Abstract
Human sporotrichosis is caused by different Sporothrix species; however, Sporothrix brasiliensis is the main species, usually related to cat transmission in urban areas. A retrospective descriptive study was conducted at the Institute of Infectology Emílio Ribas from 2010 to 2018. Demography, clinical, diagnostic, and therapeutic data were obtained from medical records. Polymerase chain reaction of the calmodulin gene was performed to identify Sporothrix species. In addition, to evaluate the spread of the disease across São Paulo metropolitan region, TerraView version 4.2.2 software was used for geocoding cases according to residence addresses. Kernell’s maps using QGIS software version 2.16.3 were constructed to determine the concentration of cases. Results: 260 cases of sporotrichosis were diagnosed between 2010 and 2018. We observed a 700% increment in the number of human cases in the 2016–2018 triennium compared with the 2013–2015 triennium. Female adults with a median age of 46 years old were the predominant infected group associated with cats’ exposition at home care, although the age range of all patients was 01 to 86 years old. The main epidemiological risk of acquiring sporotrichosis was contact with cats, reported by 96.5% of the patients. Molecular identification showed that most of the tested isolates were Sporothrix brasiliensis. Lymphocutaneous form was observed in 59.2% and fixed cutaneous form in 37.5% of the patients. Regarding treatment, itraconazole was the main drug used (94.2%) with a cure rate of 98.8%. We observed an important spread of human sporotrichosis involving cat transmission caused by Sporothrix brasiliensis in a densely populated area of São Paulo state. These results are important to alert clinicians and dermatologists about the occurrence and progression of a neglected tropical disease in an urban area and the urgent necessity to include sporotrichosis as a differential diagnosis in the clinical investigation routine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporotrichosis is a subacute or chronic disease caused by fungi of the Sporothrix genus [1]. The literature reclassified the main etiological agents of sporotrichosis as S. schenckii, S. globosa, S. brasiliensis and S. lurei, clustered in a pathogenic clade of the Sporothrix genus [2]. These new species were first described in 2007 and took part of the Sporothrix schenckii complex [3]. Sporotrichosis was former known as “the gardener’s disease”, a sapronosis acquired by traumatic implantation of mycelial elements into the skin (hyphae fragments and conidia) after trauma involving soil and vegetable debris contaminated with Sporothrix spp. [1, 4]. In the last two decades, the zoonotic transmission of the new pathogen S. brasiliensis has gained great epidemiological importance due to the high susceptibility of cats (Felis catus domesticus) that commonly develop a severe disease [5]. Feline sporotrichosis is not limited to S. brasiliensis infection as S. schenckii also infects cats, and there are some reports of cat–human transmission mainly in Asia [6, 7], although the zoonotic expansion of S. schenckii infection has low epidemiological impact [8]. On the other hand, current reports show increments of 500–600% in the number of cases over a three-year period due to S. brasiliensis cat to human transmission [9]. The human sporotrichosis paradigm has changed mainly due to S. brasiliensis zoonotic transmission that is related to, at least, three important aspects: (i) the possibility that sporotrichosis can be acquired by the inoculation into the skin of the S. brasiliensis yeast parasitic phase found in the cats’ oral cavity (body temperature) [5, 10]; (ii) the peridomestic and intradomiciliary transmission of sporotrichosis due to the human contact with sick domestic animals and the contaminated environment [5, 7]; and (iii) the zoonotic expansion of S. brasiliensis infection is also influenced by geo-epidemiological and socioeconomic aspects [11, 12]. There is no direct evidence that the clinical manifestations of human sporotrichosis change due to the etiological agent virulence, Sporothrix spp., but this was a proposed hypothesis [13]. However, in the experimental model, S. brasiliensis is clearly more virulent compared to S. schenckii or S. globosa [14,15,16]. Nonetheless, there are a higher number of reports of sporotrichosis meningitis, disseminated sporotrichosis and other severe cases related to S. brasiliensis [17,18,19,20]. Sporotrichosis is a neglected and underreported disease in Brazil as the notification of human and animal sporotrichosis is not compulsory, with exception in some Brazilian States and some municipalities. The epidemic of sporotrichosis was first detected in Rio de Janeiro State [5]. Feline and human sporotrichosis are now considered as endemic and are already registered in all Brazilian regions (from North to South) and are reported in almost all the states [21,22,23,24,25]. Sporotrichosis caused by S. brasiliensis infection is no more geographically limited to Brazil as this disease is already reported in other South America countries [26]. Hospitalizations and deaths are of great concern as epidemiological policies are still lacking as well as a consistent epidemiological database [12, 17]. In São Paulo state, Brazil, the investigation of the first feline case of sporotrichosis due to S. brasiliensis was reported in 2011, in the East urban area of São Paulo city, at Itaquera district [27]. The series of cases at Itaquera district were controlled and notified by the Center for Control of Zoonosis (CCZ) of São Paulo city [27]. Despite the efforts of the CCZ São Paulo to control the expansion of feline sporotrichosis, the number of cases has continued to grow in São Paulo city [12]. In addition, other cities located nearby São Paulo city, i.e., Diadema and Guarulhos, started to report cases between 2011 and 2012 [12]. To update the expansion of sporotrichosis in São Paulo State, in the 2014–2016 triennium the CCZ-Guarulhos has notified 685 feline cases, but this number has jumped to 3081, in the 2017–2019 triennium, an increment of 450% [12, 28]. Data related to the magnitude and challenges of human sporotrichosis in São Paulo state are still scarce in the literature. This study aimed to evaluate the expansion of human sporotrichosis in São Paulo Metropolitan area (SPMA) covering clinical–epidemiological, diagnostic, and therapeutic aspects.
