Abstract
The harsh microenvironment in wound (HMW) remains a major obstacle to chronic wound healing. Although a series of bioactive materials have been developed, few of them are multi-functional and able to accelerate wound healing via precisely remodeling the HMW. Herein, a series of dihydromyricetin (DHM)-incorporated multilayer nanofibers (termed DQHP-n, n = 0, 2, 6 and 10) are fabricated using a layer-by-layer (LBL) self-assembly technique. The average diameters of DQHP-n significantly increase from 0.30 ± 0.16 μm to 0.84 ± 0.28 μm (P < 0.05) along with the n value increased from 0 to 10, the tensile strength of that is also significantly improved from 1.12 ± 0.15 MPa to 2.16 ± 0.30 MPa (P < 0.05), and the water contact angle of that significantly decreases from 129.1 ± 1.5° to 76.6 ± 3.9° (P < 0.05). The DQHP-n are found to be biocompatible, in which DQHP-6 promoted cell migration through activation of the epithelial–mesenchymal transformation (EMT) pathway and reconstruction of the HMW by stopping bleeding, killing bacteria, eliminating inflammation, and scavenging reactive oxygen species (ROS). The in vivo evaluation is carried out via an E. coli-infected rat skin regeneration model. The DQHP-6 group demonstrates the best effect, as it healed up to 98.5 ± 1.0% of the wound area at day 15. DQHP-6 differentially regulates the mRNA expressions of several cytokines (FGF2, PDGF, IL-1α, IL-6, IL10, and TGF-β), which ends to reductions of total inflammatory cells (CD45+ cells) and M1 macrophages (CD80+ and CD86+ cells), proliferation of host cell (Ki67+ cells), and enhancement of collagen synthesis. In conclusion, DQHP-6 exhibits multifunctional properties for HMW, and can serve as a promising wound dressing for clinical transformation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Skin is the largest superficial organ of the human body, and it plays multiple vital roles in maintaining body integrity, regulating body temperature, and resisting pathogens as well as sensation [1, 2]. Chronic skin injuries (CSIs) caused by type II diabetes and drug-resistant bacterial infections are common in clinical practice [3, 4]. The management of CSI remains a great challenge, as the global burden has increased rapidly over a 10-year period [5]. Normally, the wound-healing process is divided into four successive phases, including hemostasis, inflammation, proliferation, and remodeling, with a duration of 3 weeks or more [6, 7]. However, various intrinsic and extrinsic pathogenic factors of CSI form a harsh wound microenvironment, which may lead to healing failure [8,9,10]. In recent decades, advanced tissue engineering wound dressings (TEWDs) have been widely developed and transformed [11,12,13]. These TEWDs have relatively good biocompatibility, biodegradability, and one or more specific bioactivities. However, few of them were multifunctional and would able to accelerate chronic wound-healing process for comprehensively remodeling the harsh microenvironment in wound (HMW).
The HMW consists of foreign bodies, necrotic tissue, blood clots, bacteria, immune cells, granulation tissue, and neovessels. HMW is also full of inflammatory cytokines and reactive oxygen species (ROS) products [14, 15], which negatively delay the healing process. Based on the pathophysiological characteristics of HMW, requirements of TEWDs were summarized, including hemostatic activity, broad-spectrum antibacterial activity, antioxidant activity, and anti-inflammatory activity. Nanofibers commonly have good flexibility and extracellular matrix (ECM)-like 3D network structures and are thus considered as an ideal TEWD [16]. Nanofibers are prepared by electrospinning techniques, which are rapidly developing from a single-fluid process to coaxial, tri-axial, side-by-side, and other complicated processes to endow the nanofibers with the desired functional performances [17,18,19,20,21,22]. However, single-fluid blending electrospinning is still the mainstream of this field and holds great promise for scaling up [23].
The multiple-function performances of nanofibers can be simply achieved through the encapsulation of functional ingredients [24] and the organization of deposited nanofibers in a layer-by-layer (LBL) manner [25]. LBL self-assembly is an universal method for preparing polyelectrolyte multilayers by alternately depositing substrates in two polyelectrolyte solutions with opposite charges [26]. In our previous work, quaternized chitin with a positive charge and silk fibroin with a negative charge were LBL self-assembled onto electrospun polycaprolactone (PCL) nanofibers [27]. The obtained TEWDs exhibited enhanced antibacterial activity, vascularization, collagen deposition, and hair follicle regeneration. In another work, fibroblast growth factor-2 (FGF2) protein was self-assembled into nanofiber TEWDs to improve proliferative activity [25]. Although these nanofiber TEWDs exhibited relatively well wound-healing effects, they have obvious deficiencies in remodeling the HMW, especially in eliminating inflammations and scavenging ROSs.
Traditional Chinese herbs (TCBs) are popular in China, Japan, and South Korea for thousands of years [28]. To date, a great amount of the bioactive ingredients of TCBs have been uncovered, which provides an opportunity for interdisciplinary research [29]. Tengcha, also named ampelopsis grossedentata, is distributed in the Wuling Mountain area, China [30]. Local Tujia people drink Tengcha to treat traumatic injuries. A series of Chinese medicine research, such as “The Book of Songs” and “Collection of Chinese Herbal Medicine”, recorded its medicinal value. Dihydromyricetin (DHM), chemical formula C15H12O8, is a flavonoid compound extracted from Tengcha (Fig. 1a). DHM has been confirmed to have antibacterial, anti-inflammatory, antioxidant, and antitumor activities [30, 31], and its molecular mechanisms on inflammation and oxidative stress pathways have been largely summarized [32, 33]. It can be speculated that DHM would have a potential activity for remodeling the HMW. At present, the development of DHM is confined to a preclinical stage, and no commercial product has been approved by the Food and Drug Administration (FDA, USA) or National Medical Products Administration (NMPA, China).
