Abstract
Low-temperature stress can seriously impair plant physiology. Chilling injury leads to a complex array of cellular dysfunctions, and symptoms include chlorosis, sterility, loss of vigor, wilting, and even death of the plants. Furthermore, phosphorus limitations additionally halt the growth of plants. Low-temperature adaptive plant growth–promoting microbes through various direct and indirect mechanisms help in the survival of plants under stress conditions. The present investigation deals with isolation of P-solubilizing psychrotrophic bacteria from diverse cultivars of wheat grown in the Keylong region of Himachal Pradesh. A total of 33 P-solubilizing bacterial isolates were obtained. P-solubilizers were screened for different plant growth–promoting (PGP) attributes of K and Zn solubilization, production of IAA, siderophores, and different hydrolytic enzymes. Among 33 P-solubilizers, 8 efficient strains exhibiting multiple PGP attributes were used as bioinoculants for wheat under low-temperature stress in different in vitro and in vivo experiments. The psychrotrophic bacterial isolates positively influenced the growth and physiological parameters as well as nutrient uptake and yield of wheat and efficiently alleviated low-temperature stress. The potential of low-temperature stress adaptive and PGP microbes can be utilized in agricultural sector for amelioration of low-temperature stress and plant growth promotion. The present study deals with the isolation of psychrotrophic P-solubilizers with multiple PGP attributes and their role in alleviation of cold stress in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extreme environments including drought, salinity, and high and low temperature greatly affect the productivity of several crop plants of commercial importance [1]. Low temperature is among the major factor limiting the geographical distribution of many species and agricultural productivity [2]. The effect of low-temperature stress on plants depends on the degree of severity and the extent of exposure. Injuries are known to occur through chilling at temperatures between 15 and 0°C [3]. The exposure of the crops to low temperatures disrupts cellular homeostasis, leads to the production of reactive oxygen species (ROS), and damages carbohydrates, DNA, lipids, and proteins eventually leading to cell death [4]. Plants possess tolerance mechanisms to cope with such non-freezing temperatures by a phenomenon referred to as cold acclimation [5]. The primary mechanisms involved in cold acclimation include the accumulation of cytosolic Ca2+, alterations in the expression of cold-related genes, activation of ROS scavenger systems, changes in protein and sugar synthesis, and accumulation of osmolytes. But, many a time, plants need the support of the microbiome they harbor for reducing their burden to combat low-temperature stress. In fact, the association of plants with microbes plays a key role in the existence of both partners in stress conditions.
Another major problem faced by the plants is the unavailability of phosphorus in sufficient amounts for their uptake. Phosphorus is among the essential macronutrients for plants and thus supplied in the form of phosphatic fertilizers. However, a large portion of soluble Pi supplied in chemical form is immobilized rapidly and also becomes unavailable to plants. P-solubilizing microbes are efficient in releasing P from inorganic and organic pools of total soil P through solubilization and mineralization) [6]. A range of P-solubilizing microbes have been reported from wheat [7,8,9]. Psychrotrophic P-solubilizers have been also reported [10, 11].
Numerous tools of biotechnology have been also broadly applied for crop improvement under abiotic stress conditions and nutrient limitations of which the role of plant growth–promoting microbes (PGPMs) in the alleviation of stress has become of paramount importance [12]. Diverse plant growth–promoting microbial genera including Achromobacter, Acinetobacter, Arthrobacter, Bacillus, Brevundimonas, Enterobacter, Exiguobacterium, Flavobacterium, Jeotgalicoccus, Klebsiella, Kluyvera, Kocuria, Leclercia, Lysinibacillus, Methylobacterium, Mrakia, Naganishia, Paenbacillus, Pantoea, Planococcus, Pontibacillus, Providencia, Pseudomonas, Rhodotorula, Sporosarcina, Staphylococcus, Stenotrophomonas, and Virgibacillus have been reported from low-temperature environments [1, 9, 13].
PGPMs positively influence plants under stress conditions and promote their growth by diverse mechanisms. These mechanisms include the production of different plant growth regulators, siderophores, and solubilization of insoluble and unavailable various macro- and micronutrients. Additionally, there is a production of ACC deaminase enzyme which lowers the levels of ethylene and accumulation of osmolytes for water uptake and ROS scavenging enzymes [14]. The utilization of PGPMs as bioinoculants to mitigate the negative effects of low temperatures is an innovative and cost-effective strategy and has drawn the attention of the scientific community throughout the world [15]. These beneficial PGPMs if judiciously formulated and allowed to colonize the target crops will surely work for the well-being of the crops and enhance the yield under all conditions [16].