Methods
Ethics statement
The Research Ethics Committee of the Instituto de Infectologia Emílio Ribas (IIER), São Paulo, Brazil, has approved this study under the protocol number CAAE: 09,815,619.0.0000.0061.
Study design
We conducted a retrospective study by reviewing the human cases of sporotrichosis admitted to the IIER, a reference infectious diseases public hospital of São Paulo state, Brazil, between January 2010 and December 2018. Medical records of all patients who were diagnosed with sporotrichosis were accessed for the study period. Patients of all ages who were suspected or diagnosed for sporotrichosis based on clinical–epidemiological or clinical–laboratory characteristics were included. The SPMA area has a total of 39 municipalities, including São Paulo city, and an estimated population 21.6 million of inhabitants, according to the last Brazilian census performed by IBGE (Brazilian Institute of Geography and Statistics), in 2018. TerraView version 4.2.2 software was used for geocoding cases according to residence addresses, using the cartographic base of the Center for Metropolis Studies (CEM, 2018). Three-year cumulative cases were considered to construct the Kernell’s maps in QGIS software version 2.16.3.
Diagnosis of sporotrichosis
Suspect cases of sporotrichosis were defined by epidemiological data (contact with cat diagnosed with sporotrichosis) and the presence of cutaneous lesions or subcutaneous nodules. Confirmed cases were defined by clinical–epidemiological criteria associated with the detection of fungi by direct examination or culture. Patients who presented response to treatment, even without detection of fungi, were considered confirmed. For a total of 260 patients, 178 had cutaneous lesions specimens collected by biopsy for laboratory diagnosis by direct microscopy, fungus culture and histopathology analyses.
Histopathology
Paraffin-embedded tissue was stained with Grocott, hematoxylin–eosin, PAS and analyzed by optical microscopy. Histopathology was considered suggestive of sporotrichosis if a granulomatous infiltrate was present, and rarely rounded yeast-like cells with multiple budding, cigar-shaped cells, asteroid bodies were observed.
Direct microscopy
Imprint from lesion was stained with Giemsa, and fungal elements were searched by optical microscopy.
Mycological test
Biopsy fragments were incubated on Sabouraud Dextrose Agar and Mycosel Agar (Difco Laboratories, Detroit, MI) for 30 days and assessed weekly. Identification of Sporothrix spp. was based on macro- and micromorphology aspects of fungal isolates and was also based on dimorphism confirmation [12].
Polymerase Chain Reaction to detect DNA of Sporothrix spp.
To determine the etiological agent genotype, DNA samples were extracted directly from mycelial colonies using Trichoderma harzianum lysing enzyme (L-1412, Sigma) and the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. DNA content was measured in a spectrophotometer NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA). Species-specific primers that targeted the calmodulin gene of three Sporothrix species were employed for PCR amplifications: Sbra-F and Sbra-R (S. brasiliensis) that generated a 469-bp amplicon; Ssch-F and Ssch-R (S. schenckii) that amplified a 331-bp region, and Sglo-F and Sglo-R (S. globosa) that generated a 243-bp amplicon [29]. Thermal cycling conditions were as follows: an initial denaturing step of 5 min at 95 °C, followed by 35 cycles of 1 min at 95 °C, 1 min at the annealing temperature (touchdown PCR), and 1 min at 72 °C, followed by a final extension step of 10 min at 72 °C [29]. PCR products were analyzed on 2% agarose gel, stained with GelRed™ (Biotium, Fremont, CA, USA) and visualized with UVITEC gel documentation system (Cleaver Scientific, Rugby, Warks, UK).
