane fluxes in a tropical forested peatland. Biogeochemistry 142:231–245. https://doi.org/10.1007/s10533-018-0531-1
Gravel V, Dorais M, Ménard C (2013) Organic potted plants amended with biochar: Its effect on growth and Pythium colonization. Can J Plant Sci 93:1217–1227. https://doi.org/10.4141/CJPS2013-315
Hoover BK (2018) Herbaceous perennial seed germination and seedling growth in biochar-amended propagation substrates. HortScience 53:236–241. https://doi.org/10.21273/HORTSCI12624-17
Huang L, Gu M (2019) Effects of biochar on container substrate properties and growth of plants—a review. Horticulturae 5:1–25. https://doi.org/10.3390/horticulturae5010014
Intani K, Latif S, Islam S, Müller J (2019) Phytotoxicity of corncob biochar before and after heat treatment and washing. Sustainability 11:30. https://doi.org/https://doi.org/10.3390/su11010030
Kaudal BB, Aponte C, Brodie G (2018) Biochar from biosolids microwaved-pyrolysis : Characteristics and potential for use as growing media amendment. J Anal Appl Pyrolysis 130:181–189. https://doi.org/10.1016/j.jaap.2018.01.011
Kern J, Tammeorg P, Shanskiy M, et al (2017) Synergistic use of peat and charred material in growing media—an option to reduce the pressure on peatlands ? J Environ Eng Landsc Manag 6897:. https://doi.org/https://doi.org/10.3846/16486897.2017.1284665
Keskinen R, Hyväluoma J, Sohlo L et al (2019) Fertilizer and soil conditioner value of broiler manure biochars. Biochar 1:259–270. https://doi.org/10.1007/s42773-019-00020-7
Landis TD, Jacobs DF, Wilkinson KM, Luna T (2009) Growing media. In: Nursery manual for native plants: a guide for tribal nurseries. USDA, Forest Service, pp 100–121
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strateg Glob Chang 11:403–427. https://doi.org/10.1007/s11027-005-9006-5
Leng L, Xu X, Wei L et al (2019) Biochar stability assessment by incubation and modelling: methods, drawbacks and recommendations. Sci Total Environ 664:11–23. https://doi.org/10.1016/j.scitotenv.2019.01.298
Lévesque V, Rochette P, Ziadi N et al (2018) Mitigation of CO2, CH4 and N2O from a fertigated horticultural growing medium amended with biochars and a compost. Appl Soil Ecol 126:129–139. https://doi.org/10.1016/j.apsoil.2018.02.021
Margenot AJ, Griffin DE, Alves BSQ et al (2018) Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind Crops Prod 112:160–169. https://doi.org/10.1016/j.indcrop.2017.10.053
Mašek O, Buss W, Roy-Poirier A et al (2018) Consistency of biochar properties over time and production scales: a characterisation of standard materials. J Anal Appl Pyrolysis 132:200–210. https://doi.org/10.1016/j.jaap.2018.02.020
Méndez A, Paz-ferreiro J, Gil E, Gascó G (2015) The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci Hortic (Amsterdam) 193:225–230. https://doi.org/10.1016/j.scienta.2015.07.032
Mumme J, Getz J, Prasad M et al (2018) Toxicity screening of biochar-mineral composites using germination tests. Chemosphere 207:91–100. https://doi.org/10.1016/j.chemosphere.2018.05.042
Munzuroglu O, Geckil H (2002) Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus Cucumis sativus. Arch Environ Contam Toxicol 213:203–213. https://doi.org/10.1007/s00244-002-1116-4
Nemati MR, Simard F, Fortin J-P, Beaudoin J (2015) Potential use of biochar in growing media. Vadose Zo J 14:1–8. https://doi.org/10.2136/vzj2014.06.0074
Nieto A, Gascó G, Paz-ferreiro J et al (2016) The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci Hortic (Amsterdam) 199:142–148. https://doi.org/10.1016/j.scienta.2015.12.012
Page SE, Rieley JO, Banks CJ (2011) Global and regional importance of the tropical peatland carbon pool. Glob Chang Biol 17:798–818. https://doi.org/10.1111/j.1365-2486.2010.02279.x
Pavel VL, Sobariu DL, Diaconu M, et al (2013) Effects of heavy metals on Lepidium sativum germination and growth. Environ Eng Manag 12:. Doi: https://doi.org/10.30638/eemj.2013.089
Prasad M, Chrysargyris A, McDaniel N et al (2020) Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 10:1–17. https://doi.org/10.3390/agronomy10010010
Rathnayake D, Ehidiamhen P, Egene C et al (2021) Investigation of biomass and agricultural plastic co-pyrolysis: effect on biochar yield and properties. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2021.105029
Rathnayake D, Maziarka P, Ghysels S et al (2020a) How to trace back an unknown production temperature of biochar from chemical characterization methods in a feedstock independent way. J Anal Appl Pyrolysis. https://doi.org/10.1016/j.jaap.2020.104926
