Abstract
As portable and wearable electronic devices are rapidly developing, there is an urgent need for flexible and robust thermally conductive electromagnetic interference shielding materials to address the associated electromagnetic pollution and overheating issues. Herein, multifunctional poly(p-phenyl-2,6-phenylene bisoxazole) nanofiber/boron nitride nanosheet/Ti3C2Tx MXene nanosheet (PBO/BN/MXene) composite papers are prepared by a gel microparticle-mediated ordered assembly process with the aid of vacuum-assisted filtration. Nacre-like “brick and mortar” structure, segregated structure and sandwich structure are integrated into the composite paper, so that efficient thermally and electrically conductive networks have been established. When the BN and MXene contents are 29.2 wt% and 41.7 wt%, the 13 μm thick composite paper exhibits an EMI shielding performance of 31.8 dB and a thermal conductivity of 26.1 W/mK, markedly superior to those of the control samples without the ordered structures. Meanwhile, because of the unique architecture and inherent advantages of the building blocks, the composite paper exhibits extremely low coefficient of thermal expansion (~ 1.43 ppm/K), excellent mechanical properties, and outstanding thermal stability and flame retardance, making it highly advantageous for practical applications in electronic devices. This work offers a promising approach for fabricating high-performance multifunctional composites by constructing efficient filler networks.
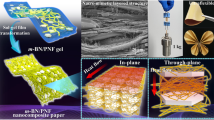
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The constant development of multifunctional miniaturized electronics with high levels of integration and power density has led to widespread concern about electromagnetic interference (EMI) and high-energy hotspots [1, 2]. Specifically, electromagnetic radiation from electronic devices can not only cause malfunctions in surrounding electronic equipment, but also threaten human health [3,4,5]. High-energy hotspots also affect equipment performance and lifespan, as well as cause fires in severe cases [6,7,8]. The conventional EMI shielding and/or heat dissipation materials consisting of metals and ceramics have disadvantages of high stiffness, high brittleness and high density [9]. Therefore, flexible and lightweight polymer composites with high EMI shielding effectiveness (EMI SE) and thermal conductivity (TC) are very desirable.
MXene (Ti3C2Tx) nanosheets with surface functional groups (− OH, − O and − F) are widely used to build high-performance EMI shielding materials because of their metallic electrical conductivity (15,100 S cm− 1) and good mechanical properties [10,11,12]. Recently, MXenes have been composited with polymers to form nacre-like structures by blending [13, 14] or alternating layer-by-layer approaches [15, 16], to reduce EMI intensity through multiple internal reflections of electromagnetic radiation. For the blending method, an effective conductive pathway is usually formed at high filler contents, since MXene nanosheets are distributed in a disordered manner in the polymer matrix. For the alternating layer-by-layer approach, the as-formed highly concentrated MXene layer allows for a more efficient conductive pathway to be easily achieved, thus significantly reducing the amount of MXene required. In addition, MXene nanosheets can form segregated structure in the composites to enhance the multiple reflections of electromagnetic radiation, and the excluded volume effect of the structure can significantly improve the effective concentration of the filler [17, 18]. Limited by the relatively low TC of MXene [19, 20], most of the reported MXene-based composites have a TC < 10 W/mK. Different from MXene, boron nitride (BN), another 2D sheet, has excellent TC (~ 600 W/mK). Therefore, BN is widely used to construct high thermally conductive composites [21, 22]. However, the BN-based composites are generally transparent to EM waves because of their inherent electrical insulating nature [23].
As far as the polymer matrix is concerned, poly(p-phenyl-2,6-phenylene bisoxazole) (PBO) has attracted great research interest in the construction of high-performance composites due to its remarkable fire safety, mechanical properties and TC [24,25,26,27,28,29]. For example, Liu et al. developed a superhydrophobic wave-transparent nanocomposite paper with a tensile strength of 271.6 MPa using PBO nanofibers and polytetrafluoroethylene (PTFE) particles [26]. Zhang et al. developed a temperature-resistant phase change composite with a TC of 22.38 W/mK using 50 wt% PBO fibers distributed in D-mannitol directionally [28]. Therefore, several recent reports have demonstrated laminating PBO with MXene or BN nanosheets to fabricate EMI shielding and/or TC composites with excellent overall performance. Wang et al. prepared MXene/PBO nanofiber films by the sol-gel-film conversion method, exhibiting an EMI SE of 35 dB, an in-plane TC of 5.82 W/mK, a tensile strength of 125.1 MPa, and flame retardancy at 70 wt% filler content [30]. Chen et al. prepared BN/PBO nanocomposite paper by the same method, demonstrating an in-plane TC of 21.34 W/mK, a tensile strength of 206 MPa, and flame retardancy at 10 wt % filler content [31]. For practical application, a low coefficient of thermal expansion (CTE) to reduce thermal-induced expansion mismatch with electronic components is also desirable [32], but has yet to be reported in thermally conductive EMI shielding multifunctional composites.
In the study, we sequentially assembled PBO nanofiber/BN nanosheet (PBN) gel microparticles and PBN@MXene gel microparticles to prepare multifunctional nanocomposite papers. The orderly assembled nanocomposite paper was featured with three structures: a nacre-like “brick and mortar” structure, a segregated structure, and a sandwich structure, which contributed greatly to the establishment of the efficient electrical/thermal conductive networks. A combination of excellent EMI SE and in-plane TC has been obtained as a result. Because of the unique architecture and the inherent advantages of the building blocks, the composite papers also exhibited outstanding mechanical properties, low CTE, electrical insulation, thermal stability and flame retardancy. Therefore, the novel nanocomposite paper provides a new platform for EMI shielding and heat dissipation in high-power flexible electronics.
Experimental Section
Materials
Commercial hexagonal boron nitride (h-BN, purity > 99.5%, size ≤ 45 μm) was purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). PBO fibers were got from the Zhongke Jinqi New Material Technology Co., Ltd. (Zhejiang, China). MAX (Ti3AlC2) (200 Meshes, 98%) was bought from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Hydrochloric acid (HCl, 37 wt%) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Lithium fluoride (LiF, AR, 98.5%) was bought from Alfa Aesar Chemicals Co., Ltd. (Shanghai, China). Methane sulfonic acid (MSA, 99%) and trifluoroacetic acid (TFA, 99.9%) were bought from J & K Scientific Co., Ltd. (Beijing, China). Ethyl acetate (EA, GR) and isopropanol (IPA, GR) were purchased from Concord Technology Co., Ltd. (Tianjin, China).
