Abstract
In recent years, annual cases of gastroenteritis have been reported in the world at high rates, suggesting an association with the consumption of shellfish with enteric viruses in their tissues. Anthropic activities are considered a source of environmental pollution and the main responsible for contamination by pathogenic microorganisms in aquatic environments. The objective of this study was to evaluate, by RT-semi-nested PCR, the presence of astrovirus (AstV) and norovirus genogroup II (NoV GII) in mussels (Mytella falcata) and oysters (Crassostrea brasiliana) collected in two sites of the Lagunar Complex of Cananéia, State of São Paulo, Brazil. A total of 150 samples of mussels and oysters (75 samples each) were analyzed. AstV was not identified in any shellfish sample. NoV GII was detected in 21 samples (14%), 8 mussel samples (38%), and 13 oyster samples (62%). From the 21 positive samples, 16 were analyzed by nucleotide sequencing. The molecular characterization revealed that Brazilian samples were grouped into clades along with other sequences from Brazil, Japan, and Mexico. There was 93.8–100% amino acid sequence similarity among the samples in this study and > 94.9% when compared with the strains isolated from clinical cases in Brazil. The screening of shellfish for the presence of health-significant enteric viruses can help prevent outbreaks among consumers and contribute to the improvement of the estuarine environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The quality assurance of any kind of food is a critical point for food industries and other areas related to food safety [1]. Currently, aquaculture is emerging as one of the most profitable cultures regarding economic and nutritional importance worldwide [2]. However, anthropic activities are directly related to the contamination of aquatic ecosystems due to the release of numerous chemicals from agricultural and urban waste [3]. These contaminants cause environmental degradation and a decrease in the water quality [4]. The distribution of different species of shellfish and the efficiency of their filter-feeding behavior makes these species susceptible to bioaccumulation of pathogens (bacteria, viruses, and parasites) [5] and pollutants as heavy metals [6]. In fact, shellfish, particularly oysters, mussels, and cockles, are successfully used to assess marine pollution [7, 8].
The risk of foodborne disease by shellfish consumption has been recognized in various countries by the food industry [9] and health agencies [10]. The Codex Alimentarius commission released guidelines on the general principles of food hygiene to control viruses in food, with Annex I specifically focusing on controlling norovirus (NoV) and hepatitis A virus (HAV) in shellfish [11]. The guidelines recommended the countries to monitor NoV and HAV in bivalve mollusks following outbreaks of shellfish-borne diseases and high-risk events [12], such as sewage pollution due to system failures and malfunctioning, extreme rainfall events overloading the treatment capacity of sewerage systems, and provision of feces in the water source margins [13]. In fact, when shellfish are grown in polluted water, they tend to bioaccumulate environmentally stable enteric viruses, such as NoV, HAV, and enterovirus (EV) [14].
A review between 1980 and 2012 showed 368 foodborne viral outbreaks associated with shellfish [15]. The oysters (58.4%) were the most frequent shellfish implicated in outbreaks [16]. Shellfish are considered an exquisite and highly marketable product often consumed raw or undercooked [17] and then with a high probability of getting involved in foodborne disease [18]. A large number of shellfish-associated outbreaks have been attributed to enteric viruses, particularly NoV (83.7%) and HAV (12.8%) [16, 19,20,21,22,23,24], causing outbreaks in several countries such as France, Singapore, Japan, and the USA. Astrovirus (AstV) have also been isolated from shellfish, but in lower frequency [24,25,26].
Brazil has relatively few studies on the microbiological quality of shellfish from natural banks. Besides, these studies mainly evaluate coliforms counting in growing water and shellfish tissue [27,28,29,30,31,32] and virus detection in natural banks. Until 2020, few studies focused on detecting enteric viruses in shellfish from farms, mangroves, and estuaries in Brazil [2, 33,34,35,36].
Concentrations of Escherichia coli (E. coli) and coliforms, used as regulatory food safety criteria [37], in shellfish and growing waters can be reduced within a few days due to elimination and inactivation under tidal and environmental influences [38], while viruses can survive for weeks to months in the marine environment [39]. In the mariculture industry, some measures are adopted as an attempt for purification and elimination of pathogens from shellfish flesh before they are placed on the market. Some techniques like depuration are widely used in many countries, where shellfish may be kept in tanks or in natural seawater in order to be purified and suitable for human consumption [40]. Currently, there are methods available for detecting HAV and NoV by real-time RT-PCR [41], but there are no standards establishing the maximum concentrations allowed for these viruses in water samples or food.
The Cananéia region is a source of natural resources that have been exploited by local fishermen for several generations. In recent years, fishermen have observed environmental degradation, resulting in the disappearance of mussels and oysters from their usual collection locations. Estuaries are essential to the establishment of many organisms since this ecosystem is considered a natural habitat for fish, birds, and mammals [42].
Therefore, this investigation analyzed the presence of AstV and NoV GII in mussels (Mytella falcata) and oysters (Crassostrea brasiliana) collected from natural banks of the Estuary Lagunar Complex of Cananéia in the State of São Paulo, southeastern Brazil.
Material and methods
Description of the study area
The research site was Estuary Lagunar Complex of Cananéia, located in the State of São Paulo, in the southeast region of Brazil, between latitudes 24° 40′ S and 25° 05′ S and longitudes 47° 25′ W and 48° 00′ W [43]. The climate is characterized as super humid without a dry season and with excessive rainfall in the summer [44]. The average annual temperature is 23.8 °C, and the average annual rainfall is 2300 mm [43]. This region is recognized as an Atlantic Forest Biosphere Reserve by UNESCO (United Nations Educational, Scientific and Cultural Organization), and it has been described as the third most productive estuary in the world in terms of primary productivity [36].