Wheat is the major cereal crop of India and its products play a progressively more significant role in managing India’s food economy. It is the staple food of millions of Indians, particularly in the northern and northwestern parts of the country. It is nutrient-rich and provides balanced food. India is the second largest producer as well as the consumer of wheat [17]. The major producers of wheat are Bihar, Gujarat, Haryana, MP, Punjab, Rajasthan, and Uttar Pradesh. In HP, wheat is cultivated as both rabi and kharif crops. Sirmaur district is among the major producers of wheat in Himachal Pradesh. The total area under the crop is about 29.8 million hectares in the country. Wheat requires an ideal winter temperature of 10 to 15°C. Longer durations of very low temperatures can cause damage to wheat which is most sensitive during the reproductive period. Factors such as temperature reached and the duration of the cold stress are directly related to the amount of damage inflicted. The selection and successful application of low-temperature adaptive microbes with P-solubilizing capability will encourage the development of climate-specific bioinoculants. Cold-tolerant P-solubilizers also exhibit the possibility of being an alternative to phosphatic fertilizers needed to increase agricultural productivity, especially for the regions disturbed by low temperatures during the winter season.
Materials and methods
Sample collection
Keylong, 32.58°N, 77.03°E, in Great Himalayas located 120 km from Indo-Tibetan border at an elevation of 10,100ft was chosen for sample collection. Seven different wheat varieties (HS240, UP2338, HahnWR, PBW 343YR, Pavon 1-96-1, HD2967, and PBW 343-Lr24-GPC-B1) with their rhizospheric soil were collected from the Keylong region in sterile polyethene bags and stored in ice packed boxes and brought to Baru Sahib for further analysis.
Area of study and isolation of rhizospheric microbes
Baru Sahib, 30.7537° N, 77.2965° E, Valley of Divine Peace, located in a remote corner of Sirmaur district, Himachal Pradesh, India, was chosen as the area of study. The culturable microbes were isolated from the wheat rhizospheric region by standard serial dilution plating technique using different growth media [18]. The pure cultures were maintained on slants of nutrient agar and glycerol stock at 4°C and −80°C respectively.
Screening of rhizospheric microbes for P-solubilization attributes and other plant growth–promoting attributes
The isolates were screened qualitatively for P-solubilization on Pikovskaya agar supplemented with three different insoluble forms of phosphorus (rock phosphate (RP), apatite (AP), and tricalcium phosphate (TCP)) [19]. The microbial isolates solubilizing all three insoluble sources of phosphorus were further screened for multiple PGP attributes. Potassium solubilization was done by the method described by Hu et al. [20]; zinc solubilizing ability was carried by the method by Saravanan et al. [21]. IAA production was conducted according to the method of Bric et al. [22]. The siderophore production was analyzed on chrome-azurol-S (CAS) agar medium by Schwyn and Neilands [23]. The production of ammonia (NH3) was examined in peptone water as described by Cappucino [24]. The microbial isolates were also screened for enzyme production such as amylases by the method of Castro et al. [25], cellulase activity by Zhou et al. [26], and pectinases and proteases by Kanekar et al. [27] respectively.
On the basis of P-solubilization and other attributes of plant growth promotion, the selected isolates were quantified for phosphorus on tricalcium phosphate by the method of Murphy and Riley [28]. The amount of phosphorus solubilized was expressed in mg/L.
Identification of microbial isolates
Bacterial genomic DNA was extracted by the method described earlier [8] with minor modifications in the protocol. DNA samples were subjected to PCR amplification of conserved gene (16S rRNA) gene and carried out as described by Yadav et al. [1] followed by purification by Quiaquick purification kit (USA).
Accession numbers
The selected P-solubilizers were identified and partial 16S rDNA sequences were submitted to NCBI GenBank and accession numbers were assigned.
Evaluation of plant growth–promoting ability under in vitro and in vivo conditions
Seed germination plate assay
The seed germination bioassay was done on wheat. The selected psychrotrophic P-solubilizing strains were tested for their capability to inhibit/promote seedling growth using the method described by Elliott and Lynch [29]. The selected strains were grown for 48h in their respective growth medium containing at least 107 cells mL−1. The seeds were surface-sterilized with 0.1% HgCl2 followed by consecutive washing with sterile distilled water. The seeds of wheat were kept for 10min in the culture medium. Sterilized soft agar was poured into the sterilized plates and 10 seeds were placed in each soft agar plate along with control plates which contained seeds treated with a respective sterilized medium. After 3 days, the root radical length and shoot length were recorded.
Plant growth promotion under in vitro conditions
The evaluation of the PGP ability of selected psychrotrophic P-solubilizers on wheat was determined under greenhouse conditions. The experiment was carried out in the month of January when the temperature was observed to be 20°C during daytime and less than 5°C during nighttime. The efficient strains were grown in nutrient broth at 10°C. Seeds of wheat were dipped in inoculums for 1h. The seeds were surface-sterilized before putting in inoculums. The seeds were kept in a 48-h grown culture containing at least 107 cells mL−1 for 1h prior to sowing. Six seeds were sown in each pot and autoclaved water was given according to need. After the emergence of the first leaf, plant density was reduced to 4 plants per pot.
