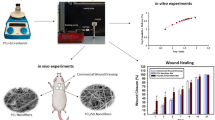
Abstract
A good wound dressing needs to promote wound healing and tissue repair when the skin is injured. In this study, a self-made spherical section free surface electrospinning device was used to produce large quantities of electrospun porous polylactic acid (PLA)/chitosan (CS)/aloin nanofiber membranes (NFMs) for antibacterial wound dressing. The porous structures of PLA-based nanofibers were controlled by adjusting the weight ratios of mixed solvent and solute. The results showed that high-quality porous PLA/CS/aloin (PCA) NFMs were obtained when the weight ratios of chloroform/N, N-dimethylformamide and PLA: CS were 90/10 and 7:1, respectively. The porous PCA NFMs exhibited high porosity, acceptable mechanical properties, moderate hydrophobicity, good swelling property, and high water vapor transmission rate. Moreover, they also showed excellent blood coagulative, antibacterial, biocompatible properties, which had the potential to be used in the application of antibacterial wound dressings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Skin is the outermost organ covering the entire body, that is in direct contact with the external environment and has functions of protection, excretion, regulation of temperature and so on. However, cuts, burns, surgical incisions or infections can all affect the structure and functions of this organ [1]. As shown in fig. S1, the four stages of wound healing for skin injuries are hemostasis, inflammation, proliferation, and remodeling [2, 3]. Traditional wound dressings are typically made of low-cost raw materials, that do not provide a moist environment and adhere to the wound, causing painful sensations [4]. Therefore, appropriate wound dressing materials should possess a skin-like structure, bioactivity and biodegradability.
Polylactic acid (PLA) is usually used to improve the mechanical properties of wound dressings because of its attractive mechanical properties, biocompatibility and biodegradability [5, 6]. Nevertheless, the high hydrophobicity and brittleness of pure PLA restrict its application in wound dressings. Chitosan (CS) is a kind of carbohydrate polymer produced by the alkaline deacetylation of natural chitin [7], which contains N-acetyl glucosamine, a major component of dermal tissue [8]. Hydrophilicity, biodegradability, biocompatibility, non-toxicity, antibacterial ability, hemostasis, anti-inflammatory, and remodeling behavior have all been demonstrated for CS [9,10,11,12]. However, poor mechanical properties of CS, as well as its rapid degradation rate limit its applications [13]. It has been found that biomaterials with excellent mechanical properties, wettability, biodegradability, and biocompatibility can be obtained by blending CS with PLA [13,14,15,16,17]. Aloe vera (AV) has been used as a wound healing medicinal plant for its anti-inflammatory, antioxidant, immunomodulatory, and antimicrobial activity [18, 19]. However, the properties of AV are greatly influenced by many factors, such as its production origin, growth period, extraction process, and transportation [20]. Aloin is the main anthraquinone in AV and contains polyphenols, which have a simple structure, better stability, excellent water-solubility, antibacterial activity, bioactivity, anti-inflammatory and anti-cancer properties [20,21,22], making it useful for wound healing.
The structure of micro/nanofibers is similar to that of collagen and conducive to cell proliferation and remodeling during wound healing [4, 23]. Wound dressings with micro/nanofiber structure have a large surface area, excellent exudate absorption capability, oxygen permeability, and antibacterial property. Electrospinning (ES) has been widely considered as one of the simplest and most efficient methods of producing micro/nanofibers. Electrospun fibers with unique structures, such as core–shell, hollow, porous, helical, and other, can be used as dermal substitutes, inducing the migration and proliferation of cells along the fiber scaffold to form new collagen [13, 24]. Besides, loading functional nanoparticles, nanowires or drugs on the fibers can be used for cell therapy, detection, and sensing [25,26,27,28,29]. Porous nanofibers with higher specific surface area than non-porous nanofibers can promote drug release, antibacterial activity, cell attachment, and other effects [30, 31]. There are two main methods to prepare porous nanofibers by ES. One method is to selectively remove a single component from the composite fibers, which may destroy the fiber structure and affect its subsequent application [32, 33]. Another method for achieving phase separation in ES process is to adjust ES parameters [34], which does not require any post-treatment processes and is more in practicality [33]. Currently, the ES technology for preparing porous nanofibers is mostly single-needle ES, but its low yield limits industrial application. As a result, many researches have focused on improving the ES device to enhance the nanofiber yield, as illustrated in a comparative table (see Table S1).