Sampling and sample processing
Mussels (Mytella falcata) and oysters (Crassostrea brasiliana) wild-caught were sampled bimonthly and analyzed for detection of AstV and NoV GII. The mussels were chosen for this study because of their abundance, wide distribution, and frequent consumption along the Brazilian coast. Moreover, mussels represent an important nutritional resource for low-income populations living in coastal areas. Cananéia is one of the most important oyster-producing areas in São Paulo. The native oysters (Crassostrea brasiliana) are produced in a natural and sustainable way in the region [2], destined for the market, and representing the main source of income for the autochthonous population [36].
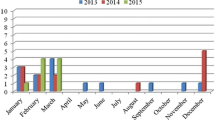
A total of 150 samples constituted by mussels (Mytella falcata; n = 75) and oysters (Crassostrea brasiliana; n = 75) were collected at the Cananéia Estuary (Fig. 1) in two sites: site 1 “Itapanhoapima” (sampling in June and August 2016) and site 2 “Resex do Mandira” (sampling in October 2016, December 2016, and February 2017), adding up to five samplings at the end of the experiment. Each sample was composed of a pool of 25 mussels or 10 oysters randomly chosen. The stomach and digestive diverticula (DT) were isolated by dissection and pooled to obtain 25 g of tissue.
Virus recovery and RNA extraction
Two grams of digestive tissue were used for viral particle elution following the methods described by Keller et al. [35] with the following modifications: mussels and oyster tissues were homogenized with 1:7 (w/v) glycine buffer, pH 9.5 (0.1 M glycine/0.3 M NaCl). After centrifugation at 10,000 × g for 30 min at 4 °C, the pH of the supernatant was adjusted to 7.5, and the same volume of PEG-NaCl (16%, 0.6 M) was added and incubated overnight at 4 °C. Viruses were recovered by centrifugation at 6700 × g for 30 min at 4 °C and the pellet was suspended in 3 mL of Na2HPO4 buffer (0.15 M, pH 9.0). The suspension was clarified by centrifugation at 6700 × g for 30 min at 4 °C. An aliquot of 400 μL supernatant was stored at − 80 °C prior to nucleic acid extraction.
The supernatant was subsequently processed using the TRIzol™ Reagent (Life Technologies, USA), according to the manufacturer’s instructions. Briefly, an aliquot of 200 μL supernatant, 1 mL TRIzol™ reagent, and 200 µL chloroform was added before shaking vigorously for 30 s. The mixture was allowed to stand for 25 min at 8 °C. The mixture was centrifuged at 12,000 × g for 25 min at 4 °C. The supernatant was transferred to a new tube, and 650 μL isopropanol was added. The mixture stands for at least 8 h at − 70 °C. Subsequently, the mixture was centrifuged at 12,000 × g for 25 min at 4 °C. The pellet obtained was briefly washed with 70% ethanol before air drying. The mixture was centrifuged at 12,000 × g for 25 min at 4 °C. The pellet was then resuspended in 20-μL nuclease-free water and frozen at − 80 °C until use.
Reverse transcription
Reverse transcription was performed using an ImProm-II™ Reverse Transcription System (Promega, USA) according to the manufacturer’s instructions. In summary, 4.0-µL viral RNA and 0.5 µg Random Primer (Promega, EUA) were mixed and heated to 70 °C for 5 min, followed by cooling at 4 °C for 5 min. The following reagents were then added: 3.2-µL nuclease-free water (GE Healthcare, USA), 4.0 µL ImProm-II™ 5 × Reaction Buffer, 4.8 µL MgCl2, 1.0 µL dNTP mix, 1.0 µL recombinant RNasim™ Ribonuclease Inhibitor, and 1.0 µL ImProm-II™ Reverse Transcriptase enzyme. The reactions were incubated at 25 °C for 5 min, 42 °C for 60 min, and 70 °C for 15 min. Nuclease-free water was used as a negative control. The synthesized cDNA was used directly in the PCR reactions or frozen at − 80 °C until use.
PCR amplification and semi-nested PCR
Astrovirus
Primers were designed to amplify one ORF1b fragment of the AstV genome [45]. Concentrate fecal samples containing AstV, kindly provided by Dr. Ricardo Luiz Moro de Sousa (FZEA, USP, Brazil), were used as external control. The primer sequences used were as follows: AstV-F 5′-GAYTGGACBCGHTWTGATGG-3′ and AstV-R 5′-KYTTRACCCACATNCCAA-3′ (432-bp amplicon). PCR reactions were performed using GoTaq™ Colorless Master Mix (Promega, USA), according to the manufacturer’s instructions. In summary, 3.0 µL cDNA was mixed with 12.5 µL GoTaq™ Colorless Master Mix 2X, 1.0 µL 10 µM specific primer, sense (AstV-F) and antisense (AstV-R), and 7.5 µL nuclease-free water (GE Healthcare, USA). Reactions, where cDNA was replaced by nuclease-free water, were used as negative controls. The amplification profile for the AstV ORF1b fragment was initial denaturation at 94 °C for 7 min, followed by 50 cycles at 94 °C for 1 min, 48 °C for 1 min, and 72 °C for 1 min and final extension at 72 °C for 10 min, in a Swift™ MaxPro Thermal Cycler (Esco Technologies Inc., EUA) [45].