Preparation of PBO Nanofibers, BN Nanosheets, and Ti3C2Tx MXene Nanosheets
PBO nanofibers were obtained by exfoliating PBO fibers [33]. For example, 1 g PBO fibers were added to a mixture of MSA (50 g) and TFA (50 g) and stirred at room temperature for at least three days, and then a homogeneous PBO nanofiber sol (1 wt%) was obtained.
BN nanosheets were prepared by exfoliating h-BN bulks [34]. The original h-BN powder was thermally annealed at 900 °C for 2 h, and then dispersed in IPA and sonicated for 24 h. Finally, the upper BN nanosheets were collected by centrifugation and dried in an oven at 30 °C.
Ti3C2Tx MXene nanosheets were prepared by etching the Ti3AlC2 MAX phase [35]. Typically, 3.2 g LiF was added to 40 mL 9 M HCl solution. Then, 2 g MAX powders were added slowly to the above solution. The obtained mixture was stirred at 35 °C for 24 h and then washed several times with ultrapure water under the assistance of centrifugation (3500 rpm, 5 min) until the pH was close to 7. Next, the precipitate was dispersed into 30 mL ethanol under sonication for 60 min and centrifuged at 10,000 rpm for 10 min. The obtained precipitate was treated in 30 mL water under sonication for 20 min in a nitrogen atmosphere. The mono- and/or few-layered MXene dispersion can be obtained after centrifugation at 5000 rpm for 5 min. The resulting MXene dispersion was freeze-dried for further use.
Preparation of O-PBN/MXene Nanocomposite Papers
Dispersion of BN nanosheets was obtained by adding 35 mg BN nanosheets to MSA/TFA (3.25 g/3.25 g) mixture and sonicating for 10 min. Then, 3.5 g of 1 wt% PBO nanofiber sol was added to the BN dispersion, and a homogeneous PBO/BN acid sol was obtained after 15 min of sonication as well as 10 min of high-speed mixing with a planetary mixer (2000 rpm). Next, a mixture of MSA/EA/H2O (7.66 g/2.33 g/0.30 g) was added dropwise to the acid sol under stirring to induce gelation. The resulting acid gel was replaced with IPA for 12 h and then processed under an ultrasonic cell grinder for 1 h to obtain a dispersion of PBN microparticles (1.2 mg/mL, the mass ratio of PBO to BN is 1:1). For comparison, PBN microparticles with mass fractions of BN nanosheets of 0, 10, 30, and 70%, respectively, were prepared. MXene dispersions were obtained by dispersing different qualities of MXene nanosheets into 20 mL of ultrapure water. Then, 5.83 mL of PBN dispersion (microparticle containing 50 wt% of BN) was mixed with MXene dispersion at 2000 rpm with the planetary mixer for 10 min to obtain PBN@MXene dispersion.
O-PBN/MXene papers were manufactured by a step-by-step vacuum-assisted filtration method. Briefly, to form the bottom layer, 5.83 mL of PBN dispersion was filtered through a hydrophilic PTFE microfiltration membrane with 0.44 μm pore size. Then, the PBN@MXene dispersion was filtered to obtain the middle layer. Next, 5.83 mL of the PBN dispersion was filtered again to form the upper layer. After filtration, the samples were compressed at 20 MPa to remove most of the solvent and then dried in a vacuum oven at 30 °C. O-PBN/MXene papers with 0, 12.5, 25.0, 41.7, and 54.3 wt% of MXene, named O-0%, O-12.5%, O-25.0%, O-41.7%, and O-54.3%, respectively, were obtained. In the control experiment, PBN/MXene composite papers without sandwich structure (P1-41.7% and P1-54.3%) were obtained by a one-step filtration of the mixture of PBN and PBN@MXene dispersions. PBO/BN/MXene (PBM) microparticles were obtained using the preparation method of the PBN microparticles. The PBN/PBM/PBN composite papers with a sandwich structure (P2-41.7% and P2-54.3%) were prepared by stepwise filtration, and PBO/BN/MXene composite paper (B-41.7%) was fabricated by one-step filtration of PBM microparticles. The detailed formulations were summarized in Table S1. The structural features of the different samples (O-0%, O-41.7%, P1-41.7%, P2-41.7% and B-41.7%) are shown in Fig. S1.