Data collection and analysis
The data collected included information concerning residence, demographics, clinical form, duration of symptoms, underlying medical illnesses, occupational and other exposure risks, history of trauma and response to therapy. Cases of sporotrichosis were classified clinically as lymphocutaneous (LC), cutaneous fixed (CF), disseminated forms (DF) and extracutaneous form (EF). The outcome was determined by the clinician based on clinical criteria. Successful outcome was defined as the complete healing of cutaneous lesions and therapeutic failure as the stability or worsening of the initial lesion after 1–2 months of treatment. Relapse was considered in those patients with previous improvement of the cutaneous lesion in which a new therapeutic regimen was required.
The data collected for each patient were analyzed using the software STATA, version 13.0. Statistical methods aimed to calculate frequencies, mean, median, variance, and standard deviation (SD).
Results
Patient series
Two hundred and sixty cases of sporotrichosis were followed and diagnosed during the period of 2010–2018 at the IIER with a historical mean annual frequency of 28.9 cases, ranging from 4 to 108 annual cases (Fig. 1). The majority of these patients were from São Paulo metropolitan area (98.8%), and two patients declared residence in other cities of São Paulo state. No geographical data were available in one case.
When analyzed triennially, we observed an important increase in the number of sporotrichosis cases between 2016 and 2018, 700% when compared with the 2013–2015 triennium (Fig. 2). In addition, we also observed a spread of human cases to several regions of the SPMA (Fig. 3). The geocoded cases (n = 255) were also distributed according to triennium of symptoms onset: 2010–2012 (Fig.3A); 2013–2015 (Fig. 3B); 2016–2018 (Fig. 3C). In the first two triennia analyzed (Fig. 3Aand 3B), the cases were concentrated in the East zone of the São Paulo city and its borders.. In the last triennium (2016–2018), there was a remarkable increase in the number of cases in SPMA and a dispersion of cases to the North and South zones of São Paulo city (Fig. 3). The metropolitan area corresponds to the city of São Paulo and other municipalities. On the maps, this expansion can clearly be seen in the municipalities of Guarulhos and Itaquaquecetuba municipalities, for example.
Age range and gender
Most patients were adults (88.4%), and the main age groups ranged from 40 to 60 years old (40.3%). However, the patients’ age interval ranged from 01 to 86 years old (median of 46 years old). Concerning the gender, the disease was predominant in females (69.9%) with a female/male ratio of 2.3:1.0.
Comorbidities
Data regarding comorbidities and/or medical illnesses were reported in 45% (n = 118) of all patients (n = 260). The most frequent comorbidities reported were arterial hypertension (33 cases), diabetes mellitus (16 cases) and hypothyroidism (7 cases). One patient was under immunosuppressive therapy for systemic lupus erythematosus. Two patients had HIV infection and virus C-associated liver cirrhosis.
Occupation
This information was available for 116 patients. Among these, housekeeper was the most common occupation (28.4%) followed by veterinary clinics workers (18.1%). The other 53.5% of patients of this cohort was distributed in a wide range of occupations.
Transmission
An expressive number of patients, 251 out of 260 (96.5%), reported contact with cats, before the appearance of skin lesions. Among the nine patients that did not report contact with cats, two had traumas caused by a piece of wood and a thorny plant and three were in contact with dogs without associated trauma.
We could correlate these human cases with two hundred and twenty-nine cats that were sick, with clinical manifestations compatible with sporotrichosis. Trauma involving cat was reported in 184 human cases (73.3%), either by scratch (n = 137) or by bite (n = 47). In the case of all other patients who reported contact with cats, sixty-seven individuals (26.7%) did not report any skin trauma, fifty-nine of these individuals (88.1%) reported contact with sick cats presenting cutaneous lesions and four patients reported contact with apparently healthy cats.
Clinical Presentation
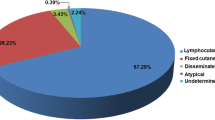
The most common clinical presentation was the LC (Fig. 4A) observed in 154 patients (59.2%), followed by the CF (Fig. 4B) reported in 97 patients (37.3%). Three patients presented the disseminated cutaneous form (Fig. 4C) and one the extracutaneous form (osteoarticular involvement, two phalanges of the second finger of the left hand). Atypical presentations due to hypersensitivity reactions were also observed: three patients developed erythema nodosum and two developed Sweet’ syndrome (Fig. 4D). All the 16 diabetic patients presented non-severe forms of the disease: 11 (68.7%) had the LC form and 5 (31.2%) the CF form. Similarly, for the two HIV patients, one patient with CD4 T lymphocytes of 463 cells/μl and undetectable viral load presented the CF form, and a second patient with CD4 T lymphocyte of 176 cells/μl and detectable viral load presented the LC form.