Norovirus
Primers were designed to amplify a highly conserved region in the RNA-dependent RNA-polymerase (RdRp) in the ORF1 of the NoV genome [46]. Concentrate fecal samples containing NoV genogroup II, kindly provided by Dra. Marize P. Miagostovich (FIOCRUZ, RJ, Brazil), were used as external control. The primer sequences used were as follows: NoV GII specific primers NI 5′-GAATTCCATCGCCCACTGGCT-3′ and NV-4611 5′-CWGCAGCMCTDGAAATCATGG-3′ [47] and a broadly reactive primer NVp110 5′-ACDATYTCATCATCACCATA-3′ [19]. First-round PCR was carried out with primers NVp110 and NV-4611 (273-bp amplicon) using GoTaq™ Colorless Master Mix (Promega, USA), according to the manufacturer’s instructions. In summary, 3.0 µL of cDNA was mixed with 12.5 µL GoTaq™ Colorless Master Mix 2X, 1.0-µL 10-µM specific primer, sense (NV-4611) and antisense (NVp110), and 7.5-µL nuclease-free water (GE Healthcare, USA). Reactions, where cDNA was replaced by nuclease-free water, were used as negative controls. The amplification profile for the 273-bp fragment was initial denaturation at 94 °C for 7 min, followed by 40 cycles at 94 °C for 30 s, 48 °C for 1.20 min, 68 °C for 1 min, and final extension at 68 °C for 10 min. A semi-nested PCR, specific for genogroup II, was performed with primers NVp110 and NI, in order to amplify a 120-bp fragment using GoTaq™ Colorless Master Mix (Promega, USA), according to the manufacturer’s instructions. In summary, 3.0 µL of cDNA was mixed with 12.5 µL GoTaq™ Colorless Master Mix 2X, 1.0-µL 10-µM specific primer, sense (NI) and antisense (NVp110), and 7.5-µL nuclease-free water (GE Healthcare, USA). Reactions, where cDNA was replaced by nuclease-free water, were used as negative controls. The amplification profile for the 120-bp fragment was initial denaturation at 94 °C for 10 min, followed by 25 cycles at 94 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s, and final extension at 72 °C for 10 min. All the PCRs were performed in a Swift™ MaxPro Thermal Cycler (Esco Technologies Inc., EUA).
Analysis of PCR products
Amplicons were subjected to 1% (AstV) and 2% (NoV) agarose gel electrophoresis in Tris–acetate/EDTA buffer. The gels were stained with SYBR™ Gold Nucleic Acid Gel Stain (Life Technologies, USA) for 20 min and visualized under UV light. The images were analyzed using the L-Pix ST photodocumentation system and L-Pix Image Software (Loccus Biotechnology, Brazil). PCR products were purified using ExoSAP-IT™ (USB-Affymetrix), according to the manufacturer’s instructions.
Sequencing reactions were performed in an ABI Prism Genetic Analyzer 3130 (PerkinElmer, Applied Biosystems, EUA) using a BigDye™ Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Life Technologies, EUA), according to the manufacturer’s instructions. Initially, sequence comparisons among the sequences obtained and marked NoV sequences were performed using BLAST software, version 2.0 [48]. Editing and alignment of nucleotide and deduced amino acid sequences were performed using ClustalW software, version 1.4 [49], implemented in BioEdit sequence alignment editor software, version 7.0.9 [50]. Distance matrices were generated from percentages of similarity/identity between nucleotide and deduced amino acid sequences using the global alignment algorithm tool in MatGAT software, version 2.0 [51]. Phylogenetic reconstruction using deduced amino acid sequences was carried out by the neighbor-joining (NJ) method with the JTT + G substitution method. In this analysis, we used bootstrap nodal support for 1000 pseudoreplicates in MEGA 6 software, version 5.0 [52].
Reference strains
The following reference strains NoV GII (with GenBank accession numbers in parentheses) were included in the analysis: GII.1, Hawaii (U07611); GII.2, Melksham (X81879); GII.3, Toronto (U02030); GII.4, Common Florida (AF080549); GII.5, Vermont (AF414423); GII.6, Florida 1993 (AF414407); GII.7, Pennsylvania (AF414409); GII.8, Idaho (AY054299); GII Arg320, (AF190817); Hu/GII/ICB1241/1996/Brazil (DQ386921); Hu/GII/ICB2109/1996/Brazil (DQ386957); Hu/GII/ICB1467/1996/Brazil (DQ386941); Hu/GII/ICB1434/1996/Brazil (DQ386933); Hu/GII/ICB2670/1996/Brazil (DQ386955); Hu/GII.4/Sydney348/97O/AU (DQ078829); Hu/NLV/OCS960352/1996/JP (AB089860); HMO6-201,308-NOR (KX019854); YURI 32,073 (AB083781); Hu/NV/Hokkaido/47/2000/JP (AB240173) Hu/NV/Hokkaido/44/2000/JP (AB240174); NV/Saitama T24eGII/01/JP (AB112306); GII, Swine NoV Sw43/1997/JP (AB074892); GIII, Jena (AJ011099).
Nucleotide sequence accession numbers
The nucleotide sequence data of the RNA-dependent RNA-polymerase (RdRp) in the ORF1 of the NoV genome have been submitted to GenBank and assigned accession numbers MH444874 to MH444889.
Results and discussion
This was the first study evaluating AstV in shellfish in the Cananéia region. Then, this study is important in the knowledge of enteric viruses on the coast, verifying the impact of human occupation in the proximity of the sea, as it can compromise the quality of ecosystems. The AstV was not identified in any of the shellfish samples analyzed in this study (Table 1). An important aspect is that the extracts were not controlled for the presence of inhibitors to avoid false-negative results [25]. Similar results were reported by La Rosa et al. [18], who did not find AstV in fresh and frozen mussels and clams analyzed during official control monitoring programs, collecting from fish markets, harvesting areas, restaurants, and shellfish markets in southern Italy (Sicily).
A few reports have linked AstV infections to shellfish consumption [53]; however, we can find several reports on AstV detection around the world and in different situations. Studies, where the main species studied were pacific oysters, clams, and mussels, there was AstV at a detection rate from 6 to 61% [26, 54]. In a 3-year study conducted in southern France, AstV was detected in 17% of oysters (Crassostrea gigas) located in areas occasionally impacted by sewage and in 50% of mussels (Mytilus galloprovincialis) collected in areas subjected to sewage discharge [25]. One hundred and thirty-seven shellfish, including 61 clams (Tapes decussates and semidecussatus), 54 mussels (Mytilus galloprovincialis), and 22 oysters (Crassostrea gigas), were sampled for environmental monitoring and from the market, and AstV was detected in 18.2% shellfish [55]. AstV was detected in a large number of oysters samples (n = 41) analyzed following a flooding event close to a shellfish production lagoon, and 205 cases of gastroenteritis were related to oyster consumption [56]. From January 2001 to March 2012, 286 fecal specimens were collected from patients in 88 oyster-associated outbreaks with acute nonbacterial gastroenteritis in Osaka City, Japan, with eight strains of AstV detected from five outbreaks [24].