Characterization
Scanning electron microscope (SEM) images were obtained on a JEOL JSM-7500 F at an acceleration voltage of 5 kV. Energy dispersive spectroscopy (EDS) elemental mapping were obtained on a JEOL JSM 7200 F. Transmission electron microscopy (TEM) images were obtained on a JEOL-JEM 2100 F at 200 kV. Atomic force microscopy (AFM) investigation was performed on a Bruker Dimension ICON. Dynamic light scattering (DLS) on the Malvern Zetasizer Nano ZS was used to investigate the size distribution of the MXenes of the synthesized dispersions. Fourier transform infrared spectroscopy (FTIR) was acquired on BRUKER TENSOR 27 FTIR spectrometer from 400 to 4000 cm− 1. X-ray diffractometer (XRD, Empyrean) with Cu Kα radiation was employed to analyze the crystalline structure of samples at a scan speed of 5 °/min from 3° to 60°. X-ray photoelectron spectrometer (XPS, ESCALAB250XI) with a monochromatic Al Kα radiation (1486.6 eV, 500 μm spot) was used to analyze the surface chemical composition of the samples. The C1s line (284.8 eV) from adventitious carbon was commonly used for calibration. Deconvolution and peak fitting of XPS spectra was done using the Thermo Avantage software. The mechanical tests of the composite papers were performed on a UTM-16,555 tensile tester (Shenzhen Suns Technology Stock Co., Ltd.) at a rate of 1 mm/min. The linear coefficient of thermal expansion (CTE) of the composite papers in the temperature range from 30 to 400 °C was tested using a thermal mechanical analyzer (TMA, TA-Q400). The electrical conductivity and surface resistance of samples were measured by a four-probe instrument (MCP-T700) and Keithley 6517B, respectively. Thermogravimetric analysis (TGA) was carried out on a thermal gravimetric analyzer (Pyris 1, PE) with a heating rate of 10 °C/min from 30 to 800 °C in an air atmosphere. The thermal conductivity (\(\text{T}\text{C}\)) was calculated according to the equation \(\text{T}\text{C}=\alpha \times \rho \times {C}_{p}\), where \(\alpha\), \(\rho\) and \({C}_{p}\) are thermal diffusivity, density, and specific heat capacity of the papers, respectively, and \(\alpha\) was measured by LFA 447 (NETZSCH, Germany), \(\rho\) was obtained by weighing, \({C}_{p}\) was measured by DSC (TA-Q2000) using the sapphire method. EMI shielding performance in the X-Band was tested using an Agilent E5071c vector network analyzer. Based on the scattering parameters (S11 and S12), the power coefficients for reflection (R), transmission (T), absorption (A), total EMI SE (SET), reflection SE (SER), absorption SE (SEA) and thickness-specific SE (SE/t) were calculated as follows:
Results and Discussion
Fabrication Strategy and Characterization of Nanocomposite Papers
The orderly assembled PBO/BN/MXene (O-PBN/MXene) nanocomposite paper is shown schematically in Fig. 1. The procedure can be summarized in four steps. (1) Preparation of PBO nanofibers [33], BN nanosheets [34], and Ti3C2Tx MXene nanosheets [35]. (2) A homogeneous mixture of PBO nanofibers and BN nanosheets undergoes a sol-gel-solvent replacement process to form PBO/BN (PBN) gel (Fig. S2), which is then ultrasonically comminuted into PBN gel microparticles. (3) MXene nanosheets are adsorbed onto the surface of PBN microparticles via hydrogen bonding and van der Waals forces, to form eggshell-like PBN@MXene gel microparticles. (4) Sandwich structured nanocomposite papers are constructed by vacuum-assisted filtration of dispersions of PBN, PBN@MXene and PBN microparticles in turn. The nanosheets and the nanofibers form a nacre-like structure in the whole paper, in which PBN@MXene further forms a segregated structure through the excluded volume effect in the middle layer [17]. The formulations of the various composite papers prepared are shown in Table S1. It should be emphasized that benefiting from the strong interfacial interaction between the PBO nanofibers and the BN nanosheets (Fig. S3), the mechanical properties and TC of the PBN layer are optimal at a PBO to BN mass ratio of 1:1 (Fig. S4), so we have used PBN microparticles containing 50 wt% BN throughout the subsequent discussion.
The compositions and structures of the building blocks of the composite paper have been characterized in detail. PBO nanofibers exfoliated from commercial PBO fibers possess a diameter of 7–26 nm (SEM and TEM images in Fig. S5). BN nanosheets peeled from h-BN possess a lateral size of 100–500 nm and a thickness of approximately 20 nm (SEM and TEM images in Fig. S6). Fig. S7 demonstrates that Ti3C2Tx MXene nanosheets have been successfully exfoliated from Ti3AlC2 MAX (SEM, TEM and AFM images, DLS analysis and XRD patterns). In particular, the DLS technique shows that the average size of the MXene sheets in dispersion is approximately 0.80 μm. AFM investigation shows that the MXene sheet thickness is approximately 2 nm. Figure 2a shows a typical SEM image of the as-formed PBN microparticle, and the corresponding elemental mappings confirm the presence of characteristic elements B and N from BN and PBO. For PBN@MXene microparticle, the surface becomes very rough and the main enrichment elements change to Ti and F from MXene, indicating the formation of eggshell-like structure via capsulating the PBN microparticles by MXene nanosheets (Fig. 2c). TEM images clearly show the three building units of PBO, BN, and MXene in the different microparticles (Fig. 2b and d), which is consistent with the SEM images.
Cross-section of the nanocomposite papers shows a nacre-like “brick and mortar” (“nanosheet and nanofiber”) morphology of the three layers (Fig. 2e and Fig. S8). There are no obvious voids between the three layers. Elemental mappings show that element B is uniformly distributed throughout the composite paper, while the element Ti is concentrated in the middle layer of the paper, verifying the sandwich structure and good integration of the composite paper.
Figure 2f compares XRD patterns of PBO, BN, MXene, and PBN layer and PBN@MXene layer in the composite paper. The peaks of PBN and PBN@MXene layers are generally consistent with the crystal plane locations of the pristine PBO, BN and MXene, indicating good preservation of the original crystal structures during the assembly process. The intensity ratios of (002) to (100) peak for BN in PBN and PBN@MXene layers are as high as 58 and 85, respectively, far exceed that of the pristine BN (with a ratio of 10), demonstrating the orientation of BN in the composite paper [36].
Figure S9 exhibits FTIR spectra of MXene, PBN and PBN@MXene. The − OH and C = O stretching vibration peaks of MXene are located at 3453 cm− 1 and 1639 cm− 1, respectively. Meanwhile, the stretching vibration of C-O (1020 cm− 1) and the bending vibration of -OH (1362 cm− 1) can be found. This result indicates that the synthesized MXene has a large number of oxygen-containing groups [37]. The spectra of PBN exhibits the stretching vibrations of -OH (-NH) and C = O at 3434 cm− 1 and 1628 cm− 1, respectively. Vibration peaks of other oxygen-containing groups (C-O (1056 cm− 1), N-O (1010 cm− 1)) are also observed [38]. After compounding with MXene, the vibration peaks of -OH (-NH) and C = O of PBN move to 3444 cm− 1 and 1634 cm− 1, demonstrating the existence of hydrogen bonding interactions between them [39, 40].