Plant growth promotion under in vivo conditions
The evaluation of the PGP ability of selected psychrotrophic P-solubilizers on wheat was determined under field conditions. The experiment was carried out in two plots in the month of January when the temperature was observed to be 20°C during daytime and less than 5°C during nighttime. Both plots consisted of 12 blocks each of 1.5m2 area. All blocks consisted of six rows with each row consisting of 15 plants at a distance of about 25 cm. A complete randomized block design was used for the field experiment. The experiment was done in triplicates. The selected psychrotrophic P-solubilizing bacterial strains were grown in a growth medium for 48h under shaking conditions (107 cells mL−1). Wheat seeds were surface-sterilized and coated with inoculums and sugar solution (1:1 ratio) before sowing. The control seeds were coated with sterilized growth media and sugar solution. The inoculums were sprayed on the wheat plants of plot I after 30 days of sowing and plot II was left unsprayed until harvesting.
Determination of plant growth, physiological parameters, nutrient uptake, and yield
Growth parameters
Different growth parameters including shoot and root length and fresh and dry weight were studied in each experiment.
Physiological parameters
The proline content of the leaves was determined according to the method described by Bates et al. [30]. Glycine betaine (GB) estimation was done according to Grieve and Grattan [31] using dried leaf powder. Total soluble sugars (TSS) were determined by the method of Irigoyen et al. [32]. The level of lipid peroxidation was done according to Heath and Packer [33]. Superoxide dismutase (SOD) activity was determined by Dhindsa et al. [34] and glutathione reductase (GR) activity was assayed by Smith et al. [35].
Nutrient uptake in plant and yield
For the analysis of iron, zinc, and phosphorus, the grains were collected after harvesting and washed in deionized water and dried at 80°C in a hot air oven for 5h. After drying, 0.5g of seed samples was weighed and digested with 5mL concentrated nitric acid and 2mL H2O2 in a microwave digester (Anton Paar, GmbH, Austria) at a set temperature and pressure until a clear solution is obtained. For iron and zinc analysis, the digested samples were then transferred to the 50-mL graduated Falcon tubes, and the final volume was made to 25 mL with deionized water for further analysis by atomic absorption spectrophotometer (AA240FS, Agilent Technology, CA, USA) [36]. The P content after digestion was analyzed by the ammonium molybdate method [28]. The nutrient concentrations were expressed in mg/kg of dry weight. The yield of wheat was expressed in q ha−1.
Statistical analysis
The various data obtained in the study were subjected to statistical analysis using Student’s t-test. Mean comparisons were conducted using the least significant difference (LSD) test (P¼ 0.05) and critical difference (CD5% and CD1%). The standard error (SE) and LSD results were calculated.
Results
Isolation of rhizospheric microbes
The population of heterotrophic rhizospheric microbes was enumerated from different varieties of wheat cultivated in Keylong, Himachal Pradesh. Nutrient agar supported the highest population of bacteria whereas the least microbial population was supported by ammonium mineral salt agar. A total of 100 bacterial morphotypes were finally selected for further screening.
Screening of rhizospheric microbes for P-solubilization attributes
Among 100 isolates, 33 solubilized phosphorus. Among 33 P-solubilizers, 8 isolates solubilized all three insoluble sources of phosphorus. Among selected 8 isolates, strain EU-KL44 exhibited multiple plant growth–promoting attributes including the solubilization of minerals (K and Zn), production of Fe chelating compounds, IAA, and different hydrolytic enzymes (Table 1).
The amount of P-solubilized was observed in the range of 80.3±0.009 to 572.0±0.001mg L−1. The highest amount of phosphorus was solubilized by EU-KL44 and the lowest by the strain EU-KL59 (Table 1).
Identification of microbial isolates
16S rRNA sequence analysis of the strain EU-KL44 showed 99.02% similarity to Acinetobacter rhizosphaerae in the existing database of the National Center of Bioinformatics. The partial 16S rRNA gene sequence was submitted to NCBI GenBank and the assigned accession number MN733449. The strain EU-KL44 was deposited to the culture collection facility of NBAIM, Mau, Uttar Pradesh, India.
Evaluation of plant growth–promoting ability under in vitro and in vivo conditions
Seed germination plate assay
Eight psychrotrophic P-solubilizing strains were evaluated for their capability to inhibit/promote seedling growth. All the isolates positively influenced the root radical length and shoot length. Strain EU-KL44 showed the highest increment in the shoot and the root length followed by strain EU-KL78 as compared to untreated control (Table 2).
Plant growth promotion under in vitro conditions
On the basis of seed germination plate assay, three efficient strains of Acinetobacter rhizosphaerae EU-KL44, EU-KL78, and EU-KL86 were used as inoculants for wheat under greenhouse conditions. All the strains showed variations in influencing the growth of the wheat. The strains efficiently improved both the physiological and growth parameters under low-temperature conditions.