Le Guyader et al. [25] reported a greater presence of AstV in mussel samples than in oysters and a seasonal pattern of AstV presence, with a very low detection during the summer and high detection during winter. The present study did not detect AstV throughout the evaluation period, which comprised the summer and winter seasons; however, it is necessary to highlight the importance of sampling more in different seasons to verify the microbiological quality of shellfish during this time.
Therefore, studies have shown that AstV are widespread in the environment in rivers, hosts, and wastewater [57,58,59,60,61]. The studies cited above demonstrate the presence of AstV in different environments and at different levels of occurrence. However, it should be highlighted that is difficult to compare the occurrences, as the conditions are frequently different, including site conditions, sampling period, and detection methods.
The results of RT-PCR for 150 shellfish samples analyzed showed that 21 (14%) were positive for NoV GII (Table 1). Positive samples included mussels (38%) and oysters (62%) obtained from two sites: five (24%) from Itapanhoapima and 16 (76%) from Resex do Mandira. The sampling sites, Sustainable Development Reserve of Itapanhapima and Extractive Reserve Resex Mandira, had a population of approximately 12,539 inhabitants in 2018. The reserve areas represent 1242.70 ha in Itapanhapima and 1237.35 ha in Resex Mandira. The environment is characterized by dense rainforest, and the biome is Atlantic Forest. The sampling sites presented good parameters for the conservation of natural resources and similar environmental characteristics because they are part of the same Lagunar Complex. It is important to point that two samplings were carried out in Itapanhapima in autumn and winter, while three samplings were carried out in Resex Mandira in spring and summer. The differences in sampling periods were due to legal questions for accessing the areas. Further studies should compare sampling in the same period in order to verify differences in seasonality.
Positive results for NoV were obtained from the two collection sites for shellfish, which may be due to the incorrect elimination or nontreatment of wastewater from local families dedicated to the extraction of natural resources. It is important to note that enteric viruses in seawater, such as NoV, have a close relationship with the types of anthropogenic activities and discharge of sewage-contaminated effluents into the marine coast [62].
Gentry et al. [63] investigated the distribution of norovirus in an estuarine environment and showed that NoV GII represented 9.5% of positive samples identified. A study between 2005 and 2008 investigating 116 shellfish samples (clams, mussels, and oysters) presented four strains showing 100% identity to GII.4 2004 NoVs and eight showing 100% identity to GIIb/Hilversum [46]. Also, GII.4 and GIIb genotypes were found in 7% oyster and mussel samples (n = 42) between September 2003 and January 2004 in Dutch shellfish [64]. Besides, in a 1-year survey in 235 Italian shellfish samples, NoVs were noticed in 14% of samples [65].
The NoVs are often found in water with fecal contamination and are responsible for many enteric diseases associated with oyster consumption [66, 67]. A significant part of human wastewater flows can transport pathogenic microorganisms and reach coastal waters [68]. One contribution to the microbiological contamination of the estuarine environment can come from heavy rains when they are transported to rivers, lakes, and seas along with agricultural runoff [69]. This contamination can also be introduced by discharging sewage from vessels [70]. In this study, 66.6% NoV GII positive samples corresponded to the rainy season in Cananéia, from November to April [71]. The microorganisms can be adsorbed to organic matter, suspended particles, or in the sediment, contributing to their persistence in the environment [70].
In Brazil, most enteric virus detection studies were carried out in the southeast [35, 72] and south regions, mainly in the state of Santa Catarina, the highest national producer of bivalve mollusks [5, 70, 73,74,75,76]. NoV GI was detected in oysters from Vale do Ribeira estuarine complex on two different occasions and oysters’ contamination by NoV GII was also evidenced from the depuration tank [2]. However, NoV was not detected in a study with mussels in the mangrove area of Vitória, Espírito Santo [35]. Souza et al. [76] did not detect any positive samples of NoV GI or NoV GII in oysters harvested from regular cultivation areas in the South. An evaluation of natural microbiological contamination in bivalve mollusk samples over a period of 18 months showed hepatitis A virus, rotavirus A, human adenovirus, and NoV GI [5]. Guarines et al. [77] evaluated 380 mollusks (260 oysters and 120 mussels) from the coast of Pernambuco and found all samples negative for NoV GI or GII. Keller et al. [72] monitored enteric viruses in water and bivalve mollusks from two sites in Vitória Bay over a period of 13 months. They reported 27 (90%) positive samples for the virus, including 11 (41%) samples positive for one virus; nine (33%) samples positive for two viruses, mainly rotavirus A and hepatitis A virus; and seven (26%) samples positive for rotavirus A, hepatitis A virus, and NoV. In a previous study carried out in the same area [35], NoV GII was not detected in mussels. During 16 months, 77 samples of bivalve mollusks (19 oysters and 58 mussels) from Arraial do Cabo, Rio de Janeiro, showed NoV in 32 (41.5%) samples, with NoV GI and GII detected in 9.4% and 87.5%, respectively [78].
In this study, 16 samples (from 21 positives) were successfully sequenced (120 nt in length) and identified as NoVs GII-related sequences. The BLAST analysis confirmed that these sequences were similar to previously determined ORF1b sequences. Three of these sequences were found in oysters and mussels collected in August 2016 (MH444874, MH444875, and MH444876), two in oysters collected in October 2016 (MH444878 and MH444879), seven in oysters and mussels collected in December 2016 (MH444880, MH444881, MH444882, MH444883, MH444884, MH444885, and MH444886) and four in oysters and mussels collected in February 2017 (MH444887, MH444888, MH444889, and MH444877).