Surface chemical composition of BN, MXene, PBN layer, PBM layer and PBN@MXene layer was characterized by XPS investigation. As shown in Fig. 2g, compared to the PBN layer, the spectra of both PBM and PBN@MXene layers generate distinct peaks of Ti 2p and F 1s attributed to MXene. Differently, the PBN@MXene layer shows a greater increase in the surface elements Ti/F content than the simple blended PBM layer, while the B/C content representing PBN is significantly lower (Fig. S10). The results in Fig. 2c and g and Fig. S10 together suggest that a large number of PBN@MXene gel microparticles are compressed and deformed during the assembly process, forming an oriented segregated structure of the highly interconnected MXene network encapsulating the PBN. Moreover, Ti 2p spectra of original MXene and PBN@MXene layer show the peaks of Ti 2p3/2 (455.0, 455.7 and 457.0 eV), Ti 2p1/2 (460.8, 461.6 and 462.5 eV) and TiO2 (459.2 and 464.2 eV) (Fig. S11) [41]. Compared with the original MXene, there is almost no increase in the peak intensity of TiO2 for the composite paper, indicating that MXene is not oxidized during the assembly process. Figure 2 h shows the O 1s spectra of samples, delivering the presence of several characteristic peaks in MXene (O-Ti (529.0 eV), Ti-C-Ox (530.2 eV), Ti-C-OH (531.8 eV), Al-O (532.8 eV) and H2O (533.8 eV)) [42, 43]. In particular, the Ti-C-OH binding energy shifts from 531.8 eV for MXene to 531.7 eV for PBN@MXene, due to the change in the chemical environment caused by the hydrogen bonds formed between MXene (rich in − OH, − O and − F polar groups) and PBN (rich in − C = O, − N, −N-O and − B-O polar groups, Fig. S12) [44].
a SEM–EDS mapping images and b TEM image of PBN microparticle. c SEM–EDS mapping images and d TEM image of PBN@MXene microparticle. e Cross-sectional SEM–EDS mapping images of O-PBN/MXene paper (O-41.7%). f XRD patterns and g XPS spectra of various samples. h The high-resolution O 1s spectra of MXene and PBN@MXene layer
Mechanical Properties of Nanocomposite Papers
Figure 3a-c show the mechanical properties of the papers are significantly improved with the amounts of MXene in the range of 12.5–41.7 wt%. For example, O-41.7% has a tensile strength of 95.8 MPa, Young’s modulus of 7.1 GPa, strain at break of 9.1%, and toughness of 5.7 MJ m− 3. This can be ascribed to the uniform dispersion of the building units, and the sufficient hydrogen bonds and van der Waals forces generated between the building units, thus improving the stress transfer efficiency [45]. When more amount of MXene was used (54.3 wt%), the mechanical properties of the composite paper, apart from Young’s modulus, begin to deteriorate. This is because apparent agglomerates of MXene appeared in the paper (Fig. S8). In the control experiment, B-41.7% was prepared by direct filtration of the mixture of the three building units with the same composition as that of O-41.7%. The mechanical properties of B-41.7% are significantly worse because BN and MXene nanosheets were difficult to disperse evenly in PBO sol. The comparison indicates the ordered assembly has a profound influence on the mechanical properties. The composite paper with a thickness of 13 μm can be folded, bent, and cut at will, showing fascinating flexibility (Fig. 3d). Figure 3e shows the crack propagation process of O-41.7% paper. It can be clearly seen that the crack starting from the notch propagates along a “zig-zag” path. In addition to the typical crack deflection, crack branching, plastic deformation and delamination introduced by the synergistic effect of the “brick and mortar” structure can also be observed, which effectively alleviate the stress concentration in the composite paper [46, 47]. Moreover, the significant curling of the pulled-out MXene nanosheets (indicated by the red arrow) suggests the homogeneous MXene interconnected network in the segregated structure of O-41.7% may contribute to a further improvement of the load transfer efficiency [18, 48]. Therefore, the excellent mechanical properties of composite paper can be attributed to its biomimetic multi-level structural design.
a Stress–strain curves, b tensile strength and Young’s modulus, and c strain at break and toughness of O-PBN/MXene papers and control sample B-41.7%. d Optical photographs of the as-prepared O-41.7% paper, showing the foldability, bendability and cutability of the ultra-thin paper. e Typical SEM images of the propagated crack for O-41.7% paper, showing typical crack deflection, crack branching, plastic deformation and delamination structural features
EMI Shield Performance of Nanocomposite Papers
Figure 4a shows the electrical conductivity of the PBN@MXene layer (the middle layer) in the composite papers. The electrical conductivity of the O-0% paper (similar to the PBN layer) is close to 0 S/cm and the measured surface resistance is as high as 3.2 × 1013 Ω cm, indicating excellent electrical insulation. However, as the MXene content increases, the electrical conductivity of the middle layer increases rapidly. In particular, the electrical conductivity of the PBN@MXene layer incorporating 54.3 wt% of MXene reaches 723.7 S/cm. Notably, sandwiched by the PBN layers, the composite paper blocks electrical currents in the out-of-plane direction (Fig. S13), which is in accordance with the requirements for electrical insulation in the application of electronic equipment. The EMI SE of the composite paper was investigated in the frequency range of 8.2–12.4 GHz. From Fig. 4b and c, O-0% has almost no EMI shielding performance. In agreement with the change in electrical conductivity, the EMI SE of the composites increase significantly with the introduction of MXene. The EMI SE of O-41.7% and O-54.3% reach 31.8 and 38.6 dB, respectively, exceeding the commercial application standard for EMI shielding materials (> 20 dB) [49]. Furthermore, the absorption shielding effect (SEA) of the composite paper is always higher than the reflection shielding effect (SER), implying an absorption-dominated shielding mechanism inside the composite paper [44]. To illustrate the superiority of the orderly assembled method, the EMI shielding performances of control samples (with the same compositions) were investigated (Fig. 4d). The average EMI SE values for P1-54.3% paper (without sandwich structure, prepared by filtration of the mixture of PBN and PBN@MXene microparticles in one step) and P2-54.3% paper (without segregated structure, replacing PBN@MXene microparticles in O-54.3% by PBM microparticles) are 26.1 and 25.8 dB, respectively, both are significantly lower than that of the O-54.3% paper, even below O-41.7% paper. This is good evidence that the optimized structural design is more beneficial in forming an efficient electrically conductive path in the composite paper. In addition, because of the excellent mechanical properties, the average EMI SE of the O-41.7% paper reduces by only ~ 1 dB after 500 cycles of bending (Fig. 4e).