Data on the time elapsed between onset of symptoms and diagnosis, available in 249 cases, showed a wide range of time, 3 to 240 days, with a median of 30 days. In relation to the body distribution of the cutaneous lesions, the upper limbs were the most affected site (78.8%), followed by the lower limbs (19.6%), thorax and face (3.4%) each one. Other body localizations were the abdomen (1.9%) and the neck (1.1%) as well as the involvement of mucosa were described (oral, nasal and ocular mucosa).
Diagnosis
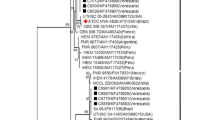
Biopsy of a cutaneous lesion was obtained from 178 patients. From 138 samples submitted to culture, 108 (78.2%) yielded Sporothrix spp. (Fig. 5). Molecular identification was performed in a subset of isolates (n = 31): 29 isolates were identified as S.brasiliensis, transmitted by cat (Fig. 6) and two others as S. globose, transmitted by environmental source. Histopathology examination was performed in 171 cases. The presence of granulomatous reaction was observed in 119 specimens (69.5%). Direct microscopy examination of the imprint of the biopsy was performed in 127 cases and revealed fungal elements in 40 cases (31.4%). One hundred and ten (42.3%) patients were diagnosed based solely on clinical and epidemiological criteria, among them 28 patients underwent biopsy, but no elements were found to suggest an etiological diagnosis of sporotrichosis, so they were treated based on clinical–epidemiological criteria.
(A) Macromorphology on Agar Sabouraud Dextrose and Agar Potato (culture of 10 days). (B) Micromorphology showing characteristic delicate septate hyaline hyphae, conidiophore forming globous hyaline to black conidia groups, in a flower-like petals arrangement (400X). (C) Imprint of lesion by Giemsa-stained demonstrated presence of elongated cigar-shaped, ovals form and spindle-shaped, intra- and extracellular (1500X)
DNA amplification of S. brasiliensis using Calmodulin as gene target. Electrophoresis on 2% agarose gel. MW: molecular weight (100 bp DNA ladder, Invitrogen); C (-): negative control; A1: sample 210; A2: sample 212; A3: sample 215; A4: sample 216; A5: sample 217; C ( +): positive control. Note that A1 to A5 amplified in the 500-bp portion of the gel, confirming that it is S. brasiliensis
Treatment
Antifungal treatment was prescribed to all except two patients who presented spontaneous regression of lesions and one patient who did not return to receive the medical prescription. Eighty-three cases (32.3%) were lost to follow-up and treatment response could not be evaluated, three months after the end of the treatment: itraconazole was prescribed to 79 patients, potassium iodide to 3 children, and amphotericin B deoxycholate was used, as induction treatment before itraconazole, in one patient with osteoarticular involvement. Treatment follow-up could be retrieved in 174 patients. Itraconazole was the drug of choice in 164 (94.2%). The median dose was 200 mg/day (range 100–400 mg) for a median of 120 days (range 30–387 days) for all clinical forms (CF and LC). For CF, 200 mg/day was used for two months and 400 mg/day was used to treat LC or disseminated forms. There was no difference in the median dose or duration of therapy between patients treated with the CF and LC forms of the disease. Failure of the initial treatment and after itraconazole dose increase was reported in seven cases (4%); all of them switched the antifungal drug. Four patients were treated with amphotericin B deoxycholate: a pregnant woman, two patients after failure to respond to itraconazole and a patient with Sweet’s syndrome who was initially misdiagnosed as having the disseminated cutaneous (treatment was switched to itraconazole). The median dose was 50 mg/day (range 40–50 mg) for a median of 16.5 days (range 5–28). All patients progressed with clinical cure. The patient with osteoarticular involvement was also treated with amphotericin B deoxycholate; however, he did not complete the treatment. Seven patients were treated with potassium iodide, three adults because of failure of treatment with itraconazole, and four children (age range 4–11 years) due to the convenience of intake. The median dose was 24 drops/day (range 15 – 30) for a median time of 135 days (range 30 – 210). All patients evolved with healing of the lesions. Because of drug interaction of itraconazole with concomitant medications, terbinafine was prescribed to three patients, at a dose of 500 or 1000 mg/day, during a median time of 116 days (range 30–197), with clinical cure in all cases. Two patients upon itraconazole treatment were switched to fluconazole due to unavailability of the drug in the health service. The dose prescribed was 300 mg/day and 150 mg/day for 30 days. Fluconazole led to clinical cure in one patient but failed in the other patient: it was then switched to potassium iodide, which resulted in cure. Topical trichloroacetic acid (50%) was used in four patients: two patients with treatment failure with itraconazole, one patient with a small fixed cutaneous lesion and one pregnant woman. Before the use of trichloroacetic acid, the crusts were removed. A median of one application/week was used, ranging from 1 to 4. All patients evolved with cure. Six patients were hospitalized. Four were children required diagnostic elucidation; one adult patient required parenteral oxacillin for secondary skin infections, while the other was the single patient with severe clinical condition who required hospitalization to receive parenteral antifungal treatment with amphotericin. From 174 patients treated in our service, one hundred and seventy-two cases (98.9%) evolved to clinical cure, but two patients presented post-treatment relapse, with the culture yielding S. globosa in one case. Both were cured after retreatment with the same dose of itraconazole (400 mg/day) from the first treatment.