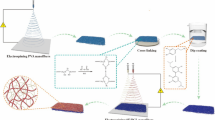
In this study, a self-made spherical section free surface electrospinning (SSFSE) device [35] was used to prepare porous PLA/CS/aloin (PCA) nanofiber membranes (NFMs) in batch for antibacterial wound dressings by controlling the solvent system and weight ratio in blends, as shown in Fig. 1. The effects of solvent and polymer ratios on the spinning solution properties and nanofiber morphology were investigated, and optimal parameters for obtaining porous NFMs (PNFMs) were determined. In addition, the mechanism of porous nanofibers formation with various morphologies was studied. Following that, the structure, yield, porosity, wettability, water vapor transmission rate (WVTR), swelling ratio, mechanical properties, antibacterial activity and biocompatibility of NFMs were characterized. The fabricated PCA PNFMs exhibited fair tensile strength, adequate hydrophobicity, high porosity, fine swelling property, ideal WVTR, great blood coagulative, and excellent antibacterial activity. Furthermore, they possessed good biocompatibility, which could promote cell attachment and growth, thereby accelerating wound healing.
Experiments Section
Materials
Chitosan (CS, 95% deacetylated powder) was provided by Macklin Biochemical Co., Ltd. Aloin (C21H22O9, 98%) was obtained from Alfa Biotechnology Co., Ltd. Polylactic acid (PLA) was acquired from Natureworks LLC (USA), with the product code of PLA 4032D. Chloroform (CF) and N,N-dimethylformamide (DMF) (Analytical Reagent) were supplied by Chinasun Specialty Products Co., Ltd. Calcium chloride anhydrous (CaCl2) was bought from Sinopharm Chemical Reagent Co., Ltd. The n-butanol was supplied by Prospect Chemical Reagent Co., Ltd. Nutrient broth medium and nutrient agar medium were purchased from SCAS Ecoscience Technology Inc. The healthy Sprague–Dawley (SD) rats, Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were obtained from Soochow University (China). Dulbecco’s modification of eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin–streptomycin (P&S) were bought from Thermo Fisher Scientific (USA). Phosphate-buffered saline (PBS, without calcium and magnesium) was offered from Corning Inc. The pentanediol solution was supplied by Chemical Reagent Co., Ltd (Table 1).
Preparation of Spinning Solutions
The PLA solutions with a PLA weight fraction of 7 wt% were prepared by mixing CF and DMF with different weight ratios of 100/0, 90/10, 80/20, 70/30 and 60/40, respectively. Then, PLA (7 wt%) and CS with different weight ratios of 7:1, 7:1.5 and 7:2 were dissolved respectively in the solvent mixture of CF and DMF with the optimal weight ratio (90/10). Finally, 0.0125 wt% aloin (relative to PLA/CS solution) was added into the PLA/CS solution with the optimal weight ratio of PLA to CS (7:1). After magnetically stirring the solutions for 12 h, the homogeneous spinning solutions were achieved. All concentrations of solutions were measured in weight/weight percentage (w/w%).
SSFSE Process
All spinning experiments were carried out at 24 ± 3 °C and the relative humidity of 70 ± 5%. The spinning parameters were the applied voltage of 50 kV and the receiving distance of 18 cm. The collecting device is a (35 cm length and 16 cm inner diameter), with a w rotational speed of 100 r/min. According to our previous work [35], the solution reservoir of SSFSE apparatus was made of a copper cylinder with a height of 40 mm and a diameter of 50 mm, which was truncated by a sphere with an optimal radius of 75 mm to prepare high-quality NFMs. When the voltage was applied, the steady-state of solution surface was broken and the jets were formed by overcoming the solution surface tension.