We further used reflection (R), absorption (A) and transmission (T) coefficients to investigate the shielding mechanism (Fig. 4f). As judged from the relative magnitude of A and R, it can be concluded that the entire shielding mechanism of the composite paper is reflection-based. Figure 4 g shows the interaction between the EMW and the composite paper. When the incident EMW reaches the surface of the conductive layer, it is first partially reflected because of the impedance mismatch [50]. Then the inside MXene network with rich charge carriers interacts with the EMW through ohmic losses, and converts it into thermal energy [13]. Moreover, the rich interfaces formed by the MXene network and PBN cores in the segregated structure enhance the multiple reflection and scattering of EMW, thus further improving the reabsorption and attenuation of EMW [18]. The structural superiority endows O-41.7% a higher thickness-specific SE value of 2,446.1 dB mm− 1 at a low MXene content (41.7 wt%), as compared with most of other reported MXene/polymer composites (Fig. 4h).
a Electrical conductivity (PBN@MXene layer), b SET and c average EMI SE of O-PBN/MXene papers. d SET of O-41.7%, O-54.3%, P1-54.3% and P2-54.3% papers. (e) SET of O-41.7% paper before and after bending for 500 cycles. f Power coefficients of O-PBN/MXene papers. g The diagram of EMI shielding mechanism of O-PBN/MXene paper. h Comparison of EMI shielding performance of O-41.7% with reported MXene/polymer composites. Detailed data are provided in Table S2
Thermal Conductivity and Coefficient of Thermal Expansion of Nanocomposite Papers
The thermal conductivities (TCs) were studied by the laser flash method. As shown in Fig. 5a, because of the intrinsically high thermal conductivity of BN and PBO nanofibers and their high orientation along the paper face, the O-0% paper without MXene (50 wt% BN) exhibits an impressive in-plane TC of 35.2 W/mK, which is better than that of many BN-based composites with the same filler loading [51,52,53]. As MXene content increases, the in-plane TC of the composite paper decreases to 20 W/mK gradually. This may be attributed to aggregates of MXene nanosheets that tend to form at high loading, which causes unfavorable interface thermal resistance with PBN for heat transfer (Fig. S14). Satisfactorily, O-41.7% exhibits optimal mechanical properties and EMI SE, and excellent in-plane TC of 26.1 W/mK. Because of the high horizontal orientation of the building units, the out-of-plane TCs of the composite papers are only 0.02–0.05 W/mK (Fig. S15). More importantly, the highly ordered structure of O-41.7% is also preferable to form an efficient thermal conductivity network than the control samples with partially ordered structures (TCs of P1-41.7% and P2-41.7% are only 13.4 and 10.8 W/mK, respectively; Fig. 5b). The heat dissipation potential of the composite paper for the high-temperature hot spot (~ 200 °C) was examined by recording the temperature change with an infrared thermal imager (Fig. 5c and d). The temperature of the hot spot on the O-41.7% paper is consistently lower during multiple heating-cooling cycles of 120 s than when exposed directly to air or with the P1-41.7% and P2-41.7% papers as the substrates. Benefiting from the optimized structure, the temperature drop of O-41.7% surpasses 30 and 10 °C during heating and cooling, respectively, compared with P1-41.7% and P2-41.7%. The excellent heat transfer capability and thermal stability have greatly advanced the practical application of the ordered assembly composite papers in flexible electronic devices. In addition, Fig. 5e indicates that our composite paper can achieve superior mechanical strength (95.8 MPa), EMI shielding performance (2,446.1 dB mm− 1), and thermal conductivity (26.1 W/mK) simultaneously than most existing multifunctional composites, further demonstrating the advantages of the ordered assembly structures.
The thermal-induced expansion mismatch with electronic components is unfavorable for the practical application of thermally conductive EMI shielding materials. In Fig. 5f and g, the TMA was used to investigate the coefficient of thermal expansions (CTEs) of the composite papers. Because of the introduction of rigid inorganic fillers, the CTEs of O-0% (50 wt% BN, − 1.35 to − 15.19 ppm/K) and B-41.7% (29.2 wt% BN and 41.7 wt% MXene, − 1.94 to − 12.87 ppm/K) composite papers are markedly lower between 30 and 400 °C than pure PBO paper (− 9.13 to − 22.16 ppm/K) [54]. Impressively, the CTEs of P1-41.7% and O-41.7% papers, which have the identical components as B-41.7%, are further reduced to 1.69 to − 3.10 ppm/K and 2.29 to − 3.71 ppm/K, respectively. This can be attributed to the synergistic effect of the optimized 3D MXene network and BN with segregated structure in the composite paper, which greatly inhibits the thermal movement of PBO chains. Meanwhile, the extraordinary shape stability of the O-41.7% paper is also indirect proof of the strong interactions between adjacent layers. Figure 5 h compares the CTE of the O-41.7% paper (the average CTE in the temperature range of 30–400 °C is − 1.43 ppm/K) with that of other electromagnetic shielding or thermally conductive composites reported and some universal materials. The CTE of O-41.7% is much lower than that of other polymer-based functional composites, and even lower than that of common metals. Excitingly, the CTE is closer to that of semiconductor Si, indicating its great potential for application in electronic devices [55].