Discussion
Human sporotrichosis cases have exponentially expanded in Brazil since the late 1990s, emerging with the cat-transmitted species S. brasiliensis [30]. To understand the dynamics of human sporotrichosis in SPMA, we carried a retrospective study focusing on the epidemiological, demographic, clinical data, and therapeutic response of patients (N = 260) registered at IIER between 2010 and 2018. The IIER is a reference center for infectious diseases, and as a result, an agreement between this institution and the centers for zoonosis control in the SPMA facilitated the patients’ admission to this institution, allowing our data to reflect the ongoing progression of the epidemic in the expanded São Paulo area. It is important to notice that the number of cases is presumably much higher, as this disease has no compulsory notification in almost all São Paulo state. Furthermore, in general, health professionals are not prepared for clinical diagnosis of this fungal infection and sporotrichosis may, in consequence, be misdiagnosed during the clinical–diagnostic evaluation. In addition, routine diagnostic methods for sporotrichosis are not easily available in public health services. Initially, we observed a slight increase in human cases up to 2015. However, after 2016 we started to observe a geometric progression in the number of cases registered at the IIER, with an 700% increment in the human cases registered during the period of 2016–2018 as compared to 2013–2015. This geometrical growth in the number of cases may be related to some factors, such as concomitant increase of feline cases or due to the active search of cases in humans, caregivers of cats that presented clinical manifestation of the disease. In the literature, a previous retrospective study reported only 25 human cases of sporotrichosis in São Paulo, between 2003 and 2013, and 40% reported contact with sick cat [31]. Prior to 2011, in São Paulo, only a single report of cat sporotrichosis with human transmission was found in the literature [32]. During the expansion of sporotrichosis in SPMA between 2016 and 2018 as reported here, we detected cases in all regions of the capital and in some neighboring cities (Fig. 3C) (Fig. 3C). This fact may be related to the greater transmission of S. brasiliensis by contact with infected cats, either by scratching, biting or contact without apparent skin damage, and also, by the probable traffic of cats between different regions of the city. Cats are known to be animals that do not remain fixed within their home and still transmit the fungus to other cats during coitus or as a result of fighting among them [33]. The relation between cats and the human infectious process is reinforced by the fact that the majority of the patients (96.5%) reported prior contact with them. Only three patients reported contact with sick dogs, without proved zoonotic transmission from dogs. Our findings are consistent with the Rio de Janeiro epidemiological data [5]. According to demographic data obtained in our series of cases, the disease predominates in middle-aged housewives, similar to the reports in the beginning of the Rio de Janeiro epidemics [33,34,35]. Altogether, the data suggest that the most affected population is in close contact with sick animals, especially when caring for them during home treatment. Regarding route of infection, trauma caused by scratch or bite of felines was described in most of the patients; however, 24.3% of our patients denied any trauma, suggesting only feline contact with their healthy skin, and however, many times microtrauma are unnoticed. These findings are in accordance with a previous study where the authors assumed that transmission might occur by direct contact, without any trauma, since feline lesions are full of S. brasiliensis yeast cells, and/or by contact with nasal and oral secretions of sick cats [34]. In the past, transmission of sporotrichosis was classically related to the rural environment. However, the current Brazilian epidemic is increasingly related to urban areas and zoonotic transmission. Furthermore, it is possible that an increased virulence of S. brasiliensis may be a factor contributing to sporotrichosis outbreak [5]. In the present study, we identified the Sporothrix species of 31 samples collected from skin lesions. The results showed that 29 isolates were genotyped as S. brasiliensis (93.5%) and two as S. globosa (6.5%). For these two patients, infected with S. globosa, no contact with sick cats was reported. One patient referred to ground handling in rural area and, the other one, had close contact with a healthy dog. Our data show similarity to a previous report in Rio de Janeiro state, whereas S. brasiliensis was identified in 90% of human samples [19] and also to data reported in the Rio Grande do Sul state, showing that S. brasiliensis was isolated in 86.6% of human cases [36]. Montenegro and collaborators analyzed specimens isolated from cats and dogs of the metropolitan region of São Paulo and found that 100% of the isolates were genotyped as S. brasiliensis [28]. To our knowledge, this is the first report of human cases of sporotrichosis in São Paulo state with the identification of S. brasiliensis and S. globosa as the major causative agents. In our study, the predominant clinical form was the lymphocutaneous (59.2%), similar to other Brazilian studies [1, 23, 24, 31, 36, 37], and for the majority of our patients the lesions were located in the upper limbs. One of these patients presented lesions in the thoracic region, without reporting any trauma. Other important clinical manifestations observed were erythema nodosum and Sweet syndrome. Almeida-Paes and collaborators showed a direct association between unusual clinical presentations of human sporotrichosis with S. brasiliensis infection, in the rising Brazilian epidemic [14].