Figure 1 depicts the forces at the point o on one of the jets that emerged from the solution surface during the SSFSE process. It could be seen that the viscous force (\(\tau = av + bv^{2}\), \(v\)—the jet velocity, a, b—the constants determined by solution viscosity) and electric field force (\(F_{E} = q_{o} E\), \(q_{o}\)-the charge at the point o, E—the electric field intensity) had an significant impact on the jet motion. The greater the solution viscosity, the greater the viscous force, resulting in the greater electric field force required for the upward movement of jets [37]. The electric field distribution in SSFSE process was simulated by Maxwell 3D, as shown in Fig. S2 (a and b). The vector plot of electric field distribution in Fig S2,, illustrated that the electric field directions were pointing towards the drum, causing the jets to move upwards. Fig. S2(b) represented the radial electric field distribution curve on the top surface center of the reservoir, indicating that the maximum electric field intensity value appeared at the top edge of the reservoir, which made it easier for more jets to form around the edge of reservoir. Fig. S2(c) and (d) depict the photographs of SSFSE processes captured by a camera, which indicating that SSFSE could provide good spinning effects for both pure PLA and PLA/CS solutions. In Furthermore, the jets of 7 wt% PLA spinning solution were mainly concentrated at the edge of solution reservoir (Fig. S2(c)), and the jets of PLA/CS (7:1) spinning solution were also all around the edge of solution reservoir, but the number of jets was less than PLA solution due to the higher solution viscosity (Fig. S2 (d)). These experimental phenomena were consistent with the findings of theoretical analysis.
Characterization of Solution Properties
The viscosity and electrical conductivity of spinning solutions were determined by a viscometer (SNB-1, China) and a conductivity meter (DDS-307A, China), respectively. The corresponding average value of five measurements at room temperature was taken.
Characterization of NFMs
The morphology of NFMs was characterized by scanning electron microscopy (SEM, Japan). 100 nanofibers were randomly selected from 5 SEM images in each sample to analyze the nanofiber diameter distribution of NFMs using Image J software (USA). The elemental detection was determined by energy-dispersive X-ray spectroscopy (EDS) analysis (TM 3030, Japan). The attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy (Nicolet 5700, USA) was used to investigate the composition of NFMs. A NFM was immersed in n-butanol for 1 h until equilibrium was reached, and then its porosity (P) was calculated by measuring its mass before (\(M_{m}\)) and after (\(M\)) absorbing n-butanol according to Eq. (1).
where \(\rho\) is the density of n-butanol, \(\rho_{m}\) is the density of the dry NFM.
The yields (W) of NFMs were calculated by formula \(W = (W_{1} - W_{0} )/t\), and the weights of aluminum foil before (W0) and after (W1) spinning for 2 min were measured using a precise electronic balance (XJ120A, China), respectively. The average yield of each sample was determined by the results of five measurements.
Assessment of NFM properties
A Krüss K100 apparatus (Germany) was used to test the water contact angles (WCAs) of NFMs. The average value of WCAs was determined by measuring five different locations on the same sample using a droplet of 6 μl.
For measuring the moisture permeability of NFMs, the water vapor transmission rate (WVTR) values of NFMs were measured by a test tube including 10 ml of deionized water. The NFM was sealed on the test tube and then placed into an incubator at 37 °C and 50% humidity for 12 h. All measurements were repeated three times, and Eq. (2) was used to estimate the WVTR [38].
Where \(W_{loss}\) is the daily weight loss of water (g/day), and \(S\) is the area of the tube orifice.
The degree of swelling ratio (%) of NFM was obtained by soaking the NFM in PBS for 24 h at 37 °C. The initial weight of NFM was denoted as W0, and its weight after removing excess water on its surface with the filter paper was measured as WS. Each sample was repeated three times, and the swelling ratio of NFM was evaluated by the following Eq. (3) [39]:
The mechanical properties of NFMs were performed by a universal electromechanical test machine Instron (model 3365, USA) with a pre-tension of 0.2 cN and a tensile speed of 10 mm/min. The NFM samples with dimensions of 40 mm × 10 mm were analyzed. All the above measurements were done three times.
In Vitro Blood Coagulation Test
In vitro blood coagulation tests were carried out using the SD rat whole blood with 3.8% sodium citrate solution at a volume ratio of 9:1. The prepared NFMs and cotton gauze (15 × 15 mm) were laid flat in tubes, and each tube was equilibrated in water bath at 37 °C for 1 h. 20 μl of whole blood was dropped onto the sample surface in the tube, then 2 μl of 0.2 M CaCl2 solution was added in the whole blood to initiate blood clotting, and it was incubated at 37 °C for 5 min and 10 min, respectively. Subsequently, 5 ml of distilled water was added dropwise into the tube without disturbing the clot. Finally, 2 ml upper layer aqueous solution was absorbed to determine the absorbance values of hemoglobin at 540 nm using a visible spectrophotometer (T5, China). Without sample as the control, the blood clotting index (BCI) was calculated as follows [40]:
Antibacterial Test
The antibacterial activities of PLA/CS and PCA NFMs against S. aureus and E. coli were tested. 150 mg samples were placed separately in a conical flask containing 14 ml of sterile PBS after sterilizing in UV light for 1 h, and the standard blank cotton was used as the control. Then 1 ml of bacterial solution with a concentration of 3 × 105–5 × 105 colony forming units (CFUs/ml) was added into the conical flask, which was shaken for 18 h in an incubator shaker (IS-RDV1, USA) at 25 °C. Finally, 1 ml of the prepared solution was spread onto a nutrient agar plate, and the bacterial colony number of each plate was determined after incubation for 24 h at 37 °C. The antibacterial activities of samples were estimated based on the following Eq. (5) [41]:
Where Nc is the bacterial colony number of NFM and Nb is the bacterial colony number of standard blank cotton.