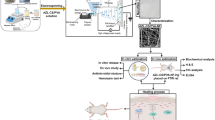
This work aims to fabricate a series of DHM-incorporated nanofiber TEWDs. The design and preparation of nanofiber TEWDs followed our previous work [25]. As shown in Fig. 1b, the electrospun PCL nanofiber films were LBL self-assembled with positively charged quaternized chitosan and negatively charged DHM/hyaluronic acid (HA). HA is a biocompatible macromolecule which are used to immobilize DHM [34]. The obtained nanofiber TEWDs were, respectively, termed as DQHP-n (n = 0, 2, 6 and 10, indicating the number of polyelectrolyte multilayers). The physical as well as chemical properties, biocompatibilities, bioactivities and mechanisms, and in vivo application effect of DQHP-n were investigated. As shown in Fig. 1c, d, it was assumed that the DQHP-n could remodel the HMW by killing bacteria, stopping bleeding, eliminating inflammation, and scavenging reactive oxygen species (ROS), thus accelerating chronic wound healing. This work will provide an new insight for skin tissue engineering.
Schematic illustration of the dihydromyricetin-incorporated multilayer nanofibers. a Dihydromyricetin (DHM) is extracted from ampelopsis grossedentata, a traditional Chinese herb. b Polycaprolactone (PCL) nanofibers are layer-by-layer self-assembled with positively charged quaternized chitosan (QC) and negatively charged DHM/hyaluronic acid (HA). c, d The obtained products can remodel the harsh wound microenvironment by killing bacteria, stopping bleeding, eliminating inflammation, and scavenging reactive oxygen species
Materials and Methods
Preparation of DHM-Incorporated Nanofibers
LBL self-assembly protocols were followed as previously reported [25]. Briefly, a 2 wt% QC solution served as a positive constituent, and a 1 wt% HA solution served as a negative constituent. DHM (10 mg) was dissolved into 1,000 mL HA solution. PCL nanofibers were immersed into DHM-HA solution for 15–20 min and then washed with PBS three times to remove the residuals. Afterward, the nanofilms were transferred into QC solution for 15–20 min and then underwent the same washing procedures. The self-assembly and rinsing protocols were repeated. In this work, the DHM-incorporated QC/HA self-assembled PCL nanofibers were termed as DQHP-n (n = 0, 2, 6 and 10, corresponding to the number of polyelectrolyte multilayers). Notably, D refers to DHM, Q refers to quaternized chitosan, H refers to hyaluronic acid, and P refers to PCL.
Physical and Chemical Characterizations
The microstructure of DQHP-n was observed using scanning electron microscope (Zeiss SIGMA). The dimensions of the nanofibers were calculated by Image-J software. Fourier transform infrared spectrum (FT-IR) was detected at wavenumbers ranging from 4000 to 400 cm− 1. For crystal analysis, X-ray diffraction (XRD) patterns were obtained at diffraction angles ranging from 4 to 60°. The water contact angle test was performed by a drop shape analyzer (Surface Science, China). Tensile tests were carried out using a universal mechanical tester (ITW, USA).
HPLC Analysis
The drug-loading capability of DQHP-n (n = 2, 6, 10) was detected by high-performance liquid chromatography (HPLC). One gram of DQHP-n sample was immersed into 10 mL HCl solution (pH = 1.2), and then constantly stirred at 37 °C for 10 days to fully release DHM. Prior to test, 1 mL of solution was collected and filtered using a 45 μm nylon filter. The samples were separated through a TAC-1 (PFP) column (4.6 × 250 mm, Hichrom, Berkshire, UK) with particle size of 5 μm. The temperature of column and sample was set to 25 °C and 5 °C. The mobile phase was composed of acetonitrile: 0.2% phosphoric acid solution (20:80, v/v). Each sample was analyzed by an isocratic gradient with a flow rate of 1 mL/min for 15 min. The samples were detected at 291 nm. All samples were compared to a calibration curve of DHM solution. The drug releasing kinetics of DQHP-n was also detected by HPLC. One gram of DQHP-n sample was immersed into 10 mL phosphate-buffer saline (PBS, pH = 7.4), and then gently stirred at 37 °C. At regular time intervals, the concentration of DHM in the PBS was detected as described before.
MTT Assay
In this work, L929 cells and HUVECs were applied as seeding cells. L929 cells and HUVECs were seeded into 96-well plates at densities of 1.5 × 103 and 3.0 × 103 cells/well, respectively. Those cells were cultured for 12–24 h, and then, the mediums were removed. Two hundred microliters of extract were added to each well. At regular time intervals, 20 µL MTT solution was added into each well, and the plates were incubated for another 4 h. After that, 150 µL DMSO in each well were added. The absorbance (OD 490 nm) of the solution was detected by a shared microplate reader (Thermo, USA).
Scratch Assay
Cells were seeded onto 6-well plates, and the cell density was adjusted to 6 × 105 cells/well. After the cell confluence reached above 80%, linear wounds were prepared over the TCPs using a 200 µL tip. The mediums were replaced with the extracts. After 24 h of incubation, these samples were stained using a Calcein-AM/PI kit, and the wound sites were photographed by a shared fluorescence microscope (IXplore, OLYMPUS, Japan).
Transwell Chamber assay
Cells were suspended in FBS-free extracts of DQHP-n and pipetted into the transwell chamber. The medium volume was set as 200 µL, and the number of seeded cells was set at 4.5 × 104. Then, 700 µL medium containing 10% FBS was added into the 24-well plates. After 24 h of incubation, the samples were quickly fixed and then stained using crystal violet solution for 30 min. The cells in the upper chamber were slowly cleared by a cotton swab, and the cells in the lower chamber were observed by a fluorescence microscope.
Cell Adhesion Assay
DQHP-n was cut into 1 × 1 cm2 pieces and then transferred into 6-well tissue culture plates. All samples were sterilized by ultraviolet radiation for 30 min. L929 cells and HUVECs were seeded onto the upper surface of DQHP-n and then incubated for another 48 h. The morphology and viability of adhered cells were visualized using a live/dead cell staining kit. This work was performed according to the manufacturer’s protocols. The images were captured by an inverted fluorescence microscope (IXplore Standard, Olympus, Japan).
Hemolysis Test
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (approved No: 2,015,029). Four milliliters of anticoagulant blood were kindly donated by a healthy volunteer and then diluted with 5 mL of normal saline. DQHP-n was immersed into 10 mL normal saline (NS) and 200 µL diluted blood at 37 ℃. After incubation for approximately 1 h, all samples were centrifuged. The absorbance (OD 545 nm) of the supernatant was detected by a shared microplate reader.
Hemostatic Test
This study was carried out according to the “Guidelines and Regulations for the Use and Care of Animals of the Review Board of Hubei Medical Laboratory Animal Center” and approved by the Animal Care & Welfare Committee of Wuhan University School of Basic Medical Sciences (approval No: 201,816). Thirty-two SD female rats, aged approximately 6–8 weeks and weighing approximately 180–200 g, were purchased from the Experimental Animal Center, Three Gorges University.