Figure 2 shows a cladogram obtained by analysis of deduced amino acid sequences. Samples of NoVs GII were clustered together in a single clade along with other NoV sequences, being phylogenetically closer to sequences from Brazil, Japan, and Mexico. The nucleotide sequences obtained in this study showed values higher than 97.2% identity with norovirus sequences detected in Brazil (Hu/GII/ICB1241/1996/Brazil, Hu/GII/ICB2109/1996/Brazil, Hu/GII/ICB1467/1996/Brazil, Hu/GII/ICB1434/1996/Brazil, and Hu/GII/ICB2670/1996/Brazil) belonging to genotype GII [72]. Another study was conducted by Morillo et al. [73] in fecal samples of patients with gastroenteritis from outbreaks of diarrhea in the State of São Paulo. Thirty-two (15.7%) samples analyzed were positive for norovirus genogroup GII. Comparison of PCR product sequences with GenBank sequences demonstrated 88.8 to 98.8% identity, suggesting the presence of norovirus genogroup GII in outbreaks of gastroenteritis in the State of São Paulo. In Brazil, the NoV GII was described, evidencing a great variety of circulating genotypes, revealing the viral diversity in this region [74].
Cladogram representing a phylogenetic reconstruction based on deduced amino acid sequences using a 120-bp fragment of NoVs RdRp. The NoVs determined in the present study are indicated by filled circles. GenBank accession numbers are shown on the tree. Statistical support was obtained by bootstrapping over 1000 replicates. The scale bar represents the phylogenetic distance among sequences
The strains were analyzed along with a selection of NoV GII strains representative of various genotypes. Large-scale epidemiological studies have documented the sequential onset of several NoV GII variants emerging consecutively worldwide [75,76,77]. It should be highlighted that the analyses were based on a small fragment of a highly conserved region of the NoV genome, then there was no variation and diversity, and all the sequences are similar. For further studies, it would be interesting to analyze another region of the NoV genome (capsid) to verify if there is a relationship with stool, oyster, and water samples worldwide.
Conclusions
The results obtained in this study demonstrate that NoV could be detected in shellfish collected in the Estuary Lagunar Complex of Cananéia, Brazil. The evaluation of sources of pollution is quite important because it may be applied as a management strategy to prevent or mitigate fecal contamination into the estuarine and provide valuable information to the knowledge of risk assessments of many infections caused by different pathogens linked with shellfish consumption.
Introducing specific diagnostic tools for viral foodborne pathogens in the food chain is pivotal to improve the control strategies and also to monitor the epidemiology of the strains circulating in the field.
References
Di Renzo L, Colica C, Carraro A, Cenci Goga B, Marsella LT, Botta R et al (2015) Food safety and nutritional quality for the prevention of non communicable diseases: the Nutrient, hazard Analysis and Critical Control Point process (NACCP). J Transl Med 13:1–13. https://doi.org/10.1186/s12967-015-0484-2
Leal Diego AG, Dores Ramos AP, Marques Souza DS, Durigan M, Greinert-Goulart JA, Moresco V et al (2013) Sanitary quality of edible bivalve mollusks in Southeastern Brazil using an UV based depuration system. Ocean Coast Manag 72:93–100. https://doi.org/10.1016/j.ocecoaman.2011.07.010
da Rocha MP, Dourado PLR, Cardoso CAL, Cândido LS, Pereira JG, de Oliveira KMP et al (2018) Tools for monitoring aquatic environments to identify anthropic effects. Environ Monit Assess 190:1–13
Ballesteros ML, Rivetti NG, Morillo DO, Bertrand L, Amé MV, Bistoni MA (2017) Multi-biomarker responses in fish (Jenynsia multidentata) to assess the impact of pollution in rivers with mixtures of environmental contaminants. Sci Total Environ 595:711–722
Souza DSM, Dominot AFÁ, Moresco V, Barardi CRM (2018) Presence of enteric viruses, bioaccumulation and stability in Anomalocardia brasiliana clams (Gmelin, 1791). Int J Food Microbiol 266:363–371. https://doi.org/10.1016/j.ijfoodmicro.2017.08.004
El Nemr A, El-Said GF, Ragab S, Khaled A, El-Sikaily A (2016) The distribution, contamination and risk assessment of heavy metals in sediment and shellfish from the Red Sea coast. Egypt Chemosphere 165:369–380. https://doi.org/10.1016/j.chemosphere.2016.09.048
Marques JA, Silva de Assis HC, Guiloski IC, Sandrini-Neto L, Carreira RS, Lana PC, Antioxidant defense responses in Mytella guyanensis (Lamarck, (1819) exposed to an experimental diesel oil spill in Paranaguá Bay (Paraná, Brazil). Ecotoxicol Environ Saf 2014(107):269–275. https://doi.org/10.1016/j.ecoenv.2014.06.001
Carter MJ (2005) Enterically infecting viruses: pathogenicity, transmission and significance for food and waterborne infection. J Appl Microbiol 98:1354–1380. https://doi.org/10.1111/j.1365-2672.2005.02635.x
Walker DI, Younger A, Stockley L, Baker-Austin C (2018) Escherichia coli testing and enumeration in live bivalve shellfish – present methods and future directions. Food Microbiol 73:29–38. https://doi.org/10.1016/j.fm.2017.12.006
Cormier J, Gutierrez M, Goodridge L, Janes M (2014) Concentration of enteric virus indicator from seawater using granular activated carbon. J Virol Methods 196:212–218
FAO. FAO 2008.