a In-plane TCs of O-PBN/MXene papers. b In-plane TCs of O-41.7%, P1-41.7% and P2-41.7% papers. c Surface temperature–time curves of the hot spot under different heat dissipation conditions. d Corresponding thermal infrared images at the 60th s and 120th s. e Comparison of mechanical strength, EMI shielding performance (SE/t) and TC for O-41.7% paper with existing thermally conductive EMI shielding composites. Detailed data are provided in Table S3. f Dimensional changes (dL/L0) and g CTE of PBO, O-0%, B-41.7%, P1-41.7% and O-41.7% papers at different temperatures. h Comparison of CTE for O-41.7% paper with reported thermally conductive or electromagnetic shielding composites and some universal materials (metals and Si). The CTE is the average value over a range of temperatures. Detailed data are provided in Table S3
Thermal Stability and Flame Retardancy of Nanocomposite Papers
Figure 6a exhibits the combustion behaviors of O-0%, P1-41.7%, and O-41.7% papers. Derived from the inherent nature of building blocks, no flame spread can be observed throughout the combustion process for all three papers, revealing their flame retardancy. In the details, most of the O-0% composite paper is thermally degraded after 180 s of combustion. At the same time, because of the large CTE, it suffers from severe shrinkage deformation, which makes it difficult to meet the requirements of practical applications. In comparison, only a small percentage of the P1-41.7% and O-41.7% papers are burnt within 240 s, demonstrating more remarkable thermal stability and flame retardancy. Their smaller deformation degrees are consistent with the lower CTEs. In addition, thermogravimetric analysis (TGA) results also confirm that the introduction of MXene improves the thermal residue of the composite paper at 800 °C (O-0% ~ 61.5%, O-41.7% ~ 74.8%) (Fig. S16). The structural features of the burned composite papers were determined by surface SEM images (Fig. 6b). The burned O-0% shows a dense and compact surface morphology consisting of BN-supported char. In contrast, because MXene can undergo severe breakage and oxidation to TiO2 nanoparticles at high temperatures [56], the morphology of the burned P1-41.7% is more sparse and porous. As expected, the burned O-41.7% paper shows a layered character corresponding to the sandwich structure. Interestingly, the TiO2 nanoparticles formed in the middle layer have a smaller size and a denser morphology than that in the burned P1-41.7% due to the presence of the PBN surface layer. Furthermore, we investigated Ti 2p XPS spectra of the samples after 30 s of combustion (Fig. 6c). The results show that MXene in P1-41.7% paper has been completely oxidized to TiO2, while MXene in O-41.7% paper still retains some of the reduced Ti, further confirming the protective effect of the surface layer on the internal electrically conductive layer. Based on these characterizations, the following combustion mechanism is postulated. When the composite paper is exposed to fire, PBN surface layer with the “brick and mortar” structure and the resulting robust BN-supported char restrict the flow of heat and oxygen to the interior, thus protecting the integrity of the internal electrically conductive layer. As the flame keeps burning, MXene is oxidized to TiO2, and MXene/TiO2 can act as inorganic ceramic bricks to further prevent the combustion of composite paper (Fig. 6d).
a Combustion snapshots and residues of O-0%, P1-41.7% and O-41.7% papers. b SEM images of (b1, b1’) O-0%, (b2 and b2’) P1-41.7% and (b3, b3’ and b3’’) O-41.7% papers after combustion. c High-resolution Ti 2p XPS spectra of P1-41.7% and O-41.7% papers after 30 s of combustion. d Illustration of the combustion model of O-PBN/MXene nanocomposite paper, showing the role of the outer layer in retarding heat and oxygen transfer and the oxidation of MXene in the middle layer
Conclusions
In summary, we have fabricated a high-performance multifunctional PBO/BN/MXene nanocomposite paper through a gel microparticle-mediated ordered assembly strategy. In virtue of the synergistic effects of the as-formed nacre-like “brick and mortar” structure, segregated structure and sandwich structure in the composites, the oriented PBO nanofiber, BN and MXene nanosheets form highly efficient electrical and thermal conductive networks, endowing the composite paper excellent EMI SE and TC of 31.8 dB and 26.1 W/mK, respectively. Besides, the structural optimizations bring other advantages: (1) the nacre-like and segregated structures realize outstanding mechanical properties, including a tensile strength of 95.8 MPa, a Young’s modulus of 7.1 GPa, a strain at break of 9.1%, and a toughness of 5.7 MJ m− 3; (2) the segregated structure enables an ultra-low CTE of − 1.43 ppm/K; (3) the sandwich structure allows for electrical insulation, as well as enhanced thermal stability and flame retardancy. This work not only demonstrates a novel nanocomposite paper with superior overall performance to most existing thermally conductive electromagnetic shielding composites, but also provides a fresh template towards the exploration of other multifunctional multi-component composites.
References
Tan X, Liu TH, Zhou W, Yuan Q, Ying J, Yan Q, Lv L, Chen L, Wang X, Du S, Wan YJ, Sun R, Nishimura K, Yu J, Jiang N, Dai W, Lin CT. Enhanced electromagnetic shielding and thermal conductive properties of polyolefin composites with a Ti3C2Tx MXene/graphene framework connected by a hydrogen-bonded interface. ACS Nano. 2022;16:9254.
He YJ, Shao YW, Xiao YY, Yang JH, Qi XD, Wang Y. Multifunctional phase change composites based on elastic MXene/silver nanowire sponges for excellent thermal/solar/electric energy storage, shape memory, and adjustable electromagnetic interference shielding functions. ACS Appl Mater Interfaces. 2022;14:6057.
Zhou Z, Liu J, Zhang X, Tian D, Zhan Z, Lu C. Ultrathin MXene/calcium alginate aerogel film for high-performance electromagnetic interference shielding. Adv Mater Interfaces. 2019;6:1802040.
Cao W, Ma C, Tan S, Ma M, Wan P, Chen F. Ultrathin and flexible CNTs/MXene/cellulose nanofibrils composite paper for electromagnetic interference shielding. Nano-Micro Lett. 2019;11:72.
Wang W, Yuen ACY, Long H, Yang W, Li A, Song L, Hu Y, Yeoh GH. Random nano-structuring of PVA/MXene membranes for outstanding flammability resistance and electromagnetic interference shielding performances. Compos B. 2021;224: 109174.
Ruan K, Guo Y, Lu C, Shi X, Ma T, Zhang Y, Kong J, Gu J. Significant reduction of interfacial thermal resistance and phonon scattering in graphene/polyimide thermally conductive composite films for thermal management. Research. 2021;2021:8438614.
Dai W, Ma T, Yan Q, Gao J, Tan X, Lv L, Hou H, Wei Q, Yu J, Wu J, Yao Y, Du S, Sun R, Jiang N, Wang Y, Kong J, Wong C, Maruyama S, Lin CT. Metal-level thermally conductive yet soft graphene thermal interface materials. ACS Nano. 2019;13:11561.
Mou PP, Zhao JC, Wang GZ, Shi SH, Wan GP, Zhou MF, Deng Z, Teng SJ, Wang GL. BCN nanosheets derived from coconut shells with outstanding microwave absorption and thermal conductive properties. Chem Eng J. 2022;437: 135285.