Cell Culture Study
Cell cultures were carried out using human umbilical vein endothelial cells (CRL-1730, ATCC). PLA, PLA/CS and PCA NFMs were cut into circular shape with a diameter of 14 mm for subsequent testing. The NFMs were rinsed with 75% ethanol for 30 min, immersed in DMEM (containing FBS and P&S) for 4 h, dried at the room temperature, and put in a 24-well plate. Then all NFMs were fixed using O-rings to avoid any movement before incubating cells, which could make cells be seeded on the top of NFMs. Each sample was cultured three times.
The initial adhesion and proliferation of cells (1 × 104 cells/well) seeded on NFMs were evaluated using CCK-8 assay at the corresponding time after cell seeding. The cell adhesion assay was incubated for 1, 3, 5 and 7 h, and the proliferation of cells was cultured for 1, 3, 5, and 7 days. On the measurement time, 500 μl of DMEM and 50 μl of CCK-8 were added to each well, which was also incubated at 37 °C in the dark for 2 h. Then, 100 μl of medium was transferred from each well to a new 96-well plate. The optical density was evaluated by a microplate reader (Synergy HT, USA) at a wavelength of 450 nm [42]. The tissue culture polystyrene (TCPS) was used as the control, and the cell adhesion rate was calculated as follows:
The morphology and survival of cells (5 × 104 cells/well) on NFMs were observed using a confocal laser scanning microscope (CLSM, Fv1000, Japan) after cells were stained with fluorescein diacetate (FDA) [43]. On the 1st, 3rd, and 5th day of cell cultures, the NFMs were washed with 1 ml PBS and then stained with 2 µl FDA (5 mg FDA/ml in acetone). After 20 min at 37 °C, the NFMs were taken out, the excess staining solutions were washed off with PBS, and the morphology and distribution of cells on NFMs were evaluated by CLSM. As an outstanding living cell indicator, FDA can only get into the cytoplasm of living cells and emit green fluorescence at a wavelength of 488 nm.
On the 3rd and 5th days of cell cultures (5 × 104 cells/well), the NFMs were washed three times with PBS and soaked in 2.5% pentanediol solution for 2 h at 4 °C. Then the NFMs were dehydrated with ethanol gradients of 25%, 50%, 75%, 90% and 100%, respectively. Finally, SEM was performed to observe the morphology and attachment of cells.
Statistical Analysis
All statistical analyses were performed by an analysis of variance (ANOVA) and the value of p lower than 0.05 meant statistically significant.
Results and Discussions
Properties of Spinning Solution
The electrical conductivity and viscosity of spinning solutions have significant impact on the yield and quality of NFMs during the ES process [44], which correlate with the compositions of mixed solution [45]. DMF is only used as an auxiliary solvent in SSFSE process because PLA is insoluble in it. Table 1 compares the physical properties of CF and DMF revealing that DFM has higher electrical conductivity and boiling point than CF. Therefore, the addition of DMF can improve the electrical conductivity and reduce the solvent volatilization speed of the solution. The electrical conductivity and viscosity of spinning solutions with different solvent weight ratios (CF/DMF) are presented in Table S2. When PLA was dissolved in pure CF, the solution had the highest viscosity, but the electrical conductivity was too low to be correctly measured. It was also observed that with the increase in DMF concentration, the viscosity of PLA solutions markedly decreased while its electrical conductivity gradually increased. After adding CS, the solution viscosity increased significantly as the CS content increased while the solution electrical conductivity remained almost unchanged. The reason for this was that the viscous CS solution increased the blend viscosity [46]. Moreover, the addition of aloin had little effect on the viscosity and electrical conductivity of the solution.