The gold standard for the diagnosis of sporotrichosis is the detection of the etiological agent. Body fluids or tissue fragments are suitable for fungus isolation in culture [38]. Direct search by microscopy could be used as an additional tool; however, the sensitivity remains understudied, due to the paucity of fungal cells [39], so we suggest a better assessment of the sensitivity of this tool so that it can be used routinely. In our case series, we observed 78.2% and 31.4% of sensitivity using the mycological test (culture) and direct microscopy, respectively. The low sensitivity of direct microscopy when compared with fungus culture is noteworthy. The histopathological findings revealed, predominantly, a granulomatous infiltrate in specimens collected from patients with fixed cutaneous and lymphocutaneous forms of the disease. These findings are similar to observations reported by other authors [33].
The majority of patients were treated with itraconazole, as first-choice drug for the treatment of sporotrichosis. To treat the patients with the fixed form, a daily dose of 200 mg was used and this dose was raised to 400 mg per day to treat the lymphocutaneous form. The treatment was kept for at least 3 months and up to 6 months. We observed high cure rate (98.9%), probably due to the azole dose used and a longer duration of treatment. Other drugs are used to treat sporotrichosis: amphotericin B, potassium iodide and terbinafine [39]. Other therapeutic option can be used when there is a poor clinical response after three months of itraconazole regular intake or contraindication to its use, such as pregnancy. Using different therapeutic options, we observed 98% of clinical cure. It is important to note that four patients treated with topical therapy, due to the contraindication for the use of itraconazole, also presented clinical cure. These data are essential, because in situations of fixed cutaneous form, in patients with contraindications for systemic treatment, a therapeutic option would be local treatment. Although there is some evidence in the literature regarding the local treatment of sporotrichosis, this result cannot be ignored.
In conclusion, in the description of human sporotrichosis cases series in SPMA, we shown a spread of the disease over the course of years, caused by Sporothrix brasiliensis. An extremely populous region, which presents full conditions for a catastrophic expansion of the disease, was associated with feline contact.
Clearly, what we describe here is the tip of the iceberg that we are seeing. Probably, the diagnosis and treatment of sporotrichosis are underestimated, due to the fact that it is not a compulsory notification disease, the lack of knowledge of a large part of health professionals about the clinical features, and the restricted access to diagnosis and treatment. Within the binomial human and animal health, sporotrichosis, in urban centers transmitted by feline contact, is a classic representation and that should be worked on as one health. We strongly encourage the inclusion of sporotrichosis as a compulsory notification disease in Brazil and that is considered a neglected disease.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Orofino-Costa R, Macedo PM, Rodrigues AM, Bernardes-Engemann AR (2017) Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol 92(5):606–20. https://doi.org/10.1590/abd1806-4841.2017279
de Beer ZW, Duong TA, Wingfield MJ (2016) The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol 83:165–191. https://doi.org/10.1016/j.simyco.2016.07.001
Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J (2007) Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol 45(10):3198–206. https://doi.org/10.1128/JCM.00808-07
Zhang Y, Hagen F, Stielow B et al (2015) Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 35:1–20. https://doi.org/10.3767/003158515X687416
Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira AS (2017) Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog 13(1). https://doi.org/10.1371/journal.ppat.1006077
Tang MM, Tang JJ, Gill P, Chang CC, Baba R (2012) Cutaneous sporotrichosis: a six-year review of 19 cases in a tertiary referral center in Malaysia. Int J Dermatol 51(6):702–708. https://doi.org/10.1111/j.1365-4632.2011.05229.x
Han HS, Kano R (2021) Feline sporotrichosis in Asia. Braz J Microbiol 52(1):125–134. https://doi.org/10.1007/s42770-020-00274-5