Rat Tail Bleeding Model
The animals were anesthetized by isoflurane inhalation and then divided into four groups, with four animals in each group. Each animal was cut 1.5 cm away from the end of the rat tail. The bleeding tails were immediately covered with a piece of DQHP-n sample. The positive control was medical gauze, and the blank control was no treatment. Two indices were recorded, including blood loss and bleeding time.
Rat Liver Bleeding Model
Sixteen anesthetized rats were fixed onto surgical corkboards. The liver was exposed, and the abdominal dropsy was cleared. A 10 mm wound was made in each liver. The wounds were immediately covered with a piece of DQHP-n. The positive control was medical gauze, and the blank control was no treatment. Two indices were recorded, blood loss and bleeding time.
Antibacterial Test
Gram-negative Escherichia coli (E. coli), Gram-positive Staphylococcus aureus (S. aureus), and methicillin-resistant Staphylococcus aureus (MRSA) were used for the antibacterial test. Bacterial precipitates were suspended using 0.85% normal saline, and the OD600 value of the bacterial suspension was adjusted to 0.5–0.6. DQHP-n samples were cut into 2 × 2 cm2 pieces and then transferred into 6-well tissue culture plates containing 2.5 mL bacterial suspension in each well. DQHP-n and the bacterial suspension were incubated at 37 ℃ with constant stirring for 12 h. As previously reported [35], the proliferation and survival abilities were evaluated by bacterial proliferation assay and clone formation assay, respectively.
Antioxidant Evaluation
Mouse monocyte macrophages (RAW264.7) were incubated with the extracts of DQHP-n for 2 h. LPS was then added at a concentration of 1 µg/mL, followed by incubation for another 12 h. Reactive oxygen species (ROS) levels were detected. Nitric oxide (NO) was preliminarily detected by a biochemical NO assay kit. The mRNA and protein expression levels of several genes were analyzed using quantitative real-time PCR and western blotting. To evaluate macrophage M1 polarization, these cells were stained with APC-labeled CD11b, PE-labeled CD80, and FITC-labeled CD86. The percentages of positively stained cells were measured.
Wound-Healing Assay
This study was performed using a Gram-negative bacteria-infected skin regeneration model. Twenty-four SD female rats, aged approximately 6–8 weeks and weighing approximately 180–200 g, were purchased from the Experimental Animal Center, Three Gorges University. The animals were first anesthetized by isoflurane inhalation. After that, four 20 mm skin wounds were made in each animal. E. coli bacterial precipitates were suspended using 0.9% normal saline, and the OD600 value of the bacterial suspension was adjusted to 2.0. Then, 100 µL of bacterial suspension was added to each wound site. The wounds were then covered with pieces of medical gauze, DQHP-0 sample or DQHP-6 sample. The blank control group was not treated. At different time points, the wound sites were photographed by a digital camera (Apple, USA). We resected the neo-skin tissues. Hematoxylin–eosin (HE) staining, Masson’s trichrome (MT) staining, immunofluorescence (Ki67) staining, and immunohistochemical (CD45, CD80, CD86) staining were performed according to general protocols. The expression levels of several cytokines were detected by quantitative real-time PCR.
Statistical Analysis
All data were calculated using SPSS 22.0 software. Quantitative results are expressed as the mean ± standard deviation. Statistical analysis was performed by one-way analysis of variance. P < 0.05 represented a significant difference.
Results and Discussion
Physiochemical Properties of DQHP-n
PCL nanofibers were prepared using a high-voltage electrospinning technique, and followed by LBL self-assembled to obtain multilayer nanofibers. A series of DHM-free DQHP-n were used for physiochemical characterization. The FT-IR spectrum of raw materials is shown in Fig. S1. PCL had four characteristic peaks, including C–H asymmetric and symmetric stretching (2944 cm−1, 2866 cm−1), C=O stretching (1720 cm−1), C–O–C asymmetric and symmetric stretching (1238 cm−1, 1163 cm−1, 1107 cm−1, 1046 cm−1), and –(CH2) n- in-plane rocking (732 cm−1). The characteristic peaks of QC were C–H stretching (1662 cm−1, 1491 cm−1) of the methyl group and the N–H in-plane blending (1560 cm−1) of the primary amine group on quaternary ammonium groups. HA also showed three characteristic peaks of -OH stretching (3416 cm-1), C=O asymmetric and symmetric stretching (1613 cm-1, 1412 cm-1), and C–O–C stretching (1151 cm-1~1042 cm-1). These characteristic absorption peaks are caused by the typical molecular structures of PCL, QC, and HA. The spectrum of DQHP-n is shown in Fig. 2a. The spectrum of DQHP-0 was similar to that of neat PCL. For DQHP-10, several characteristic peaks were observed, which were assigned to QC (C–H stretching of methyl group and N–H in-plane blending of primary amine group) and HA (-OH stretching).
The chemical composition of DQHP-n was further identified by XRD. As shown in Fig. S2, PCL exhibited two characteristic peaks at 21.3° and 23.6°. Meanwhile, the characteristic peaks of QC and HA were located at 19.8° and 18.9°, respectively. The XRD spectrum of DQHP-n is shown in Fig. 2b. As the n value increased from 0 to 10, the peak at 21.3° shifted slightly to 21.1°, and its intensity increased obviously. Notably, a new peak appeared at 29.7° in the DQHP-6 and DQHP-10 groups, which might be attributed to the crystal of the QC/HA complex. These results confirmed that QC and HA were successfully LBL self-assembled into the PCL nanofibers.
The DQHP-n samples exhibited a typical interweaved network structure (Fig. 2c). As the n value increased from 0 to 10, the diameter of the nanofibers increased obviously. The average diameter was 0.30 ± 0.16 μm for DQHP-0, 0.48 ± 0.17 μm for DQHP-2, 0.71 ± 0.25 μm for DQHP-6, and 0.84 ± 0.28 μm for DQHP-10 (Fig. S3). The hydrophilicity of nanofibers is vital for drug release. As shown in Fig. 2d, e, the water contact angle was 129.1 ± 1.5° for DQHP-0, 88.5 ± 5.6° for DQHP-2, 83.9 ± 7.6° for DQHP-6, and 76.6 ± 3.9° for DQHP-10. After LBL self-assembly, the hydrophilicity of DQHP-n was significantly improved (P < 0.05). This result revealed that the polyelectrolyte complex of QC and HA was more hydrophilic than neat PCL.