Rathnayake D, Rego F, Poucke R Van, et al (2021) Chemical stabilization of Cd contaminated soil using fresh and aged wheat straw biochar. Environ Sci Pollut Res. 28:10155–10166. https://doi.org/10.1007/s11356-020-11574-6
Reza MS, Afroze S, Bakar MSA et al (2020) Biochar characterization of invasive Pennisetum purpureum grass: effect of pyrolysis temperature. Biochar 2:239–251. https://doi.org/10.1007/s42773-020-00048-0
Robertson RA (1993) Peat, horticulture and environment. Biodivers Conserv 547:541–542
Rogovska N, Laird D, Cruse RM et al (2012) Germination tests for assessing biochar quality. J Environ Qual 41:1014–1022. https://doi.org/10.2134/jeq2011.0103
Ronsse F, Van Hecke S, Dickinson D, Prins W (2013) Production and characterization of slow pyrolysis biochar : influence of feedstock type and pyrolysis conditions. Gcb Bioenergy 5:104–115. https://doi.org/10.1111/gcbb.12018
Schmidt HP, Bucheli T, Kammann C, et al (2016) European biochar certificate-guidelines for a sustainable production of biochar
Schmilewski G (2008) The role of peat in assuring the quality of growing media. Mires Peat 3: http://www.mires-and-peat.net/pages/volumes/map03/map0302.php
Shackley S, Sohi S, Brownsort P et al (2010) An assessment of the benefits and issues associated with the application of biochar to soil. A report commissioned by the United Kingdom Department for Environment, Food and Rural Affairs, and Department of Energy and Climate Change. pp 132
Singh B, Camps-Arbestain M, Lehmann J (2017) Biochar: a guide to analytical methods. CSIRO Publishing
Sohi S, Gaunt J, Atwood J (2013) Biochar in growing media: a sustainability and feasibility assessment. A project commissioned for the Sustainable Growing Media Task Force. Defra project SP1213. Defra, London. pp 84
Steiner C, Harttung T (2014) Biochar as a growing media additive and peat substitute. Solid Earth 5:995–999. https://doi.org/10.5194/se-5-995-2014
Tian Y, Sun X, Li S et al (2012) Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata Sci Hortic (Amsterdam) 143:15–18. https://doi.org/10.1016/j.scienta.2012.05.018
Torbaghan ME (2012) Effect of salt stress on germination and some growth parameters of marigold (Calendula officinalis L.). Plant Sci J 1:7–19
van der Wal A, de Boer W (2017) Dinner in the dark: Illuminating drivers of soil organic matter decomposition. Soil Biol Biochem 105:45–48. https://doi.org/10.1016/j.soilbio.2016.11.006
Vassilev SV, Baxter D, Andersen LK, Vassileva CG (2010) An overview of the chemical composition of biomass. Fuel 89:913–933. https://doi.org/10.1016/J.FUEL.2009.10.022
Vaughn SF, Kenar JA, Thompson AR, Peterson SC (2013) Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind Crop Prod 51:437–443. https://doi.org/10.1016/j.indcrop.2013.10.010
Wallach R (2008) Physical characteristics of soilless media. In: Soilless Culture: Theory and Practice, 1st edn. Elsevier Ltd., pp 41–116
Webber CL, White PM, Gu M, et al (2018a) Sugarcane and Pine Biochar as Amendments for Greenhouse Growing Media for the Production of Bean (Phaseolus vulgaris L.) Seedlings. J Agric Sci 10:58. https://doi.org/https://doi.org/10.5539/jas.v10n4p58
Webber CL, White PM, Spaunhorst DJ et al (2018) Sugarcane biochar as an amendment for greenhouse growing media for the production of cucurbit seedlings. J Agric Sci 10:104. https://doi.org/10.5539/jas.v10n2p104
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Wu P, Wang Z, Wang H et al (2020) Visualizing the emerging trends of biochar research and applications in 2019: a scientometric analysis and review. Biochar 2:135–150. https://doi.org/10.1007/s42773-020-00055-1
Acknowledgments
The authors gratefully thank all reviewers for their comments and suggestions in improving the manuscript. Also, the authors would like to thank BSW ltd. for providing the sawmill co-products used in this study.
Funding
The study reported here received financial support from the European Union Horizon 2020 Research and Innovative Training Network program under the Marie Sklodowska-Curie grant agreement No 721991. In the UK, H. Creber received financial support from the E4 Doctoral Training Partnership of the Natural Environment Research Council and from Forest Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Rathnayake, D., Creber, H., Van Poucke, R. et al. Biochar from sawmill residues: characterization and evaluation for its potential use in the horticultural growing media. Biochar 3, 201–212 (2021). https://doi.org/10.1007/s42773-021-00092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-021-00092-4