Siew HH (2017) The Current Status of Feline Sporotrichosis in Malaysia. Med Mycol J 58(3):E107–E113. https://doi.org/10.3314/mmj.17.014
Poester VR, Mattei AS, Madrid IM et al (2018) Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health 65(7):815–821. https://doi.org/10.1111/zph.12504
Macêdo-Sales PA, Souto SRLS, Destefani CA, Lucena RP, Machado RLD, Pinto MR, Rodrigues AM, Lopes-Bezerra LM, Rocha EMS, Baptista ARS (2018) Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: a comparison between infected and non-infected populations. BMC Vet Res. Jan 18;14(1):19. https://doi.org/10.1186/s12917-018-1340-4
Lecca LO, Paiva MT, de Oliveira CSF, et al (2020) Associated factors and spatial patterns of the epidemic sporotrichosis in a high density human populated area: A cross-sectional study from 2016 to 2018. Prev Vet Med 176 https://doi.org/10.1016/j.prevetmed.2020.104939
Gremião IDF, Rocha EMS, Montenegro H, Carneiro AJB et al (2021) Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz J Microbiol 52(1):107–124. https://doi.org/10.1007/s42770-020-00365-3
Cruz ILR, Freitas DFS, de Macedo PM, Gutierrez-Galhardo MC, do Valle ACF, Almeida MA, Coelho RA, Brito-Santos F, Figueiredo-Carvalho MHG, Zancopé-Oliveira RM, Almeida-Paes R (2021) Evolution of virulence-related phenotypes of Sporothrix brasiliensis isolates from patients with chronic sporotrichosis and acquired immunodeficiency syndrome. Braz J Microbiol 52(1):5–18. https://doi.org/10.1007/s42770-020-00297-y
Cruz-Choappa R, Pérez Gaete S, Rodríguez Badilla V, VieilleOyarzo P, Opazo Sanchez H (2016) Virulencia de Sporothrix globosa en modelos murinos [Virulence of Sporothrix globosa in murine models]. Rev Argent Microbiol. 48(3):196–199. https://doi.org/10.1016/j.ram.2016.04.007 (Spanish)
Fernandes GF, dos Santos PO, Rodrigues AM, Sasaki AA, Burger E, de Camargo ZP (2013) Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 4(3):241–9. https://doi.org/10.4161/viru.23112
Castro RA, Kubitschek-Barreira PH, Teixeira PA, Sanches GF, Teixeira MM, Quintella LP, Almeida SR, Costa RO, Camargo ZP, Felipe MS, de Souza W, Lopes-Bezerra LM (2013) Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS One 8(10):e75656. https://doi.org/10.1371/journal.pone.0075656
Falcão EMM, de Lima Filho JB, Campos DP, et al (2019) Hospitalizations and deaths related to sporotrichosis in Brazil (1992–2015). Cad Saude Publica; 35(4). https://doi.org/10.1590/0102-311X00109218
Mialski R, de Almeida JN, da Silva LH, et al (2018) Chronic Meningitis and Hydrocephalus due to Sporothrix brasiliensis in Immunocompetent Adults: A Challenging Entity. Open Forum Infect Dis 5(5). https://doi.org/10.1093/ofid/ofy081
Almeida-Paes R, de Oliveira MM, Freitas DF, do Valle AC, Zancopé-Oliveira RM, Gutierrez-Galhardo MC (2014) Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis 8(9). https://doi.org/10.1371/journal.pntd.0003094
Silva-Vergara ML, de Camargo ZP, Silva PF et al (2012) Disseminated Sporothrix brasiliensis infection with endocardial and ocular involvement in an HIV-infected patient. Am J Trop Med Hyg 86(3):477–480. https://doi.org/10.4269/ajtmh.2012.11-0441
Gremião IDF, Oliveira MME, Monteiro de Miranda LH, Saraiva Freitas DF, Pereira AS (2020) Geographic Expansion of Sporotrichosis. Brazil Emerg Infect Dis 26(3):621–624. https://doi.org/10.3201/eid2603.190803
Eudes Filho J, Santos IBD, Reis CMS et al (2020) A novel Sporothrix brasiliensis genomic variant in Midwestern Brazil: evidence for an older and wider sporotrichosis epidemic. Emerg Microbes Infect 9(1):2515–2525. https://doi.org/10.1080/22221751.2020.1847001
Caus ALO, Zanotti RL, Faccini-Martínez ÁA, Paterlini GV, Falqueto A (2019) Epidemiological and Clinical Aspects of Sporotrichosis in Espírito Santo State, Southeast Brazil: A Study of Three Decades (1982–2012). Am J Trop Med Hyg 100(3):706–713. https://doi.org/10.4269/ajtmh.18-0667
Benvegnú AM, Dallazzem LND, Chemello RML, Beber AAC, Chemello D (2020) Case series of sporotrichosis at a teaching hospital in Brazil. Rev Soc Bras Med Trop 53:e20190509. https://doi.org/10.1590/0037-8682-0509-2019
Grisolia JC, Santos LA, Coelho LML, Silva RR, de Camargo ZP, Velloso TRG, Coelho LFL, Chavasco JK, Malaquias LCC (2021) Seroepidemiological survey on sporotrichosis-infection in rural areas of the south of Minas Gerais State, Brazil. Braz J Microbiol 52(1):41–47. https://doi.org/10.1007/s42770-020-00279-0