Wound dressings are required to be flexible. As shown in Fig. 2f, DQHP-n could be stretched. The tensile strength (stress) of DQHP-n increased along with strain increase (Fig. 2g). As the n value increased from 0 to 10, the tensile strength of DQHP-n also increased, but the elongation at break obviously decreased. As shown in Fig. 2h, the tensile strength at break was 1.12 ± 0.15 MPa for DQHP-0, 1.76 ± 0.33 MPa for DQHP-2, 1.57 ± 0.24 MPa for DQHP-6, and 2.16 ± 0.30 MPa for DQHP-10. As shown in Fig. S4, the Young’s modulus were 5.6 ± 3.4 MPa for DQHP-0, 20.1 ± 6.9 MPa for DQHP-2, 17.7 ± 6.3 MPa for DQHP-6, and 24.2 ± 5.9 MPa for DQHP-10. The QC/HA polyelectrolyte complexes tightly bonded the nanofibers resulting in a significant improvement in mechanical strength (P < 0.05).
Physicochemical properties of DQHP-n. a FI-IR spectrum; b XRD spectrum; c SEM photographs, scale bar: 5 μm; d, e water contact angle test and quantitative results; f representative images of the tensile test; g tensile strength–strain curves; h tensile strength at break. Values are expressed as the mean ± SD (n = 3), *P < 0.05, ***P < 0.001
Biocompatibility and Bioactivity of DQHP-6
In this experiment, a series of DHM-incorporated DQHP-n were prepared for in vitro evaluations. DHM was loaded into HA via intermolecular hydrogen bonding. The negatively charged HA containing DHM was then self-assembled with the positively charged QC to form polyelectrolyte complex on the surface of nanofibers. As a result, DHM was successfully immobilized onto the nanofibers. The drug-loading capability of DQHP-n was detected by high-performance liquid chromatography (HPLC). As shown in Fig. S5a, the drug-loading ratio were 0.0048 ± 0.0007% for DQHP-2 group, 0.0269 ± 0.0041% for DQHP-2 group, and 0.0372 ± 0.0036% for DQHP-10 group. A significant difference was observed between each group (P < 0.01). The drug releasing kinetics of DQHP-n are shown in Fig. S5b. As the n value increased from 2 to 10, the drug sustained release ability of DQHP-n was effectively improved. The results of the MTT assay are shown in Fig. 3a, b. DQHP-n was relatively good in cytocompatibility and exhibited mild cytotoxicity to L929 cells and HUVECs. Furthermore, these cells were seeded onto DQHP-n. As shown in Fig. S6, almost all cells were stained green, indicating good cytocompatibility of DQHP-n. The QC component was also reported to be cytotoxic in a dose-dependent manner [36]. Thus, the QC content should be limited by reducing the n value. The hemolysis ratio (HR) of DQHP-n must be less than 5%. As shown in Fig. 3c, DQHP-n was co-incubated with human red blood cells for 1 h, and no obvious hemolysis occurred. In conclusion, the cytocompatibility and hemocompatibility of DQHP-n met the general requirements of biomaterials.
DQHP-0 and DQHP-6 were chosen for further study. DHM is a flavonoid compound [37]. In this work, we found that the incorporation of DHM endowed DQHP-n with enhanced pro-migration activity. The results of the scratch assay are shown in Fig. 3d, e. The wound-healing rate of L929 cells was 23.3 ± 4.6% for the blank control (B.C.) group, 35.6 ± 3.3% for the DQHP-0 group, and 49.9 ± 3.1% for the DQHP-6 group. Meanwhile, the wound-healing rates of HUVECs were 19.0 ± 3.1% for B.C. group, 26.9 ± 2.8% for the DQHP-0 group, and 45.6 ± 2.4% for the DQHP-6 group. A significant difference was observed between the DQHP-6 group and the other groups (P < 0.01). The migration ability was also evaluated by Transwell chamber assay (Fig. 3f, g). The number of migrated cells per field in the DQHP-6 group was significantly greater than that in the other groups (P < 0.01).
To discover the molecular mechanism of pro-migration activity, the gene interactions of the epithelial–mesenchymal transition (EMT) pathway were detected. As shown in Fig. S7a–c, the mRNA expression levels of mesenchymal markers, such as Slug, vimentin, and N-cadherin, were significantly up-regulated in the DQHP-6 group (P < 0.01), and the protein expression levels were also up-regulated (Fig. S7d). EMT is a biological process in which epithelial cells transform into mesenchymal cells (Fig. S7e). The mesenchymal phenotype is generally associated with high migration and invasion abilities. Thus, the pro-migration activity of DQHP-6 performed the best.
Biocompatibility and bioactivity of DQHP-n. a, b MTT results of the L929 cells and HUVECs, respectively; c the results of the hemolysis test; d, e fluorescence images of the scratch assay and the results of the wound-healing rate, scale bar: 400 μm; f, g optical images of the Transwell chamber assay and the counting results of migrated cells, scale bar: 300 μm. Values are expressed as the mean ± SD (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001, N.S. = no significance
Antibacterial and Hemostatic Effects of DQHP-6
Infection prevention is the primary clinical demand of wound dressings. The antibacterial evaluation was performed using three different bacterial stains, including E. coli, S. aureus, and MRSA. As shown in Fig. 4a–c, DQHP-6 effectively inhibited bacterial proliferation. Meanwhile, the survival ability of these bacteria was also inhibited (Fig. S8a–f). Our previous work uncovered the broad-spectrum antibacterial activity and the underlying mechanism of QC [25, 38]. In this work, QC was LBL self-assembled onto multilayer nanofibers and endowed DQHP-6 with antibacterial activity in situ. DHM was also reported to be antibacterial [39]. We assumed that DHM could assist QC in killing bacteria.