Determination of plant growth and physiological parameters
Growth parameters
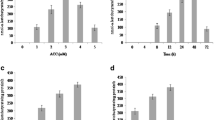
Acinetobacter rhizosphaerae EU-KL44 showed the highest increment in the shoot length and root length whereas the strain EU-KL78 showed the highest increment in the fresh weight and the highest increment in dry weight was observed in EU-KL86-treated seeds as compared to the untreated control under low-temperature conditions (Fig. 1a).
a Effect of inoculation of different strains of psychrotrophic P-solubilizing bacteria on growth parameters of wheat under cold stress conditions [biomass (gm); length (cm)]. b Effect of inoculation of different strains of psychrotrophic P-solubilizing bacteria on proline, glycine betaine, sugar content, lipid peroxidation, and enzymatic activities of wheat under cold stress conditions
Physiological parameters
All the treatments showed differences in their activities in influencing the physiological parameters of the wheat in low-temperature conditions. The highest content of proline was observed in Acinetobacter rhizosphaerae EU-KL44 (0.035 μmol g−1 of fresh weight leaf)-treated plants followed by strain EU-KL78 (0.031 μmol/g−1 of fresh weight leaf)-treated plants when compared to uninoculated control plants which showed 0.023 μmol/g−1 of proline content. The lowest content of TBARS was observed in Acinetobacter rhizosphaerae EU-KL44 (3.2 nmol g−1 of fresh weight leaf)-treated plants as compared to the control plants which showed TBARS content of about 4.0 nmol g−1. EU-KL86-treated plants showed the highest content of total soluble sugars (0.053 mg g−1 of fresh weight leaf) followed by Acinetobacter rhizosphaerae EU-KL44-treated plants (0.048 mg g−1 of fresh weight leaf) as compared to untreated control plants. The highest content of glycine betaine was observed in EU-KL86-treated plants followed by Acinetobacter rhizosphaerae EU-KL44-treated plants. The highest activity of SOD was observed in Acinetobacter rhizosphaerae EU-KL44 (97 units mL−1) treatment whereas control plants showed 80 units mL−1 of SOD activity. GR activity was also enhanced in bacterial-treated wheat plants. The highest activity was observed in Acinetobacter rhizosphaerae EU-KL44 and strain EU-KL78 treatments as compared to untreated control (Table 3, Fig. 1b).
Plant growth promotion under in vivo conditions
On the basis of plant growth promotion under in vitro conditions, Acinetobacter rhizosphaerae EU-KL44 was selected for in vivo conditions under low-temperature conditions. The strain positively influenced the growth and physiological parameters of wheat under low temperatures. The differences in each studied parameter were observed in plants sprayed with inoculums after 30 days and plants left unsprayed till harvesting.
Determination of plant growth, physiological parameters, nutrient uptake, and yield
Growth parameters
The sprayed and unsprayed plants did not show much variation in their growth parameters but the increment of growth parameters in both cases was higher than in untreated control plants. In comparison, plants left unsprayed showed slightly higher shoot and root length and fresh and dry weight (Fig. 2a).
a Effect of inoculation of Acinetobacter rhizosphaerae EU-KL44 on growth parameters of wheat under cold stress conditions [biomass (gm); length (cm)]. b Effect of inoculation of Acinetobacter rhizosphaerae EU-KL44 on proline, glycine betaine, sugar content, lipid peroxidation, and enzymatic activities as well as nutrient uptake of wheat under cold stress conditions. c Effect of inoculation of Acinetobacter rhizosphaerae EU-KL44 on yield of wheat under cold stress conditions
Physiological parameters
The proline content was higher in wheat plants sprayed with inoculum of Acinetobacter rhizosphaerae EU-KL44 (0.046 μmol g−1 of fresh weight leaf) as compared to untreated control plants (0.017 μmol g−1 of fresh weight leaf) whereas unsprayed plants showed the 0.026 μmol g−1 of proline content in their leaves.
TBARS content was observed lowest in wheat plants sprayed with inoculum of Acinetobacter rhizosphaerae EU-KL44. The wheat plants sprayed with inoculum of Acinetobacter rhizosphaerae EU-KL44 showed 0.064 mg g−1 of total soluble sugars whereas total soluble sugar content in unsprayed plants was observed to be 0.058 mg g−1 of fresh weight of leaf sample. The untreated controls of both sprayed and unsprayed plants could increase the sugar content up to 0.048 mg g−1 and 0.045 mg g−1 respectively under cold-temperature conditions. Acinetobacter rhizosphaerae EU-KL44 increased the glycine content in both sprayed and unsprayed samples though the content was observed to be higher in samples sprayed with inoculum. The glycine content was observed to be 12.9 μg g−1 in sprayed samples whereas unsprayed showed glycine content of 9.2 μg g−1. The activity of SOD did not show much variation in both sprayed and unsprayed wheat samples and reached up to 117 units mL−1 and 118 units mL−1 in sprayed and unsprayed samples respectively. Acinetobacter rhizosphaerae EU-KL44 efficiently increased the GR activity in both sprayed and unsprayed wheat plants as compared to control plants under cold stress (Table 4, Fig. 2b).
Nutrient uptake in plant and yield
The inoculation with Acinetobacter rhizosphaerae EU-KL44 efficiently improved the nutrient content and increased the yield of wheat under cold temperature conditions. The content of phosphorus in the wheat plants sprayed with inoculum after 30 days reached up to 44.90 mg kg−1 and in the case of unsprayed samples the content was 37.0 mg kg−1. The P content in untreated control plants reached up to 30 mg kg−1 and 34 mg kg−1 in sprayed and unsprayed samples respectively. Fe content in wheat samples in sprayed and unsprayed samples was observed to be 35 mg kg−1 and 32 mg kg−1 respectively. Zn content was 39.7 mg kg−1 and 32.7 mg kg−1 in sprayed and unsprayed samples respectively (Fig. 2b).