Morphology and Structure of NFMs
The morphologies of pure PLA NFMs prepared using the mixture of CF and DMF with different weight ratios were characterized by SEM, as shown in Fig. S3. The electrospun PLA fibers obtained using CF as a single solvent exhibited a porous surface structure and a wide range variation of fiber diameter. This was due to low boiling point of pure CF, which caused an excessively rapid evaporation rate, resulting in a sharply dropped temperature of the fiber surface. Meanwhile, in the high humidity environment, the water vapor could quickly condense into water droplets inside and on the surface of the fiber, finally expanding the fiber. The pores appeared on the dried fiber after the water droplets evaporated, as illustrated in Fig. S4 (a-1, a-2) [34, 47]. Furthermore, the high viscosity and extremely low conductivity of the pure CF solution resulted in jet instability and insufficient stretching during the SSFSE process [47, 48], causing the larger diameter and uneven distribution of the generated fibers, as shown in Fig. S3 (a). Adding a small amount of DMF (CF/DMF = 90/10) decreased the solution viscosity and increased the solution conductivity (Table S2), allowing the preparation of NFMs with uniform surface morphology and diameter distribution (Fig. S3 (b)). When the solvent weight ratio (CF/DMF) was 80/20, the fiber surface became rough from porous and the fiber diameter markedly decreased because of the further increase in solution conductivity and the further decrease of solution viscosity (Table S2), as displayed in Fig. S3 (c). The higher the solution conductivity, the stronger the electric field force, which made the jets to fully stretch, resulting at decrease in fiber diameter. However, the uniformity of fiber diameter distribution decreased at this time (Fig. S3 (c)), which was caused by the aggravation of jet instability in the SSFSE process as thesolution viscosity decreased. When CF/DMF ratio was 70/30, the fiber diameter decreased further and the beads appeared as a result of significant decrease in solution viscosity (Table S2 and Fig. S3 (d)). When CF/DMF ratio was 60/40, the coral-like substances were formed (Fig. S3 (e)) due to the too low solution viscosity (Table S2).
As shown in Fig. 2, the internal morphologies of pure PLA fibers prepared with ratios 90/10 and 80/20 (CF/DMF) were examined through SEM images of their cross-sections. There were pores on the surface and inside of PLA PNFM fibers obtained with ratio 90/10 caused by different evaporation rates of solvent and non-solvent, leading to the phase separation [49], as illustrated in Fig. S4 (b-1, b-2). The pores within fibers were interconnected nanochannels, with much higher specific surface area than solid fibers. When CF/DMF ratio was 80/20, the surface of fiber had no pores, but its interior was porous. This was due to the fact that the higher the DMF content, the slower the evaporation rate of the mixed solvent. Hence, the evaporation process of the mixed solvent containing more DMF could be sustained for long time to allow water vapor to penetrate the fiber surface and condense into water droplets inside the fiber. When the DMF was completely evaporated, the water droplets evaporated and formed pores within the fiber, as shown in Fig. S4(c-1, c-2). As a result, the choice of solvent had a significant impact not only on the surface morphology of the fiber but also on its internal structure. And a CF/DMF ratio of 90/10 was selected to prepare PNFMs for subsequent studies, while non-porous NFMs obtained by the mixed solvent with 80/20 were used as the control.
The morphologies of PLA/CS PNFMs with different ratios of PLA to CS and PCA PNFM, as well as their corresponding diameter distributions, are shown in Fig. 3. Because of increased viscosity of PLA/CS solution, the average fiber diameter of PLA/CS PNFMs increased with the increasing CS content, the uniformity of fiber diameter distribution decreased, and the pore structure on the fiber surface became less obvious (Table S2). Therefore, the ratio of PLA to CS (7:1) was selected as the optimal parameter and used for subsequent studies. For PCA solution, its viscosity and electrical conductivity were all similar to PLA/CS (7:1) solution, resulting in the average fiber diameter of PCA PNFM that was comparable to that of PLA/CS (7:1) PNFM.
Table 2 demonstrated that pure PLA PNFM mainly contained carbon (C) and oxygen (O). Nitrogen (N) was detected in PLA/CS and PCA PNFMs, confirming the successful blending of CS with PLA. And PCA PNFM had higher O contents due to the addition of aloin.