The hemostatic effect of DQHP-6 was evaluated using two animal models. Figure 4d shows the rat tail bleeding model. As shown in Fig. 4e, f, the bleeding time was 185.7 ± 16.2 s for the B.C. group, 83.0 ± 12.3 s for the gauze group, 96.0 ± 7.0 s for the DQHP-0 group, and 73.7 ± 7.1 s for the DQHP-6 group; blood loss was 0.50 ± 0.14 g for the B.C. group, 0.19 ± 0.03 g for the gauze group, 0.33 ± 0.06 g for the DQHP-0 group, and 0.18 ± 0.05 g for the DQHP-6 group. A significant difference was observed between the DQHP-0 and DQHP-6 groups (P < 0.05). The liver is rich in blood and bleeds more than the tail when damaged [40]. Figure 4g shows the liver bleeding model. We also found that DQHP-6 had the best hemostatic effect (Fig. 4h–i).
Bleeding occurs at the early stage of wound healing. A variety of wound dressings have been developed for hemostasis [41]. However, novel wound dressings that interact with blood cells and coagulation factors are rarely reported. In this study, DQHP-6 exerted external pressure on the wounds to reduce fresh bleeding. Hydrophilic nanofibers further transport massive plasma liquid components and enrich blood cells on one side of wounds. These blood cells as well as coagulation factors can be activated by the positively charged QC. As a result, blood clots form and seal the bleeding site tightly.
Antibacterial and hemostatic activities of DQHP-n. a–c The proliferation curves of E. coli, S. aureus, and MRSA, respectively. d Diagram of the rat tail bleeding models. e, f Bleeding time and blood loss in the tail bleeding model. g Diagram of the rat liver bleeding model. h, i Bleeding time and blood loss in the liver bleeding model. Values are expressed as the mean ± SD (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001, N.S. = no significance
Antioxidant Stress of DQHP-6
An oxidative stress model was constructed by pretreating RAW264.7 cells with 1 µg/mL LPS. The obtained cells were further incubated with extracts of DQHP-0 (L-D-0) and DQHP-6 (L-D-6). We detected the total ROS level by flow cytometry. As shown in Fig. 5a, b, the percentage of ROS+ cells was 1.94 ± 0.31% for the B.C. group, 13.09 ± 2.17% for the LPS group, 10.91 ± 1.34% for the L-D-0 group, and 7.33 ± 2.87% for the L-D-6 group. A significant difference was observed between the LPS and L-D-6 groups (P < 0.01). Nitric oxide (NO) can be released from inflammatory cells, and its concentration is highly correlated with the level of ROS [42]. As shown in Fig. 5c, the NO concentration of the L-D-6 group was significantly less than those of the LPS and L-D-0 groups (P < 0.001). These results confirmed that DQHP-6 had an antioxidant effect.
The LPS-induced inflammatory and oxidative stress pathway has been elucidated [43]. LPS first combines with LBP and CD14 to form a trimer complex, and then interacts with the TLR4–MD2 complex to transduce the signal into cells. After that, intracellular signaling pathways, such as MAPK and IKK-NFκB, are activated. To screen out the targets of DQHP-6, the expression levels of several hub genes were detected by western blotting. As shown in Fig. 5d, p-IκBα and NFκB were down-regulated. The expression of NFκB was further confirmed by immunofluorescence (IF) staining (Fig. 5e). These results suggested that DHM could inhibit the phosphorylation of IκBα and then inhibit NFκB from entering the nucleus. The mRNA expression of several pro-inflammatory factors was also detected. As shown in Fig. 5f–j, IL-18, TNF, IL-1β, and iNOS were down-regulated in the L-D-6 group (P < 0.01).
Antioxidant stress of DQHP-6. a, b Flow cytometry analysis of reactive oxygen species (ROS); c the content of extracellular NO; d the protein expression of several hub genes in the ROS pathway, β-actin served as the control; e immunofluorescence images of NFκB, scale bar: 20 μm; f–j the relative mRNA expression levels of several pro-inflammatory factors, including IL-18, TNF, IL-1β, and iNOS. Values are expressed as the mean ± SD (n = 3), **P < 0.01, ***P < 0.001, N.S. = no significance
Anti-inflammatory Activity of DQHP-6
M1 macrophages are the main source of NO and pro-inflammatory cytokines [44]. M1 macrophages can be activated by a variety of factors, such as LPS, GM-CSF, and IFN-γ [45]. Activated M1 macrophages can phagocytize a large number of pathogens and kill intracellular bacteria [46]. However, they also aggravate the inflammatory processes that are harmful to wound healing [47]. In this study, the influence of DQHP-6 on macrophage M1 polarization was evaluated by flow cytometry. The markers of myeloid cells (CD11b) and M1 macrophages (CD80 and CD86) were labeled by different fluorescent dyes [48]. As shown in Fig. 6a, b, the percentage of CD11b+/CD80+ cells in the L-D-6 group was significantly less than those in the LPS and L-D-0 groups (P < 0.001). The percentage of CD11b+/CD86+ cells showed a similar trend (Fig. 6c, d). These results indicated that macrophage M1 polarization was effectively inhibited. To provide further support for these results, we further sorted out CD11b+ cells and counted the proportion of CD80+/CD86+ cells. As shown in Fig. 6e, f, the percentage of CD80+/CD86+ cells was 1.87 ± 0.47% for B.C. group, 7.73 ± 1.10% for the LPS group, 8.87 ± 0.85% for the L-D-0 group, and 3.98 ± 0.56% for the L-D-6 group. It could be safely concluded that DQHP-6 diminished LPS-induced inflammation by inhibiting macrophage M1 polarization.
Promotion of Wound Healing in vivo
A Gram-negative bacteria-infected skin injury model was prepared by smearing E. coli bacterial fluid onto wound sites. Optical images of wound sites at different time points are shown in Fig. 7a. The infected wounds were almost completely healed within 15 days. To reveal the dynamic process of wound healing, traces of wound closures were marked [49]. As shown in Fig. 7b, the wounds in the DQHP-6 group healed faster. The quantitative results are shown in Fig. 7c. At day 5, the wound closure rate was 37.9 ± 6.9% for the B.C. group, 45.4 ± 5.6% for the gauze group, 47.4 ± 6.4% for the DQHP-0 group, and 57.5 ± 7.6% for the DQHP-6 group. A significant difference was observed between DQHP-6 and the other groups (P < 0.05). DQHP-6 promoted wound healing mainly in the early stage of wound healing.