Torok V, Hodgson K, McLeod C, Tan J, Malhi N, Turnbull A (2018) National survey of foodborne viruses in Australian oysters at production. Food Microbiol 69:196–203. https://doi.org/10.1016/j.fm.2017.08.014
Campos CJA, Kershaw S, Morgan OC, Lees DN (2017) Risk factors for norovirus contamination of shellfish water catchments in England and Wales. Int J Food Microbiol 241:318–324. https://doi.org/10.1016/j.ijfoodmicro.2016.10.028
Mesquita JR, Vaz L, Cerqueira S, Castilho F, Santos R, Monteiro S et al (2011) Norovirus, hepatitis A virus and enterovirus presence in shellfish from high quality harvesting areas in Portugal. Food Microbiol 28:936–941. https://doi.org/10.1016/j.fm.2011.01.005
Bellou M, Kokkinos P, Vantarakis A (2013) Shellfish-borne viral outbreaks: a systematic review. Food Environ Virol 5:13–23. https://doi.org/10.1007/s12560-012-9097-6
Hodgson KR, Torok VA, Turnbull AR (2017) Bacteriophages as enteric viral indicators in bivalve mollusc management. Food Microbiol 65:284–293. https://doi.org/10.1016/j.fm.2017.03.003
Padovan AC, Neave MJ, Munksgaard NC, Gibb KS. Multiple approaches to assess the safety of artisanal marine food in a tropical estuary. Environ Monit Assess 2017;189. https://doi.org/10.1007/s10661-017-5842-5.
La Rosa G, Fratini M, Vennarucci VS, Guercio A, Purpari G, Muscillo M (2012) GIV noroviruses and other enteric viruses in bivalves: a preliminary study. New Microbiol 35:27–34
Le Guyader F, Neill FH, Estes MK, Monroe SS, Ando T, Atmar RL (1996) Detection and analysis of a small round-structured virus strain in oysters implicated in an outbreak of acute gastroenteritis. Appl Environ Microbiol 62:4268–4272. https://doi.org/10.1128/aem.62.11.4268-4272.1996
Le Guyader FS, Neill FH, Dubois E, Bon F, Loisy F, Kohli E et al (2003) A semiquantitative approach to estimate Norwalk-like virus contamination of oysters implicated in an outbreak. Int J Food Microbiol 87:107–112. https://doi.org/10.1016/S0168-1605(03)00058-8
Ng TL, Chan PP, Phua TH, Loh JP, Yip R, Wong C et al (2005) Oyster-associated outbreaks of norovirus gastroenteritis in Singapore. J Infect 51:413–418. https://doi.org/10.1016/j.jinf.2004.11.003
Le Guyader FS, Krol J, Ambert-Balay K, Ruvoen-Clouet N, Desaubliaux B, Parnaudeau S et al (2010) Comprehensive analysis of a norovirus-associated gastroenteritis outbreak, from the environment to the consumer. J Clin Microbiol 48:915–920. https://doi.org/10.1128/JCM.01664-09
Lunestad BT, Maage A, Roiha IS, Myrmel M, Svanevik CS, Duinker A (2016) An outbreak of norovirus infection from shellfish soup due to unforeseen insufficient heating during preparation. Food Environ Virol 8:231–234. https://doi.org/10.1007/s12560-016-9245-5
Iritani N, Kaida A, Abe N, Kubo H, Sekiguchi J, Yamamoto SP et al (2014) Detection and genetic characterization of human enteric viruses in oyster-associated gastroenteritis outbreaks between 2001 and 2012 in Osaka City. Japan J Med Virol 86:2019–2025
Le Guyader F, Haugarreau L, Miossec L, Dubois E, Pommepuy M (2000) Three-year study to assess human enteric viruses in shellfish. Appl Environ Microbiol 66:3241–3248. https://doi.org/10.1128/AEM.66.8.3241-3248.2000
Ming HX, Fan JF, Wu LJ, Liang YB (2013) Prevalence of human enteric viruses and a potential indicator of contamination in shellfish in China. J Food Saf 33:209–214. https://doi.org/10.1111/jfs.12041
de Farias MF, de Almeida R-B, de Carvalho FCT, Silva CM, dos Reis EMF, Costa RA et al (2010) Condições Microbiológicas De Tagelus Plebeius (Lightfoot, 1786) (Mollusca: Bivalvia: Solecurtidae) E Da Água No Estuário Do Rio Ceará, Em Fortaleza - CE. Bol Do Inst Pesca 36:135–142
Ramos RJ, Pereira MA, Miotto LA, de Faria LFB, Silveira Junior N, Vieira CRW (2010) Microrganismos indicadores de qualidade higiênico-sanitária em ostras (Crassostrea gigas) e águas salinas de fazendas marinhas localizadas na Baía Sul da Ilha de Santa Catarina. Brasil Rev Do Inst Adolfo Lutz 69:29–37
Sande D, Melo TA, Oliveira GSA, Barreto L, Talbot T, Boehs G et al (2010) Prospecção de moluscos bivalves no estudo da poluição dos rios Cachoeira e Santana em Ilhéus, Bahia, Brasil. Brazilian J Vet Res Anim Sci 47:190–196
da Silveira MA, Leão MVP, dos Santos SSF, Jorge AOC, Gonçalves CR, Qualidade sanitária da água e de bivalves Iphigenia brasiliensis (Lamarck, (1818) na praia do Jabaquara, Paraty. RJ Rev Biociências 2011:17
Barbieri E, Bondioli AC, Woiciechovski E, Zapotoski SMK (2012) Microbiology quality of the oysters cultivation water marketed in Cananeia-SP. Brazil O Mundo Da Saúde 36:541–547
Doi SA, de Cardoso Oliveira AJF, Barbieri E (2015) Determinação de coliformes na água e no tecido mole das ostras extraídas em Cananéia, São Paulo. Brasil Eng Sanit e Ambient 20:111–118. https://doi.org/10.1590/S1413-41522015020000125658
Sincero TCM, Levin DB, Simões CMO, Barardi CRM (2006) Detection of hepatitis A virus (HAV) in oysters (Crassostrea gigas). Water Res 40:895–902
Rigotto C, Victoria M, Moresco V, Kolesnikovas CK, Corrêa AA, Souza DSM et al (2010) Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis. South Brazil J Appl Microbiol 109:1979–1987
Keller R, Justino JF, Cassini ST (2013) Assessment of water and seafood microbiology quality in a mangrove region in Vitória. Brazil J Water Health 11:573–580
Leal DAG, Souza DSM, Caumo KS, Fongaro G, Panatieri LF, Durigan M et al (2018) Genotypic characterization and assessment of infectivity of human waterborne pathogens recovered from oysters and estuarine waters in Brazil. Water Res 137:273–280
Brake F, Ross T, Holds G, Kiermeier A, McLeod C (2014) A survey of Australian oysters for the presence of human noroviruses. Food Microbiol 44:264–270. https://doi.org/10.1016/j.fm.2014.06.012
da Silva Luz I, Miagostovich MP. Norovírus em alimentos. Vigilância Sanitária Em Debate Soc Ciência Tecnol (Health Surveill under Debate Soc Sci Technol Em Debate 2017;5:100–15.