A 34–35% increase in yield was observed in Acinetobacter rhizosphaerae EU-KL44-treated wheat plants (Fig. 2c) (Table 5).
Discussion
Plants are frequently exposed to a multiplicity of environmental stresses. Low temperature constitutes the chief factor in influencing the growth, development, and productivity of plants. It is a well-known fact that about two-thirds of the world’s cumulative terrestrial area is annually affected by below-freezing point temperatures and a three-fourth portion of the Earth’s biosphere’s temperature is below 5°C [37]. There is an estimation that 50% of the loss in global productivity of agricultural crops is due to abiotic stresses. Chilling leads to disturbances in enzymatic activity, impairment of photosynthesis, membrane rigidification, accumulation of ROS, and leakage across membranes. Confronted to fluctuations in temperatures, plants have evolved mechanisms to readjust their biochemical makeup to adapt and survive [38]. The microbiomes of diverse cold habitats play an indispensable role in the growth and adaptation of plants to low temperatures. These microbiomes are naturally gifted with different strategies to allow higher tolerance and in addition promote plant growth under abiotic stress conditions of low temperature. The identification of psychrotrophic microbes with the capability to support plant growth at low temperatures is a worldwide trend in the field of agricultural inoculation technology [39]. The present study deals with the isolation of psychrotrophic P-solubilizing bacteria from wheat grown in the Keylong region of the Great Himalayas, their PGP attributes, and their role in the alleviation of cold stress in wheat.
In the present investigation, 100 psychrotrophic bacteria were isolated from different wheat cultivars grown in Keylong. In high-altitude agriculture, the psychrotrophic microbes are of vast significance due to their survival and adaptation to cold conditions. A huge diversity of psychrotrophic bacteria has been explored including Acinetobacter sp., Aeromonas sp., Alcaligens sp., Flavobacterium sp., Micrococcus sp., Moraxella sp., Pseudomonas sp., Vibrio sp., and Xanthomonas sp. from Antarctic marine waters [40], Janthinobacterium lividum from Alaskan soil [41], Arthrobacter sp., Bacillus sp., E. coli, Micrococcus sp., Paenibacillus sp., Pseudomonas sp., and Staphylococcus sp. from the soil of Jammu region [42]. Przemieniecki et al. [43] reported psychrotrophic Arthrobacter sp. ad Pseudomonas sp. from root zone of winter wheat. Verma et al. [9] explored the diversity of psychrotrophic bacteria associated with wheat and total morphotypes which belonged to genera Achromobacter, Acinetobacter, Arthrobacter, Bacillus, Brevundimonas, Enterobacter, Exiguobacterium, Flavobacterium, Klebsiella, Kocuria, Kluyvera, Leclercia, Methylobacterium, Pantoea, Planococcus, Providencia, Pseudomonas, Stenotrophomonas, and Staphylococcus.
Among 100 isolates, 33 were efficient in solubilizing different insoluble forms of phosphorus. Many Indian soils have a low content of organic phosphorus and thus their bioavailability is also low. On average, the phosphorus content in soil is about 0.05%, from which 0.1% is accessible to plants. The poor mobility and low concentration of plant-available phosphorus in soil demand the application of chemical phosphatic fertilizers. The application of chemical fertilizers to agricultural land though has improved soil fertility and increased crop yield but simultaneously disturbed the P cycling event [44] and also has led to other adverse effects on the environment. P-solubilizing microbes play an amazing role in the nutrition of plants by increasing the phosphate uptake by plants and reducing the demands of chemical phosphatic fertilizers.
P-solubilizers in addition to increasing the bioavailability of P also stimulate the growth of the plants by solubilizing many other unavailable macro- and micronutrients, producing plant growth regulators and various hydrolytic enzymes. In the present study, 8 efficient P-solubilizing psychrotrophic bacteria also possessed multiple PGP activities such as solubilization of K and Zn, production of siderophores, IAA, ammonia, and various hydrolytic enzymes. Verma et al. [9] reported P-solubilizing psychrotolerant bacteria with multifarious PGP attributes allied with wheat from the northern hills zone of India. Yadav et al. [1] reported psychrotrophic Bacilli with P-solubilizing ability and other PGP traits from the cold desert of the northwestern Indian Himalayas. P-solubilizers are thus efficient bioinoculants for agricultural crops to increase yield and reduce the use of chemical fertilizers.