The regenerated skin tissues were resected for histological analysis [50]. The HE staining images are shown in Fig. 7d. At day 5, a few exudates were observed on the upper surface of wounds, indicating that the infected skin injury model was successfully prepared. The wounds in the DQHP-6 group showed fewer inflammatory cells. This phenomenon could be attributed to the anti-infection and anti-inflammatory effects of QC and DHM. On days 5 and 10, we found more granulation tissue in the DQHP-6 group. At day 15, complete dermal and epidermal layers formed in the DQHP-6 group but not in the other groups. These results revealed that DQHP-6 was beneficial for tissue proliferation and re-epithelization.
The mRNA expression levels of several cytokines were detected at day 5. As shown in Fig. 7e, fibroblast growth factor-2 (FGF2) and platelet-derived growth factor (PDGF) were significantly up-regulated in the DQHP-6 group (P < 0.05). FGF2 and PDGF are both important for cell proliferation and angiogenesis. As shown in Fig. 7f, g, pro-inflammatory factors (IL-1α and IL-6) were down-regulated, and anti-inflammatory factors (IL-10 and TGF-β) were up-regulated in the DQHP-6 group. Notably, TGF-β is necessary for the synthesis and secretion of collagen. However, the abnormal expression of TGF-β may lead to scar formation in some cases [51]. In this study, TGF-β was slightly up-regulated in the DQHP-6 group, which was beneficial to collagen formation but not to scar formation.
Accelerated wound healing by DQHP-6. a Optical images of wound sites, scale bar: 1 cm. b Dynamic traces of wound sites, scale bar: 1 cm. c Wound closure rate. d HE staining images of the regenerated skin tissue, scale bar: 500 μm. e, g The relative expression of several regeneration-related genes, including FGF2, PDGF, IL-1α, IL-6, IL10, and TGF-β. Values are expressed as the mean ± SD (n = 3), **P < 0.01, ***P < 0.001, N.S. = no significance
In this experiment, a series of immunohistochemical (IHC) staining was performed to identify the degree of inflammatory cell infiltration. Total inflammatory cells were marked by CD45. The quantitative results of CD45+ cells are shown in Fig. 8a. At each time point, the DQHP-6 group exhibited the fewest CD45+ cells. M1 macrophages secrete a variety of cytokines to enhance the inflammatory response [52]. M1 macrophages were marked by CD80 and CD86. The quantitative results of CD80+ cells are shown in Fig. 8b. At day 10, the percentage of CD80+ stained area was 9.27 ± 0.47% for the B.C. group, 7.65 ± 0.58% for the gauze group, 9.24 ± 0.87% for the DQHP-0 group, and 4.68 ± 0.49% for the DQHP-6 group. A significant difference was observed in DQHP-6 (P < 0.001). The results of CD86+ cells are shown in Fig. 8c and exhibit a similar tendency. In conclusion, fewer CD80+ cells and CD86+ cells were found in the DQHP-6 group.
The collagen content of the neo-skin tissue was visualized by Masson’s staining assay. As shown in Fig. 8d, the collagen fibers were dyed blue. As the repair time increased from 5 to 15 days, the content of collagen fibers increased gradually. No pathological scar tissue was found in any sample. The quantitative results of collagen content are shown in Fig. 8e. The DQHP-6 group showed the most collagen content at each time point. At day 15, the percentage of collagen-occupied area was 32.2 ± 3.9% for the B.C. group, 41.3 ± 1.5% for the gauze group, 39.9 ± 3.1% for the DQHP-0 group, and 56.3 ± 7.9% for the DQHP-6 group. A significant difference was observed in DQHP-6 (P < 0.01).
Ki67 is a nuclear protein encoded by the MKI67 gene, and plays a key role in the cell cycle, cell proliferation, and ribosomal RNA transcription [53]. In this study, Ki67 immunofluorescence staining was performed to visualize proliferative cells. As shown in Fig. 8f, the nucleus was dyed blue, and the Ki67-positive (Ki67+) cells were dyed red. We found that the DQHP-6 group had the most Ki67+ cells. The counting results of Ki67+ cells are shown in Fig. 8g. At day 15, the number of Ki67+ cells was 7.7 ± 1.5 for the B.C. group, 13.3 ± 2.1 for the gauze group, 14.0 ± 2.0 for the DQHP-0 group, and 21.3 ± 2.5 for the DQHP-6 group. A significant difference was observed in DQHP-6 (P < 0.01).
The in vivo and in vitro results demonstrated that DQHP-6 could remodel the harsh wound microenvironment and further accelerate skin regeneration. The excellent performance of DQHP-6 included stopping bleeding, killing bacteria, reducing oxidative stress, and inhibiting macrophage M1 polarization, which could be attributed to the comprehensive effect of physical structure (nanofiber) and chemical composition (DHM and QC). For example, DHM played a vital role in the antibacterial and antioxidant effects of DQHP-6 and the inhibition of macrophage M1 polarization by DQHP-6, and the application effect was desirable. Notably, DHM is a flavonoid compound that has been widely applied for antitumor chemotherapy. Thus, the cytotoxicity of DHM is nonnegligible. It was previously revealed that intraperitoneal injection of DHM at a dosage of 20 mg/kg every third day for 21 days did not lead to obvious toxic effects [54]. In our work, the content of DHM in DQHP-n was significantly lower than that in chemotherapeutic reagents, and there was sustained release of DHM from DQHP-n. Thus, it could be safely inferred that the safety of DQHP-n containing DHM was relatively good to meet the general requirements of biomedical devices. DQHP-n exhibited great potential for clinical transformation. However, the clinical efficacy and safety of DQHP-n remain to be synthetically investigated by follow-up clinical trials.
In vivo mechanism of DQHP-6. a–c Quantitative results of the immunohistochemical staining of CD45, CD80, and CD86, respectively. d, e Optical images of Masson’s staining and the quantitative results. Scale bar: 100 μm; f, g Immunofluorescence images of Ki67 staining and the quantitative results. Scale bar: 50 μm Values are expressed as the mean ± SD (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001, N.S. = no significance
Conclusions
In summary, we successfully fabricated a series of LBL self-assembled multilayer nanofiber dressings (DQHP-n, n = 0, 2, 4 and 6) using positively charged QC and negatively charged DHM-HA. The obtained DQHP-n exhibited a relatively good hydrophilicity, flexibility, and biocompatibility. In particular, DQHP-6 activated the EMT pathway to promote host cell migration, which was beneficial for wound healing. We also found that DQHP-6 could effectively stop bleeding, kill bacteria, eliminate inflammation, scavenge ROS, and systematically remodel the HMW. The application potential of DQHP-6 was validated, which demonstrated its effects and mechanisms in vivo wound healing. This work is of great significance for the design of novel bioactive materials by combining a variety of chemical compositions and spatial structures. The products developed by this work provide an effective way to repair infected wounds and may be extended to other complicated wounds.