Lodder-Verschoor F, de Roda Husman AM, Van Den Berg H, Stein A, van Pelt-Heerschap HML, Van der Poel WHM (2005) Year-round screening of noncommercial and commercial oysters for the presence of human pathogenic viruses. J Food Prot 68:1853–1859
FDA. FDA 2007.
ISO. ISO 15216–1, 2017. n.d.
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
del Favero JM, Dias JF (2015) Juvenile fish use of the shallow zone of beaches of the Cananéia-Iguape coastal system, southeastern Brazil. Brazilian J Oceanogr 63:103–114
De Oliveira FC, Hanazaki N (2011) Ethnobotany and ecological perspectives on the management and use of plant species for a traditional fishing trap, southern coast of São Paulo. Brazil J Environ Manage 92:1783–1792. https://doi.org/10.1016/j.jenvman.2011.02.002
Tse H, Chan W-M, Tsoi H-W, Fan RYY, Lau CCY, Lau SKP et al (2011) Rediscovery and genomic characterization of bovine astroviruses. J Gen Virol 92:1888–1898
Terio V, Martella V, Moschidou P, Di Pinto P, Tantillo G, Buonavoglia C (2010) Norovirus in retail shellfish. Food Microbiol 27:29–32. https://doi.org/10.1016/j.fm.2009.07.005
Yuen LKW, Catton MG, Cox BJ, Wright PJ, Marshall JA (2001) Heminested multiplex reverse transcription-PCR for detection and differentiation of Norwalk-like virus genogroups 1 and 2 in fecal samples. J Clin Microbiol 39:2690–2694. https://doi.org/10.1128/JCM.39.7.2690-2694.2001
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser., vol. 41, [London]: Information Retrieval Ltd., c1979-c2000.; 1999, p. 95–8.
Campanella JJ, Bitincka L, Smalley J (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4:1–4
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725–9.
Yamashita T, Kobayashi S, Sakae K, Nakata S, Chiba S, Ishihara Y et al (1991) Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis 164:954–957. https://doi.org/10.1093/infdis/164.5.954
Elamri DE, Aouni M, Parnaudeau S, Le Guyader FS (2006) Detection of human enteric viruses in shellfish collected in Tunisia. Lett Appl Microbiol 43:399–404. https://doi.org/10.1111/j.1472-765X.2006.01978.x
Gabrieli R, Macaluso A, Lanni L, Saccares S, Di Giamberardino F, Cencioni B et al (2007) Enteric viruses in molluscan shellfish. New Microbiol 30:471–475
Le Guyader FS, Le Saux JC, Ambert-Balay K, Krol J, Serais O, Parnaudeau S et al (2008) Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J Clin Microbiol 46:4011–4017. https://doi.org/10.1128/JCM.01044-08
Miagostovich MP, Ferreira FFM, Guimarães FR, Fumian TM, Diniz-Mendes L, Luz SLB et al (2008) Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia. Brazil Appl Environ Microbiol 74:375–382
Fumian TM, Vieira CB, Leite JPG, Miagostovich MP (2013) Assessment of burden of virus agents in an urban sewage treatment plant in Rio de Janeiro. Brazil J Water Health 11:110–119
Nuñez LFN, Parra SHS, Carranza C, Astolfi-Ferreira CS, Buim MR, Ferreira AJP (2016) Detection and molecular characterization of chicken astrovirus associated with chicks that have an unusual condition known as “white chicks” in Brazil. Poult Sci 95:1262–1270
Siqueira JAM, de Souza OD, de Carvalho TCN, Portal TM, Justino MCA, da Silva LD et al (2017) Astrovirus infection in hospitalized children: molecular, clinical and epidemiological features. J Clin Virol 94:79–85
Alves CDBT, Budaszewski RF, Torikachvili M, Streck AF, Weber MN, Cibulski SP et al (2018) Detection and genetic characterization of Mamastrovirus 5 from Brazilian dogs. Brazilian J Microbiol 49:575–583
Gularte JS, Girardi V, Demoliner M, de Souza FG, Filippi M, Eisen AKA et al (2019) Human mastadenovirus in water, sediment, sea surface microlayer, and bivalve mollusk from southern Brazilian beaches. Mar Pollut Bull 142:335–349
Gentry J, Vinjé J, Guadagnoli D, Lipp EK (2009) Norovirus distribution within an estuarine environment. Appl Environ Microbiol 75:5474–5480. https://doi.org/10.1128/AEM.00111-09
Boxman ILA, Tilburg JJHC, te Loeke NAJM, Vennema H, Jonker K, de Boer E et al (2006) Detection of noroviruses in shellfish in the Netherlands. Int J Food Microbiol 108:391–396. https://doi.org/10.1016/j.ijfoodmicro.2006.01.002
Croci L, Losio MN, Suffredini E, Pavoni E, Di Pasquale S, Fallacara F et al (2007) Assessment of human enteric viruses in shellfish from the northern Adriatic sea. Int J Food Microbiol 114:252–257. https://doi.org/10.1016/j.ijfoodmicro.2006.09.015
Lees D (2000) Viruses and bivalve shellfish. Int J Food Microbiol 59:81–116. https://doi.org/10.1016/S0168-1605(00)00248-8
Atmar RL (2010) Noroviruses: state of the art. Food Environ Virol 2:117–126
Le Saux JC, Serais O, Krol J, Parnaudeau S, Salvagnac P, Delmas G, et al. Evidence of the presence of viral contamination in shellfish after short rainfall events. J Shellfish Res Open Access Version Http//Archier Ifremer Fr/Doc/00066/17736 2009.