Rossow JA, Queiroz-Telles F, Caceres DH et al (2020) A One Health Approach to Combatting Sporothrix brasiliensis Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J Fungi (Basel) 6(4):247. https://doi.org/10.3390/jof6040247
Silva EA, Bernardi F, Mendes MCNC, et al. (2015) Surto de esporotricose em gatos – investigação e ações de controle, município de São Paulo/SP. Vol. 113. Boletim Epidemiológico Paulista, 1–16 (article in Portuguese)
Montenegro H, Rodrigues AM, Dias MA, da Silva EA, Bernardi F, de Camargo ZP (2014) Feline sporotrichosis due to Sporothrix brasiliensis: an emerging animal infection in São Paulo. Brazil BMC Vet Res 10:269. https://doi.org/10.1186/s12917-014-0269-5
Rodrigues AM, Najafzadeh MJ, de Hoog GS, de Camargo Z (2015) Rapid Identification of Emerging Human-Pathogenic Sporothrix Species with Rolling Circle Amplification. Front Microbiol 6:1385. https://doi.org/10.3389/fmicb.2015.01385
Lopes-Bezerra LM, Mora-Montes HM, Zhang Y et al (2018) Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol 56:126–143. https://doi.org/10.1093/mmy/myx103
Marques GF, Martins AL, Sousa JM, Brandão LS, Wachholz PA, Masuda PY (2015) Characterization of sporotrichosis cases treated in a dermatologic teaching unit in the state of São Paulo - Brazil, 2003–2013. An Bras Dermatol 90(2):273–275. https://doi.org/10.1590/abd1806-4841.20153447
Marques SA, Franco SR, de Camargo RM, Dias LD, Haddad Júnior V, Fabris VE (1993) Sporotrichosis of the domestic cat (Felis catus): human transmission. Rev Inst Med Trop Sao Paulo 35(4):327–30 ((article in Portuguese))
Barros MB, Schubach AO, do Valle AC et al (2004) Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis 38(4):529–535. https://doi.org/10.1086/381200
Schubach A, Schubach TM, Barros MB, Wanke B (2005) Cat-transmitted sporotrichosis, Rio de Janeiro. Brazil Emerg Infect Dis 11(12):1952–1954. https://doi.org/10.3201/eid1112.040891
Silva MB, Costa MM, Torres CC et al (2012) Urban sporotrichosis: a neglected epidemic in Rio de Janeiro, Brazil. Cad Saude Publica 28(10):1867–80 ((article in Portuguese))
Brandolt TM, Madrid IM, Poester VR et al (2018) Human sporotrichosis: A zoonotic outbreak in southern Brazil, 2012–2017. Med Mycol. https://doi.org/10.1093/mmy/myy082
Veasey JV, Neto MFN, Ruiz LRB, Zaitz C (2021) Clinical and laboratory profile of urban sporotrichosis in a tertiary hospital in the city of São Paulo. An Bras Dermatol 96(2):245–248. https://doi.org/10.1016/j.abd.2020.07.010
Ramos-e-Silva M, Vasconcelos C, Carneiro S, Cestari T (2007) Sporotrichosis. Clin Dermatol 25(2):181–187. https://doi.org/10.1016/j.clindermatol.2006.05.006
Mahajan VK (2014) Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. https://doi.org/10.1155/2014/272376
Author information
Authors and Affiliations
Contributions
AAB contributed with conception, design, data collection and writing. LKMO contributed with data collection and patient assessment. RFC contributed with geocoding of human cases and epidemiological data. RNP contributed with histopathological analysis. GB, LMLB, and RB contributed with writing and review. VMFG, GMBDN, LPMS, and RSFX contributed with microbiology and molecular biology tests. JALL contributed with conception, design, data collection, writing and review.
All authors read and approved the final and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Research Ethics Committee of the Instituto de Infectologia Emílio Ribas, São Paulo, Brazil, approved this study under the protocol number 36/2018.
Consent to participate
The informed consent form was applied to the participant and, when the subject was not available, the consent was waived, and a researcher responsibility and confidentiality term were used for all cases.
Consent for publications
Not applicable for that section.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa
Rights and permissions
About this article
Cite this article
Bittencourt, A.A., Oyafuso, L.K.M., Cavalin, R.F. et al. A neglected disease. Human sporotrichosis in a densely populated urban area in São Paulo, Brazil: clinical–epidemiological and therapeutic aspects. Braz J Microbiol 53, 739–748 (2022). https://doi.org/10.1007/s42770-022-00713-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00713-5