Abbreviations
- HMW:
-
Harsh Microenvironment in Wound
- DHM:
-
Dihydromyricetin
- QC:
-
Quaternized chitosan
- HA:
-
hyaluronic acid
- LBL:
-
Layer-by-layer
- EMT:
-
Epithelial–mesenchymal transformation
- ROS:
-
Reactive oxygen species
- CSI:
-
Chronic skin injuries
- TEWDs:
-
Tissue engineering wound dressings
- ECM:
-
Extracellular matrix
- PCL:
-
Polycaprolactone
- FGF2:
-
fibroblast growth factor-2
- PDGF:
-
Platelet-derived growth factor
- TCBs:
-
Traditional Chinese herbs
- FDA:
-
the Food and Drug administration
- NMPA:
-
National Medical Products Administration
- FT-IR:
-
Fourier transform infrared spectrum
- XRD:
-
X-ray diffraction spectrum
- NS:
-
Normal saline
- E. coli :
-
Escherichia coli
- S. aureus :
-
Staphylococcus aureus
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- NO:
-
Nitric oxide
- HR:
-
Hemolysis ratio.
References
Zeng QK, Qian YN, Huang YJ, Ding F, Qi XL, Shen JL. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioactive Mater. 2021;6:2647.
Liang YP, He JH, Guo BL. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15:12687.
Chen JH, Bao XF, Meng T, Sun J, Yang XR. Zeolitic imidazolate framework-67 accelerates infected diabetic chronic wound healing. Chem Eng J. 2022;430:10.
Wang ZJ, Ke MF, He L, Dong Q, Liang X, Rao J, Ai JJ, Tian C, Han XW, Zhao YA. Biocompatible and antibacterial soy protein isolate/quaternized chitosan composite sponges for acute upper gastrointestinal hemostasis. Regenerative Biomater. 2021;8:12.
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545.
Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci. 2019;6:13.
Kim K, Mahajan A, Patel K, Syed S, Acevedo-Jake AM, Kumar VA. Materials and cytokines in the healing of diabetic foot ulcers. Adv Ther. 2021;4:18.
Mao JY, Chen L, Cai ZW, Qian ST, Liu ZM, Zhao BF, Zhang YG, Sun XM, Cui WG. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv Funct Mater. 2021;25:2111003.
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev. 2019;99:665.
Xu ZJ, Han SY, Gu ZP, Wu J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv Healthc Mater. 2020;9:11.
Ahmed MK, Zayed MA, El-dek SI, Hady MA, El Sherbiny DH, Uskokovic V. Nanofibrous epsilon-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioactive Mater. 2021;6:2070.
Yang ZF, Huang RK, Zheng BN, Guo WT, Li CK, He WY, Wei YG, Du Y, Wang HM, Wu DC, Wang H. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv Sci. 2021;8:12.
El-Aassar MR, Ibrahim OM, Fouda MMG, Fakhry H, Ajarem J, Maodaa SN, Allam AA, Hafez EE. Wound dressing of chitosan-based-crosslinked gelatin/ polyvinyl pyrrolidone embedded silver nanoparticles, for targeting multidrug resistance microbes. Carbohydr Polym. 2021;255:12.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425.
Kim YE, Kim J. ROS-scavenging therapeutic hydrogels for modulation of the inflammatory response. ACS Appl Mater Interfaces. 2022. https://doi.org/10.1021/acsami.1c18261.
Ke MF, Wang ZJ, Dong Q, Chen FX, He L, Huselstein C, Wang XH, Chen Y. Facile fabrication of soy protein isolate-functionalized nanofibers with enhanced biocompatibility and hemostatic effect on full-thickness skin injury. Nanoscale. 2021;13:15743.
Zhang BL, Gao Y, Yang R, Ouyang ZJ, Yu HW, Wang H, Shi XY, Shen MW. Tumor-anchoring drug-loaded fibrous microspheres for MR imaging-guided local chemotherapy and metastasis inhibition. Adv Fiber Mater. 2022. https://doi.org/10.1007/s42765-022-00137-8.
He H, Wu MA, Zhu JW, Yang YY, Ge RL, Yu DG. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv Fiber Mater. 2022;4:305.
Liu YB, Chen XH, Liu YY, Gao YH, Liu P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers. 2022;14:13.
Wang ML, Hou JS, Yu DG, Li SY, Zhu JW, Chen ZZ. Electrospun tri-layer nanodepots for sustained release of acyclovir. J Alloys Compd. 2020;846:9.
Zhang MX, Song WL, Tang YX, Xu XZ, Huang YN, Yu DG. Polymer-based nanofiber-nanoparticle hybrids and their medical applications. Polymers. 2022;14:27.
Yu DG, Wang ML, Ge RL. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdisc Rev-Nanomed Nanobiotechnol. 2021;14:27.
Wang MY, Tan YL, Li D, Xu GW, Yin D, Xiao YC, Xu TG, Chen XF, Zhu XY, Shi XY. Negative isolation of circulating tumor cells using a microfluidic platform integrated with streptavidin-functionalized PLGA nanofibers. Adv Fiber Mater. 2021;3:192.
Shen YZ, Zhang YY, Gao ZF, Ye YW, Wu QP, Chen HY, Xu JJ. Recent advances in nanotechnology for simultaneous detection of multiple pathogenic bacteria. Nano Today. 2021;38:26.
Wang ZJ, Hu WK, You WJ, Huang G, Tian WQ, Huselstein C, Wu CL, Xiao Y, Chen Y, Wang XH. Antibacterial and angiogenic wound dressings for chronic persistent skin injury. Chem Eng J. 2021;404:13.
Wagberg L, Erlandsson J. The use of layer-by-layer self-assembly and nanocellulose to prepare advanced functional materials. Adv Mater. 2021;33:13.