Wang M, Tang XH, Cai JH, Wu H, Shen JB, Guo SY. Construction, mechanism and prospective of conductive polymer composites with multiple interfaces for electromagnetic interference shielding: A review. Carbon. 2021;177:377.
Zhang J, Kong N, Uzun S, Levitt A, Seyedin S, Lynch PA, Qin S, Han M, Yang W, Liu J, Wang X, Gogotsi Y, Razal JM. Scalable manufacturing of free-standing, strong Ti3C2Tx MXene films with outstanding conductivity. Adv Mater. 2020;32: e2001093.
Wyatt BC, Rosenkranz A, Anasori B. 2D MXenes: Tunable mechanical and tribological properties. Adv Mater. 2021;33: e2007973.
Quero F, Rosenkranz A. Mechanical performance of binary and ternary hybrid MXene/nanocellulose hydro- and aerogels—a critical review. Adv Mater Interfaces. 2021;8:2100952.
Shahzad F, Alhabeb M, Hatter CB, Anasori B, Man Hong S, Koo CM, Gogotsi Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science. 2016;353:1137.
Wu N, Zeng Z, Kummer N, Han D, Zenobi R, Nystrom G. Ultrafine cellulose nanofiber-assisted physical and chemical cross-linking of MXene sheets for electromagnetic interference shielding. Small Methods. 2021;5: e2100889.
Ma Z, Kang S, Ma J, Shao L, Zhang Y, Liu C, Wei A, Xiang X, Wei L, Gu J. Ultraflexible and mechanically strong double-layered aramid nanofiber-Ti3C2Tx MXene/silver nanowire nanocomposite papers for high-performance electromagnetic interference shielding. ACS Nano. 2020;14:8368.
Jin X, Wang J, Dai L, Liu X, Li L, Yang Y, Cao Y, Wang W, Wu H, Guo S. Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem Eng J. 2020;380: 122475.
Sun R, Zhang H-B, Liu J, Xie X, Yang R, Li Y, Hong S, Yu Z-Z. Highly conductive transition metal carbide/carbonitride(MXene)@polystyrene nanocomposites fabricated by electrostatic assembly for highly efficient electromagnetic interference shielding. Adv Funct Mater. 2017;27:1702807.
Xu J, Liu T, Zhang Y, Zhang Y, Wu K, Lei C, Fu Q, Fu J. Dragonfly wing-inspired architecture makes a stiff yet tough healable material. Matter. 2021;4:2474.
Gholivand H, Fuladi S, Hemmat Z, Salehi-Khojin A, Khalili-Araghi F. Effect of surface termination on the lattice thermal conductivity of monolayer Ti3C2Tz MXenes. J Appl Phys. 2019;126: 065101.
Wang A, Li SH, Zhang XY, Bao H. Roles of electrons on the thermal transport of 2D metallic MXenes. Phys Rev Mater. 2022;6: 014009.
Weng Q, Wang X, Wang X, Bando Y, Golberg D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem Soc Rev. 2016;45:3989.
Kuang Z, Chen Y, Lu Y, Liu L, Hu S, Wen S, Mao Y, Zhang L. Fabrication of highly oriented hexagonal boron nitride nanosheet/elastomer nanocomposites with high thermal conductivity. Small. 2015;11:1655.
Tu H, Xie K, Lin XH, Zhang RQ, Chen F, Fu Q, Duan B, Zhang LN. Superior strength and highly thermoconductive cellulose/boron nitride film by stretch-induced alignment. J Mater Chem A. 2021;9:10304.
He JF, Ren M, Dong LZ, Wang YL, Wei XL, Cui B, Wu YL, Zhao YR, Di JT, Li QW. High-temperature-tolerant artificial muscles using poly(p-phenylene benzobisoxazole) composite yarns. Adv Fiber Mater. 2022;4:1256.
Liu Z, Fan X, Zhang J, Chen L, Tang Y, Kong J, Gu J. PBO fibers/fluorine-containing liquid crystal compound modified cyanate ester wave-transparent laminated composites with excellent mechanical and flame retardance properties. J Mater Sci Technol. 2023;152:16.
Tang L, Tang Y, Zhang J, Lin Y, Kong J, Zhou K, Gu J. High-strength super-hydrophobic double-layered PBO nanofiber-polytetrafluoroethylene nanocomposite paper for high-performance wave-transparent applications. Sci Bull. 2022;67:2196.
Wang XJ, Ho V, Segalman RA, Cahill DG. Thermal conductivity of high-modulus polymer fibers. Macromolecules. 2013;46:4937.
Zhang YZ, Wu K, Fu Q. A structured phase change material with controllable thermoconductive highways enables unparalleled electricity via solar-thermal-electric conversion. Adv Funct Mater. 2022;32:2109255.
Chen X, Wu K, Zhang Y, Liu D, Li R, Fu Q. Tropocollagen-inspired hierarchical spiral structure of organic fibers in epoxy bulk for 3D high thermal conductivity. Adv Mater. 2022;34: e2206088.
Wang L, Ma Z, Zhang Y, Qiu H, Ruan K, Gu J. Mechanically strong and folding-endurance Ti3C2TX MXene/PBO nanofiber films for efficient electromagnetic interference shielding and thermal management. Carbon Energy. 2022;4:200.
Chen Y, Zhang H, Chen J, Guo Y, Jiang P, Gao F, Bao H, Huang X. Thermally conductive but electrically insulating polybenzazole nanofiber/boron nitride nanosheets nanocomposite paper for heat dissipation of 5G base stations and transformers. ACS Nano. 2022;16:14323.
Hu Y, Chen C, Wen Y, Xue Z, Zhou X, Shi D, Hu G-H, Xie X. Novel micro-nano epoxy composites for electronic packaging application: Balance of thermal conductivity and processability. Compos Sci Technol. 2021;209: 108760.
Hao X, Zhu J, Jiang X, Wu H, Qiao J, Sun W, Wang Z, Sun K. Ultrastrong polyoxyzole nanofiber membranes for dendrite-proof and heat-resistant battery separators. Nano Lett. 2016;16:2981.