P-solubilizers are also known to be good stress alleviators for which P-solubilizers possess specific mechanisms to support the growth and adaptation of the plants. Cold stress disturbs the natural soil nutrient cycling and reduces soil fertility [45]. Exposure to cold stress interrupts cellular homeostasis in plants and ROS including hydrogen peroxide and singlet oxygen are among the major products of stress-induced cellular changes [46]. ROS damage biomacromolecules ultimately leading to total cell death in plants [47]. An effectual solution for protecting the plants from cold stress and enhancing their growth includes the application of cold-adapted and cold-loving PGP microbes as bioinoculants which are capable of tolerating the low temperature. PGP microbes use a range of mechanisms such as increasing the accumulation of the proline, glycine betaine, and sugars, activities of the ROS scavenging enzymes, and decreasing lipid peroxidation to improve the adaptability and growth of plants under stress conditions.
The accumulation of compatible solutes protects plants from stress through diverse mechanisms including cellular osmotic adjustment, protection of membrane integrity, detoxification of ROS, and protection and stabilization of proteins [48]. Proline is a water-soluble amino acid that accumulates in plants under different conditions of abiotic stress. It has been well established that the accumulation of proline within plants under stress conditions plays a noteworthy role in developing stress tolerance capacity simultaneously acting as an osmoregulatory molecule [49]. Proline is also known to be a way to store carbon and nitrogen. It is also known to play an additional role of molecular chaperone stabilizing the structure of proteins [50]. Proline accumulation is more significant in photosynthetic tissues though it also takes place in roots [51]. Glycine betaine is another important cryoprotective which protects the enzyme and protein activities and stabilizes membranes. It is also known to play a very important role in the adjustment and protection of the thylakoid membrane thus maintaining photosynthetic efficiency [48]. The tolerance to cold stress has been associated with the accumulation of glycine betaine in numerous plant species [52]. Sugars serve as important osmoprotectants and also protect the cellular membranes through interacting with the lipid bilayer from damage caused due to freezing [53]. In the present study, the accumulation of all three important compatible solutes viz. proline and glycine betaine was observed in wheat exposed to cold stress and inoculated with efficient psychrotrophic bacteria. In the study of Mishra et al. [2], the alleviation of cold stress in wheat inoculated with psychrotolerant pseudomonads has been reported. The study showed an increase in proline content in wheat along with other metabolites. The study of Subramanian et al. [4] showed increased tolerance capacity of tomato plants with inoculation of psychrotolerant bacteria and exposure to cold stress. The tomato plants showed a reduction in membrane damage, antioxidant enzyme activation, and proline accumulation. Thus, the increased concentrations of these three osmoprotectants may have protected the wheat plants from cold stress.
In the present study, the activities of ROS scavengers including SOD and GR and less lipid peroxidation were also observed in inoculated wheat. The abiotic stress is associated with the generation of ROS which are deleterious chemical entities with the capability to induce cellular damage by degrading the proteins and inactivating the enzymes. ROS also interfere in various pathways which are of metabolic importance. Loss of crop productivity under abiotic stress has been related to high ROS production [54]. Cold stress also enhances the production of peroxidized membranes. SOD and GR play a critical role in the detoxification of ROS and limit oxidative stress in plants. SOD provides protection against the toxic effects of oxidative stress by acting as a scavenger of superoxide radicals [55]. The involvement of antioxidative enzymes in protecting plants from stress conditions has been documented through various studies [56]. GR also plays an essential role in cell defense against reactive oxygen metabolites and confers abiotic stress tolerance in plants [57]. GR efficiently maintains the intracellular glutathione pool in the reduced state and functions as an antioxidant and facilitates ROS scavenging [58]. The study of Tiryaki et al. [59] reported less lipid peroxidation and stimulated activities of SOD as well as GR in beans inoculated with psychrotolerant bacteria.
The psychrotrophic P-solubilizing strain EU-KL44 identified as Acinetobacter rhizosphaerae by 16S rRNA gene sequencing efficiently alleviated the negative effects of cold stress by diverse mechanisms in wheat plants and promoted their growth and enhanced yield. Acinetobacter rhizosphaerae has been reported as a P-solubilizer from cold deserts of the Himalayas by Gulati et al. [60]. But much work has not been done on inoculation of Acinetobacter rhizosphaerae and mitigation of cold temperature stress in crops. Probably to the best of our knowledge, this is the first report on the utilization of psychrotrophic P-solubilizing Acinetobacter rhizosphaerae as a bioinoculant for the alleviation of cold stress in wheat. Thus, psychrotrophic P-solubilizing microbes exhibiting PGP attributes can be isolated from regions of low temperature and utilized for the development of biofertilizers for the mitigation of cold stress in mountain agriculture.