The porosities of PLA (80/20), PLA (90/10), PLA/CS and PCA NFMs were obtained by Eq. (1), which were 46.87 ± 2.42%, 52.81 ± 3.1%, 48.42 ± 2.66%, and 48.48 ± 2.75%, respectively. PLA/CS and PCA PNFMs had similar porosity because of their similarity in fiber diameter and structure. The fabricated PNFMs with higher porosity and interconnected pores could provide better nutrient and oxygen transfer for cell culture.
ATR-FTIR spectra of PLA, CS, PLA/CS and PCA NFMs are presented in Fig. 4a, which were used to assess the appearance of new peaks or peak shifts between PLA, CS, and aloin. PLA displayed a –CH asymmetric stretch at 2992 cm−1, a –C–O stretching at 1759 cm−1, a –CH bending in –CH3 at 1454 cm−1, and a –C–C stretching at 870 cm−1. Compared to PLA, the characteristic peak at 1084 cm−1 represented C–O–C glycosidic bond, the peak at 1034 cm−1 associated with the C–O stretching, and the band intensity at 1759 cm−1 were promoted in PLA/CS and PCA. And for CS, a characteristic peak at about 1664 cm−1 was assigned to C=O stretching vibration [50], which was offset to 1673 cm−1 in PLA/CS and PCA, indicating the existence of CS in them. In addition, the presence of aloin in PCA further increased the intensity at about 1664 cm−1, but no additional bands were observed.
Yield of NFMs
The yield of NFMs has a significant influence on their subsequent industrial application. In this study, the yield of pure PLA (90/10) PNFMs was 25.28 ± 3.08 g/h, the yield of pure PLA (80/20) NFMs was 36.16 ± 1.32 g/h, and the yield of PLA/CS PNFMs was 23.34 ± 7.04 g/h. This was due to the fact that increasing the DMF concentration could effectively improve the solution conductivity while reducing the solution viscosity, whereas addition of CS would decrease the solution conductivity while increasing the solution viscosity, as reflected by the changes in Table S2. As obvious in Fig. S2(c), the higher the solution conductivity, the greater the electric field force, the more charged jets were produced, and the faster the jets moved in the SSFSE process, leading to the higher yield of NFMs. moreover, the addition of aloin has little effect on the solution properties, resulting in the yield of PCA PNFMs similar to that of PLA/CS PNFMs. The yields of all prepared PNFMs were more than 20 g/h, which could realize the subsequent applications.
Properties of NFMs
The WCAs of PLA, PLA/CS and PCA NFMs, as shown in Fig.4b, clearly demonstrated that these NFMs were all hydrophobic materials due to the high proportion of hydrophobic PLA. The WCA of PLA (90/10) PNFM was higher than that of PLA (80/20) NFM for pure PLA NFMs, which was due to the pore structure on the fiber surface that could further enlarge their surface area and improve their hydrophobicity [30]. After adding CS into PLA solution, the WCA of PLA/CS PNFM decreased because CS contained the amino esters and hydroxyl groups [38]. When hydrophilic aloin was added to PLA/CS solution, the hydrophobicity of PCA PNFM was reduced and it become a moderately hydrophobic material, which could reduce the adhesion between the dressing and the wound, preventing the injury caused by the removal of the dressing.
The WVTR of a dressing determines its ability to control water loss. The dressing with an appropriate WVTR is applied to establish an optimal environment for natural healing [51]. It is reported that the wound dressings with WVTR values between 2000 and 2500 g/m2/day can supply sufficient moisture without causing the risk of wound dehydration [52]. PLA, PLA/CS and PCA PNFMs with high porosity could present direct channels for water vapor transport because their nanoscale channels and mutual permeability might greatly facilitate the movement of water vapor, thus promoting water transport [53]. As indicated in Fig. 4b, PLA PNFM had a lower WVTR than PLA/CS and PCA PNFMs, which was due to the fact that the hydrophilic groups in CS and aloin enhanced water vapor permeability. The WVTRs of PLA, PLA/CS and PCA PNFMs were all around 2200–2500 g/m2/day consistent with the requirements of wound dressings and could accelerate wound healing.
Swelling is an important characteristic of dressings, because it is aids, in the absorption of the infiltration of wound tissue, the formation of a moist microenvironment, and promotion of wound healing [54]. PLA (80/20) NFM had a low swelling ratio of 24.07 ± 1.68% due to the hydrophobicity of PLA, where PLA (90/10) PNFM had a high swelling ratio of 51.42 ± 9.2%, because of the accumulation of water molecules in the porous fiber. And PLA/CS PNFM had a higher swelling ratio of 59.88 ± 11.55% due to the hydrophilicity of CS. Furthermore, the addition of aloin had a little effect on the swelling ratio of PCA PNFM, which was 62.5 ± 14.36%.