Hu WK, Wang ZJ, Zha Y, Gu X, You WJ, Xiao Y, Wang XH, Zhang SM, Wang JL. High flexible and broad antibacterial nanodressing induces complete skin repair with angiogenic and follicle regeneration. Adv Healthc Mater. 2020;9:13.
Tu YY. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angewandte Chemie-Int Edition. 2016;55:10210.
Chi J, Sun L, Cai L, Fan L, Shao C, Shang L, Zhao Y. Chinese herb microneedle patch for wound healing. Bioactive Mater. 2021;6:3507.
Geng S, Jiang ZJ, Ma HJ, Pu P, Liu BG, Liang GZ. Fabrication and characterization of novel edible Pickering emulsion gels stabilized by dihydromyricetin. Food Chem. 2021;343:10.
Zhang JY, Chen Y, Luo HQ, Sun LL, Xu MT, Yu J, Zhou QG, Meng GL, Yang SJ. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front Pharmacol. 2018;9:11.
Sun Y, Liu SS, Yang SW, Chen C, Yang YT, Lin MY, Liu C, Wang WM, Zhou XD, Ai QD, Wang W, Chen NH. Mechanism of dihydromyricetin on inflammatory diseases. Front Pharmacol. 2022;12:10.
Liu D, Mao YQ, Ding LJ, Zeng XA. Dihydromyricetin. A review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci Technol. 2019;91:586.
Castro KC, Campos MGN, Mei LHI. Hyaluronic acid electrospinning: challenges, applications in wound dressings and new perspectives. Int J Biol Macromol. 2021;173:251.
Hu W, Wang Z, Zha Y, Gu X, You W, Xiao Y, Wang X, Zhang S, Wang J. High flexible and broad antibacterial nanodressing induces complete skin repair with angiogenic and follicle regeneration. Adv Healthc Mater. 2020. https://doi.org/10.1002/adhm.202000035.
Ao HY, Yang SB, Nie BE, Fan QM, Zhang QC, Zong JJ, Guo SR, Zheng XB, Tang TT. Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J Mater Chem B. 2019;7:1951.
Liu MM, Guo H, Li ZY, Zhang CH, Zhang XP, Cui QH, Tian JZ. Molecular level insight into the benefit of myricetin and dihydromyricetin uptake in patients with Alzheimer’s diseases. Front Aging Neurosci. 2020;12:12.
Freitas ED, Moura CF, Kerwald J, Beppu MM. An overview of current knowledge on the properties, synthesis and applications of quaternary chitosan derivatives. Polymers. 2020;12:41.
Shevelev AB, La Porta N, Isakova EP, Martens S, Biryukova YK, Belous AS, Sivokhin DA, Trubnikova EV, Zylkova MV, Belyakova AV, Smirnova MS, Deryabina YI. Vivo antimicrobial and wound-healing activity of resveratrol, dihydroquercetin, and dihydromyricetin against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. Pathogens. 2020;9:22.
Lv C, Li L, Jiao Z, Yan H, Wang Z, Wu Z, Guo M, Wang Y, Zhang P. Improved hemostatic effects by Fe3 + modified biomimetic PLLA cotton-like mat via sodium alginate grafted with dopamine. Bioactive Mater. 2021;6:2346.
Liang YP, Zhao X, Hu TL, Chen BJ, Yin ZH, Ma PX, Guo BL. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15:17.
Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquiere B, Maio N, Rice CM, Rouault TA, Cassel T, Higashi RM, Lane AN, Fan TWM, Wink DA, McVicar DW. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. 2020;11:17.
Jin K, Luo ZM, Zhang B, Pang ZQ. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sinica B. 2018;8:23.
Pan YY, Yu YD, Wang XJ, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:9.
Li XY, Guo XM, Ling JB, Tang Z, Huang GN, He LZ, Chen TF. Nanomedicine-based cancer immunotherapies developed by reprogramming tumor-associated macrophages. Nanoscale. 2021;13:4705.
Martin KE, Garcia AJ. Macrophage phenotypes in tissue repair and the foreign body response: implications for biomaterial-based regenerative medicine strategies. Acta Biomaterialia. 2021. https://doi.org/10.1016/j.actbio.2021.03.038.
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao ZY, Goodman SB. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80.
Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186.
Zhang BL, He JH, Shi MT, Liang YQ, Guo BL. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem Eng J. 2020;400:14.
Qiao Y, He J, Chen WY, Yu YH, Li WL, Du Z, Xie TT, Ye Y, Hua SY, Zhong DN, Yao K, Zhou M. Light-activatable synergistic therapy of drug-resistant bacteria-infected cutaneous chronic wounds and nonhealing keratitis by cupriferous hollow nanoshells. ACS Nano. 2020;14:3299.
Zhang J, Zheng YJ, Lee J, Hua JY, Li SL, Panchamukhi A, Yue JP, Gou XW, Xia ZF, Zhu LY, Wu XY. A pulsatile release platform based on photo-induced imine-crosslinking hydrogel promotes scarless wound healing. Nat Commun. 2021;12:13.
Rao L, Zhao SK, Wen CR, Tian R, Lin LS, Cai B, Sun Y, Kang F, Yang Z, He LC, Mu J, Meng QF, Yao GY, Xie N, Chen XY. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. 2020;32:9.
Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol. 2017;35:1061.
Wang Z, Guo Z, You W, Wang W, Zhou F, Zhang R, He Z, Chen H, Wang X. Identification of chemotherapeutic dihydromyricetin with enhanced anti-tumor activity and biosafety for muscle invasive bladder cancer. Res Square. 2021;25(2):152–65.
Acknowledgements
The authors thank Prof. Weikang Hu from Huazhong University of Science and Technology for the great support. This work was financially supported by the Fellowship of China National Postdoctoral Program for Innovative Talants (BX20220240), the Improvement Project for Theranostic Ability on Difficulty Miscellaneous Disease (Tumor) from National Health Commission of China (ZLYNXM202006), the Chinese Central Special Fund for Local Science and Technology Development of Hubei Province (2018ZYYD023), and the Science and Technology Department of Hubei Province Key Project (2018ACA159).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Z., You, W., Wang, W. et al. Dihydromyricetin-Incorporated Multilayer Nanofibers Accelerate Chronic Wound Healing by Remodeling the Harsh Wound Microenvironment. Adv. Fiber Mater. 4, 1556–1571 (2022). https://doi.org/10.1007/s42765-022-00180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00180-5