References
Yadav AN, Sachan SG, Verma P, Saxena AK (2016) Bioprospecting of plant growth promoting psychrotrophic Bacilli from cold desert of north western Indian Himalayas. Indian J Exp Biol 54:142–150
Mishra PK, Bisht SC, Ruwari P, Selvakumar G, Joshi GK, Bisht JK et al (2011) Alleviation of cold stress in inoculated wheat (Triticum aestivum L.) seedlings with psychrotolerant Pseudomonads from NW Himalayas. Arch Microbiol 193:497–513. https://doi.org/10.1007/s00203-011-0693-x
Sharma P, Sharma N, Deswal R (2005) The molecular biology of the low-temperature response in plants. Bioessays 27:1048–1059. https://doi.org/10.1002/bies.20307
Subramanian P, Kim K, Krishnamoorthy R, Mageswari A, Selvakumar G, Sa T (2016) Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PloS one 11:e0161592. https://doi.org/10.1371/journal.pone.0161592
Theocharis A, Clément C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105. https://doi.org/10.1007/s00425-012-1641-y
Chen Y, Rekha P, Arun A, Shen F, Lai W-A, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41. https://doi.org/10.1016/j.apsoil.2005.12.002
Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2014) Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci 3:432–447
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56:44–58. https://doi.org/10.1002/jobm.201500459
Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK et al (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899. https://doi.org/10.1007/s13213-014-1027-4
Yadav AN, Sachan SG, Verma P, Saxena AK (2015a) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693. https://doi.org/10.1016/j.jbiosc.2014.11.006
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015b) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108. https://doi.org/10.1007/s11274-014-1768-z
Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact 13:1–10. https://doi.org/10.1186/1475-2859-13-66
Tapia-Vázquez I, Sánchez-Cruz R, Arroyo-Domínguez M, Lira-Ruan V, Sánchez-Reyes A, del Rayo S-CM et al (2020) Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol Res 232:126394. https://doi.org/10.1016/j.micres.2019.126394
Yadav AN, Rastegari AA, Yadav N (2020) Microbiomes of extreme environments: biodiversity and biotechnological applications. CRC Press, Taylor & Francis, Boca Raton, USA
Singh JS, Singh D (2013) Plant growth promoting rhizobacteria (PGPR): microbes in sustainable agriculture. In: Management of microbial resources in the environment. Springer, pp 361–385. https://doi.org/10.1007/978-94-007-5931-2_14
Naik K, Mishra S, Srichandan H, Singh PK, Sarangi PK (2019) Plant growth promoting microbes: potential link to sustainable agriculture and environment. Biocatal Agric Biotechnol 21:101326. https://doi.org/10.1016/j.bcab.2019.101326
Joshi A, Mishra B, Chatrath R, Ferrara GO, Singh RP (2007) Wheat improvement in India: present status, emerging challenges and future prospects. Euphytica 157:431–446. https://doi.org/10.1007/s10681-007-9385-7
Verma P, Yadav A, Kazy S, Saxena A, Suman A (2013) Elucidating the diversity and plant growth promoting attributes of wheat (Triticum aestivum) associated acidotolerant bacteria from southern hills zone of India. Natl J Life Sci 10:219–226
Pikovskaya R (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Hu X, Chen J, Guo J (2006) Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990. https://doi.org/10.1007/s11274-006-9144-2
Saravanan VS, Subramoniam SR, Raj SA (2004) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (zsb) isolates. Braz J Microbiol 35:121–125. https://doi.org/10.1590/S1517-83822004000100020
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Schwyn B, Neilands J (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Cappucino JC, Sherman N (1992) Nitrogen cycle. In: Microbiology: A Laboratory Manual, 4th edn. Benjamin/Cumming Pub. Co., New York, pp 311–312
Castro G, Ferrero M, Méndez B, Sineriz F (1993) Screening and selection of bacteria with high amylolytic activity. Acta Biotechnol 13:197–201. https://doi.org/10.1002/abio.370130220
Zhou X, Chen H, Li Z (2004) CMCase activity assay as a method for cellulase adsorption analysis. Enzyme Microb Technol 35:455–459. https://doi.org/10.1016/j.enzmictec.2004.07.005
Kanekar P, Nilegaonkar S, Sarnaik S, Kelkar A (2002) Optimization of protease activity of alkaliphilic bacteria isolated from an alkaline lake in India. Biores Technol 85:87–93. https://doi.org/10.1016/S0960-8524(02)00018-4
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1080/00103624.2020.1729379
Elliott L, Lynch J (1984) Pseudomonads as a factor in the growth of winter wheat (Triticum aestivum L.). Soil Biol Biochem 16:69–71. https://doi.org/10.1016/0038-0717(84)90128-7
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Grieve C, Grattan S (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413. https://doi.org/10.1016/0003-2697(88)90564-7
Sharma P, Sheikh I, Singh D, Kumar S, Verma SK, Kumar R et al (2017) Uptake, distribution, and remobilization of iron and zinc among various tissues of wheat–Aegilops substitution lines at different growth stages. Acta Physiol Plant 39:185. https://doi.org/10.1007/s11738-017-2456-z
Kushwaha P, Kashyap PL, Kuppusamy P (2020) Microbes for cold stress resistance in plants: mechanism, opportunities, and challenges. In: Microbiological Advancements for Higher Altitude Agro-Ecosystems & Sustainability. Springer, pp 269–292. https://doi.org/10.1007/978-981-15-1902-4_14