Hong H, Jung YH, Lee JS, Jeong C, Kim JU, Lee S, Ryu H, Kim H, Ma Z, Ti Kim. Anisotropic thermal conductive composite by the guided assembly of boron nitride nanosheets for flexible and stretchable electronics. Adv Funct Mater. 2019;29:1902575.
Alhabeb M, Maleski K, Anasori B, Lelyukh P, Clark L, Sin S, Gogotsi Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem Mater. 2017;29:76334.
Yang D, Wei QG, Yu LY, Ni YF, Zhang LQ. Natural rubber composites with enhanced thermal conductivity fabricated via modification of boron nitride by covalent and non-covalent interactions. Compos Sci Technol. 2021;202: 108590.
Li W, Li X, Chang W, Wu J, Liu P, Wang J, Yao X, Yu Z-Z. Vertically aligned reduced graphene oxide/Ti3C2Tx MXene hybrid hydrogel for highly efficient solar steam generation. Nano Res. 2020;13:3048.
Hao M, Hu Z, Zhang Y, Qian X, Liu L, Yang J, Wang X, Zhi J, Huang Y, Shi X. Facile preparation of ultraviolet resistant “hard armors” on poly(p-phenylene benzobisoxazole) fibers through heat-induced surface treatment. Polym Degrad Stab. 2022;199: 109896.
Wan Y, Xiong P, Liu J, Feng F, Xun X, Gama FM, Zhang Q, Yao F, Yang Z, Luo H, Xu Y. Ultrathin, strong, and highly flexible Ti3C2Tx MXene/bacterial cellulose composite films for high-performance electromagnetic interference shielding. ACS Nano. 2021;15:8439.
Feng S, Yi Y, Chen B, Deng P, Zhou Z, Lu C. Rheology-guided assembly of a highly aligned MXene/cellulose nanofiber composite film for high-performance electromagnetic interference shielding and infrared stealth. ACS Appl Mater Interfaces. 2022;14:36060.
Shuck CE, Sarycheva A, Anayee M, Levitt A, Zhu Y, Uzun S, Balitskiy V, Zahorodna V, Gogotsi O, Gogotsi Y. Scalable synthesis of Ti3C2Tx MXene. Adv Eng Mater. 2020;22:1901241.
Halim J, Cook KM, Naguib M, Eklund P, Gogotsi Y, Rosen J, Barsoum MW. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl Surf Sci. 2016;362:406.
Näslund L-Å, Persson I. XPS spectra curve fittings of Ti3C2Tx based on first principles thinking. Appl Surf Sci. 2022;593: 153442.
Wang J, Ma X, Zhou J, Du F, Teng C. Bioinspired, high-strength, and flexible MXene/aramid fiber for electromagnetic interference shielding papers with joule heating performance. ACS Nano. 2022;16:6700.
Cao WT, Chen FF, Zhu YJ, Zhang YG, Jiang YY, Ma MG, Chen F. Binary strengthening and toughening of MXene/cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano. 2018;12:4583.
Zeng F, Chen X, Xiao G, Li H, Xia S, Wang J. A bioinspired ultratough multifunctional mica-based nanopaper with 3D aramid nanofiber framework as an electrical insulating material. ACS Nano. 2020;14:611.
Sun C, Huang Y, Shen Q, Wang W, Pan W, Zong P, Yang L, Xing Y, Wan C. Embedding two-dimensional graphene array in ceramic matrix. Sci Adv. 2020;6:1338.
Zhou T, Wu C, Wang Y, Tomsia AP, Li M, Saiz E, Fang S, Baughman RH, Jiang L, Cheng Q. Super-tough MXene-functionalized graphene sheets. Nat Commun. 2020;11:2077.
Hu D, Wang S, Zhang C, Yi P, Jiang P, Huang X. Ultrathin MXene-aramid nanofiber electromagnetic interference shielding films with tactile sensing ability withstanding harsh temperatures. Nano Res. 2021;14:2837.
Zhang Y, Ma Z, Ruan K, Gu J. Multifunctional Ti3C2Tx-(Fe3O4/polyimide) composite films with janus structure for outstanding electromagnetic interference shielding and superior visual thermal management. Nano Res. 2022;15:5601.
Zhou J, Yu Z, Lv Y, Wang C, Hu P, Liu Y. Highly thermal conductivity of PVA-based nanocomposites by constructing MWCNT-BNNS conductive paths. Compos A. 2022;163: 107195.
Mazumder MRH, Mathews LD, Mateti S, Salim NV, Parameswaranpillai J, Govindaraj P, Hameed N. Boron nitride based polymer nanocomposites for heat dissipation and thermal management applications. Appl Mater Today. 2022;29: 101672.
Hu BY, Zhang W, Guo H, Xu S, Li Y, Li M, Li BA. Nacre-mimetic elastomer composites with synergistic alignments of boron nitride/graphene oxide towards high through-plane thermal conductivity. Compos A. 2022;156: 106891.
Fu K, Yang J, Cao C, Zhai Q, Qiao W, Qiao J, Gao H, Zhou Z, Ji J, Li M, Liu C, Wang B, Bai W, Duan H, Xue Y, Tang C. Highly multifunctional and thermoconductive performances of densely filled boron nitride nanosheets/epoxy resin bulk composites. ACS Appl Mater Interfaces. 2021;13:2853.
Zhang X, Shi Z, Zhang X, Wang K, Zhao Y, Xia H, Wang J. Three dimensional AlN skeleton-reinforced highly oriented graphite flake composites with excellent mechanical and thermophysical properties. Carbon. 2018;131:94.
Dall’Agnese C, Dall’Agnese Y, Anasori B, Sugimoto W, Mori S. Oxidized Ti3C2 MXene nanosheets for dye-sensitized solar cells. New J Chem. 2018;42:16446.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51733008), the Chinese Academy of Sciences (Grant No. QYZDB-SSW-SLH032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhao, N. & Xu, J. Mechanically Strong and Flame-Retardant PBO/BN/MXene Nanocomposite Paper with Low Thermal Expansion Coefficient, for Efficient EMI Shielding and Heat Dissipation. Adv. Fiber Mater. 5, 1657–1670 (2023). https://doi.org/10.1007/s42765-023-00298-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-023-00298-0