Figure 4c illustrates the mechanical properties of PLA, PLA/CS and PCA NFMs. PLA (80/20) NFM had the lowest tensile strength and the largest elongation break, which was because PLA molecular chains were arranged neatly after the solvent phase separation, resulting in the improvement of the tensile strength of PNFMs. After adding CS into PLA solution, the tensile strength of PLA/CS PNFM increased, which could be attributed to the good interaction between CS and PLA laeding to the increase in tensile strength [55]. But the addition of CS might cause local stress concentration and reduce the elongation at break [55]. In addition, there was no significant difference between the mechanical properties of PCA and PLA/CS PNFMs, illustrating that a small amount of aloin had no obvious effect on the mechanical properties of PLA/CS PNFM.
Whole Blood Coagulation Assay
Blood coagulation can effectively seal off damaged blood vessels for hemostasis and minimize blood loss [56]. As depicted in Fig. 4d, PLA/CS and PCA PNFMs had lower BCI values at 5 min compared to gauze with superhydrophilicity and blood absorption, indicating a lower free hemoglobin level and faster coagulation rate. This was because the positively charged CS accelerated the aggregation of negatively charged platelets, thereby increasing the blood viscosity at the initial stage of hemostasis [57]. Moreover, as the time passed the BCI value decreased, and PCA PNFM showed the lowest BCI value, indicating faster clotting at 10 minutes. Above all, the fabricated PCA PNFM had the best hemostatic effect in vitro, which could lead to rapid progression of inflammatory stage of wound.
Antibacterial Activity of PNFMs
The antibacterial activities of PLA/CS and PCA PNFMs against both S. aureus and E. coli were investigated. These two bacteria represent the two major prokaryotic taxa involved in skin wound infection, Gram-negative and Gram-positive [54]. Blank cotton was used as a control sample. As indicated in Fig. S5 and Table 3, the inhibition rate of PLA/CS PNFM against S. aureus was 38.89%, which was higher than that against E. coli, indicating its weak antibacterial activity due to the addition of a small amount of CS. Adding aloin into PNFMs could significantly improve the antibacterial activities of PNFMs against S. aureus and E. coli, both of which were 99.9%.
Behavior of Cells on the NFMs
The initial cell adhesion is critical for subsequent cell proliferation and migration. Figure 5a portrays the cell adhesion culture on PLA, PLA/CS and PCA NFMs. It could be seen that the cell adhesion rates of all NFMs were more than 80% at 1, 3, 5 and 7 h. At 1, 5 and 7 h, more than 90% of cells adhered to PLA (90/10), PLA/CS and PCA PNFMs, and the cell adhesion rate of PLA (80/20) NFM was lower than that of PNFMs, which proved that the porous structure could promote the initial cell adhesion on NFMs. After cultured for 7 h, the cell adhesion of PCA PNFM was the highest because the addition of aloin could enhance the cell adhesion.
Cell adhesion of human umbilical vein endothelial cells cultured on PLA, PLA/CS and PCA PNFMs (a), the proliferation of cells cultured on PLA, PLA/CS and PCA PNFMs by CCK-8 assay (n = 3, *p < 0.05, **p < 0.01, ***p < 0.001) (b) and CLSM pictures of human umbilical vein endothelial cells cultured on PLA, PLA/CS and PCA PNFM on 1, 3 and 5 days (c)
As depicted in Fig. 5b, the CCK-8 assay was used to evaluate the cell proliferation on NFMs and TCPS, respectively. All NFMs showed an improved cell proliferation with increasing culture time from 1 to 7 days. The optical density value of PLA (90/10) PNFM was higher than that of PLA (80/20) NFM within 7 days because the larger specific surface area allowed filopodia to anchor more easily and tightly [58, 59]. The PLA/CS and PCA PNFMs all exhibited significantly higher optical density values than PLA NFMs and TCPS on the third, fifth and seventh days, indicating the fact that the addition of hydrophilic CS could promote the cell proliferation. And the optical density value of PCA PNFM was higher than that of PLA/CS PNFM on the fifth and seventh days, which proved that aloin could obviously improve the cell proliferation as well as behavior.