Ruelland E, Zachowski A (2010) How plants sense temperature. Environ Exp Bot 69:225–232. https://doi.org/10.1016/j.envexpbot.2010.05.011
Vega-Celedón P, Bravo G, Velásquez A, Cid FP, Valenzuela M, Ramírez I et al (2021) Microbial diversity of psychrotolerant bacteria isolated from wild flora of Andes Mountains and Patagonia of Chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 9:538. https://doi.org/10.3390/microorganisms9030538
De Souza M-J, Nair S, Bharathi PL, Chandramohan D (2006) Metal and antibiotic-resistance in psychrotrophic bacteria from Antarctic Marine waters. Ecotoxicology 15:379–384. https://doi.org/10.1007/s10646-006-0068-2
Schloss PD, Allen HK, Klimowicz AK, Mlot C, Gross JA, Savengsuksa S et al (2010) Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol 29:533–541. https://doi.org/10.1089/dna.2010.1020
Maharana AK, Ray P (2013) Isolation and screening of cold active extracellular enzymes producing psychrotrophic bacteria from soil of Jammu City. Biosci Biotechnol Res Asia 10:267–273. https://doi.org/10.13005/BBRA/1120
Przemieniecki SW, Kurowski TP, Korzekwa K, Karwowska A (2014) The effect of psychrotrophic bacteria isolated from the root zone of winter wheat on selected biotic and abiotic factors. J Plant Prot Res 54:407–413. https://doi.org/10.2478/jppr-2014-0061
Mitra D, Anđelković S, Panneerselvam P, Senapati A, Vasić T, Ganeshamurthy A et al (2020) Phosphate-solubilizing microbes and biocontrol agent for plant nutrition and protection: current perspective. Commun Soil Sci Plant Anal 51:645–657
Uphoff N, Ball AS, Fernandes E, Herren H, Husson O, Laing M et al (2006) Biological approaches to sustainable soil systems. CRC Press, Taylor & Francis, Boca Raton, London, New York
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51. https://doi.org/10.1111/j.0031-9317.2005.00582.x
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Siddique A, Kandpal G, Kumar P (2018) Proline accumulation and its defensive role under diverse stress condition in plants: an overview. J Pure Appl Microbiol 12:1655–1659. https://doi.org/10.22207/JPAM.12.3.73
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino acids 35:753–759. https://doi.org/10.1007/s00726-008-0061-6
Forlani G, Trovato M, Funck D, Signorelli S (2019) Regulation of proline accumulation and its molecular and physiological functions in stress defence. In: Osmoprotectant-mediated abiotic stress tolerance in plants. Springer, pp 73–97. https://doi.org/10.1007/978-3-030-27423-8_3
Nayyar H, Chander K, Kumar S, Bains T (2005) Glycine betaine mitigates cold stress damage in chickpea. Agron Sustain Dev 25:381–388
Yuanyuan M, Yali Z, Jiang L, Hongbo S (2009) Roles of plant soluble sugars and their responses to plant cold stress. Afr J Biotechnol 8
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681. https://doi.org/10.4161/psb.23681
Aydin SS, Büyük I, Aras S (2013) Relationships among lipid peroxidation, SOD enzyme activity, and SOD gene expression profile in Lycopersicum esculentum L. exposed to cold stress. Genet Mol Res 12:3220–3229. https://doi.org/10.4238/2013
Kumar V, Yadav SK (2009) Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant 31:261–269. https://doi.org/10.1007/s11738-008-0227-6
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I et al (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212. https://doi.org/10.1016/j.plaphy.2013.05.032
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In: Abiotic stress responses in plants. Springer, pp 149–158. https://doi.org/10.1007/978-1-4614-0634-1_8
Tiryaki D, Aydın İ, Atıcı Ö (2019) Psychrotolerant bacteria isolated from the leaf apoplast of cold-adapted wild plants improve the cold resistance of bean (Phaseolus vulgaris L.) under low temperature. Cryobiology 86:111–119. https://doi.org/10.1016/j.cryobiol.2018.11.001
Gulati A, Vyas P, Rahi P, Kasana RC (2009) Plant growth-promoting and rhizosphere-competent Acinetobacter rhizosphaerae strain BIHB 723 from the cold deserts of the Himalayas. Curr Microbiol 58:371–377. https://doi.org/10.1007/s00284-008-9339-x
Acknowledgements
The authors are grateful to Department of Biotechnology, Dr. Khem Singh Gill Akal College of Agriculture, Eternal University, Baru Sahib and the Department of Environment, Science & Technology (DEST), Shimla-funded project “Development of Microbial Consortium as Bio-inoculants for Drought and Low Temperature Growing Crops for Organic Farming in Himachal Pradesh” for providing the facilities, to undertake the investigations.
Funding
The Department of Environment, Science & Technology (DEST), Shimla-funded project “Development of Microbial Consortium as Bio-inoculants for Drought and Low Temperature Growing Crops for Organic Farming in Himachal Pradesh” provided financial support, to undertake the investigations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Aleksander Westphal Muniz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kour, D., Yadav, A.N. Alleviation of cold stress in wheat with psychrotrophic phosphorus solubilizing Acinetobacter rhizosphaerae EU-KL44. Braz J Microbiol 54, 371–383 (2023). https://doi.org/10.1007/s42770-023-00913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00913-7