Bigoraj E, Kwit E, Chrobocińska M, Rzeżutka A (2014) Occurrence of norovirus and hepatitis A virus in wild mussels collected from the Baltic Sea. Food Environ Virol 6:207–212. https://doi.org/10.1007/s12560-014-9153-5
Moresco V, Viancelli A, Nascimento MA, Souza DSM, Ramos APD, Garcia LAT et al (2012) Microbiological and physicochemical analysis of the coastal waters of Southern Brazil. Mar Pollut Bull 64:40–48
Ferro de Godoy D, Andriolo A, de Fatima Filla G. The influence of environmental variables on estuarine dolphins (Sotalia guianensis) spatial distribution and habitat used in the Estuarine Lagunar Complex of Cananéia, southeastern Brazil. Ocean Coast Manag 2015;106:68–76. https://doi.org/10.1016/j.ocecoaman.2015.01.013.
Keller R, Pratte-Santos R, Scarpati K, Martins SA, Loss SM, Fumian TM et al (2019) Surveillance of enteric viruses and thermotolerant coliforms in surface water and bivalves from a mangrove estuary in southeastern Brazil. Food Environ Virol 11:288–296
Corrêa A de A, Rigotto C, Moresco V, Kleemann CR, Teixeira AL, Poli CR, et al. The depuration dynamics of oysters (Crassostrea gigas) artificially contaminated with hepatitis A virus and human adenovirus. Mem Inst Oswaldo Cruz 2012;107:11–7.
Souza DSM, Piazza RS, Pilotto MR, do Nascimento M de A, Moresco V, Taniguchi S, et al. Virus, protozoa and organic compounds decay in depurated oysters. Int J Food Microbiol 2013;167:337–45.
Sobral Marques Souza D, Miura T, Le Mennec C, Barardi CRM, Le Guyader FS. Retention of rotavirus infectivity in mussels heated by using the French recipe Moules Marinières. J Food Prot 2015;78:2064–9.
Souza DSM, Ramos APD, Nunes FF, Moresco V, Taniguchi S, Leal DAG et al (2012) Evaluation of tropical water sources and mollusks in southern Brazil using microbiological, biochemical, and chemical parameters. Ecotoxicol Environ Saf 76:153–161
Guarines KM, Mendes RPG, Cordeiro MT, Miagostovich MP, Gil L, Green KY, et al. Absence of norovirus contamination in shellfish harvested and commercialized in the Northeast coast of Brazil. Brazilian J Med Biol Res 2020;53.
Sarmento SK, Guerra CR, Malta FC, Coutinho R, Miagostovich MP, Fumian TM. Human norovirus detection in bivalve shellfish in Brazil and evaluation of viral infectivity using PMA treatment. Mar Pollut Bull 2020;157:111315.
Castilho JG, Munford V, Resque HR, Fagundes-Neto U, Vinjé J, Rácz ML (2006) Genetic diversity of norovirus among children with gastroenteritis in São Paulo State. Brazil J Clin Microbiol 44:3947–3953. https://doi.org/10.1128/JCM.00279-06
Morillo SG, Audrey C, Carmona R de CC, Timenetsky M do CST. Identification and molecular characterization of norovirus in São Paulo State, Brazil. Brazilian J Microbiol 2008;39:619–22.
da Silva PT, Peiró JR, Mendes LCN, Ludwig LF, de Oliveira-Filho EF, Bucardo F et al (2016) Human norovirus infection in Latin America. J Clin Virol 78:111–119. https://doi.org/10.1016/j.jcv.2016.03.016
Gallimore CI, Iturriza-Gomara M, Xerry J, Adigwe J, Gray JJ (2007) Inter-seasonal diversity of norovirus genotypes: emergence and selection of virus variants. Arch Virol 152:1295–1303. https://doi.org/10.1007/s00705-007-0954-9
Vinje J, Koopmans MPG (1996) Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis 174:610–615
Lopman B, Vennema H, Kohli E, Pothier P, Sanchez A, Negredo A et al (2004) Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 363:682–688
Acknowledgements
The authors want to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the doctoral scholarship and to the Laboratory of Molecular and Cellular Biology of Microorganisms, under the responsibility of Dr. Sandro Roberto Valentini and Prof. Dr. Cleslei Fernando Zanelli, from the Faculty of Pharmaceutical Sciences of the Paulista State University “Julio de Mesquita Filho,” Araraquara Campus for the sequencing of samples.
Author information
Authors and Affiliations
Contributions
AVG and AMF conceived the study and wrote the manuscript; AMF and RLMS designed the study protocol; JEMB and SHSG performed the microbiology experiments and interpretation of the data; EB assessed and acquired samples of mussels and oysters. All authors contributed to the analysis and interpretation of the data and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasquez-García, A., Mejia-Ballesteros, J.E., de Godoy, S.H.S. et al. Norovirus GII and astrovirus in shellfish from a mangrove region in Cananéia, Brazil: molecular detection and characterization. Braz J Microbiol 53, 317–326 (2022). https://doi.org/10.1007/s42770-021-00631-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00631-y