Figure 5c displays the CLSM micrographs of human umbilical vein endothelial cells cultured on PLA, PLA/CS and PCA NFMs after 1, 3 and 5 days, respectively. The CLSM results revealed that the number of cells increased with the culture time, indicating that the cells had good adhesion and proliferation on the scaffolds. During 1, 3, and 5 days, the number of cells attached on PLA (80/20) NFM was significantly lower than that on PLA (90/10) PNFM, demonstrating that the fibers with porous surface structure could promote cell viability. This was because nanopores provided additional anchorage points for cells to attach. And once the cells were attached, the cell–matrix adhesion was stronger due to the increase of the surface area of nanoporous fibers [60]. Moreover, on the first and third days, the number of cells attached on PLA PNFM was lower than that on PLA/CS and PCA PNFMs, illustrating that the addition of CS and aloin had a significant effect on promoting the cell proliferation. But on the fifth day, the cells had completely covered PLA, PLA/CS and PCA NFMs, which meant that these NFMs had similar good biocompatibility and all could promote the cell proliferation. This also indicated that there was an effective interaction between cells and fibers, which created a decent environment for cell growth.
The morphologies of human umbilical vein endothelial cells on the PLA, PLA/CS and PCA NFMs after 3 and 5 days of culture were further observed using SEM, as illustrated in Fig. S6. The SEM results demonstrated that the cells were flattened on the surface of NFMs after 3 days of culture, and PCA PNFM showed the highest cell proliferation compared to other NFMs. After 5 days of culture, the morphology of cells on PLA (80/20) NFM was round-shaped and did not spread. Contrarily, the cells spread well on the surface of PLA, PLA/CS, and PCA PNFMs, exhibiting a flattened phenotype. And the cells covered on PCA PNFM displayed a multilayer cell sheet, which was due to the highest cell proliferation after 5 days of culture. Combined with the CCK-8 assay and CLSM micrographs of cells, these results confirmed that the fabricated PCA PNFM could promote the cell growth and migration.
Conclusions
The SSFSE device was successfully used in this study to obtain PCA PNFMs with high yield and quality for antibacterial wound dressings. Firstly, the effects of the solvent weight ratio (CF/DMF) on the PLA solution properties were investigated by measuring the viscosity and electrical conductivity of spinning solution. Then, the surface morphology and internal structure of the fabricated PLA fibers using the solvent mixture of CF and DMF with various weight ratios were characterized by SEM. In addition, the mechanism of porous nanofibers formation with different morphologies was investigated. The results that high-quality PLA PNFMs were prepared when the solvent weight ratio (CF/DMF) was 90/10.
Following that, PLA/CS and PCA PNFMs were fabricated using a 90/10 solvent mixture of CF and DMF. The effects of the polymer weight ratio (PLA:CS) on the spinning solution properties and the surface morphology of fabricated PNFMs were explored, and 7:1 was determined to be the optimal weight ratio. Therefore, the solvent weight ratio of 90/10 and the polymer weight ratio of 7:1 was selected to prepare PCA PNFMs.
Finally, PLA, PLA/CS and PCA PNFMs were characterized and compared in terms of their structure, yield, porosity, wettability, WVTR, swelling ratio, mechanical property, hemostatic effect, antibacterial activity, and biocompatibility. The results illustrated that PCA PNFMs with aloin incorporation have good tensile properties, appropriate hydrophobicity, high porosity, fine swelling ratio, ideal WVTR, excellent blood coagulation, antibacterial activity, and good biocompatibility, indicating that the fabricated PCA PNFMs could be used in wound dressing applications.
References
Muhlstadt M, Thome C, Kunte C. Rapid wound healing of scalp wounds devoid of periosteum with milling of the outer table and split-thickness skin grafting. Br J Dermatol 2012;167:343–7.
Yang QW, Xie ZG, Hu JL, Liu YC. Hyaluronic acid nanofiber mats loaded with antimicrobial peptide towards wound dressing applications. Mater Sci Eng C 2021;128:112319.
Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol 2020;10:200223.
Li H, Wang Z, Zhang H, Pan Z. Nanoporous PLA/(chitosan nanoparticle) composite fibrous membranes with excellent air filtration and antibacterial performance. Polymers 2018;10:1085.
Liu S, Qin SH, He M, Zhou DF, Qin QD, Wang H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos B 2020;199:108238.
Gomaa SF, Madkour TM, Moghannem S, El-Sherbiny IM. New polylactic acid/ cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int J Biol Macromol 2017;105:1148–60.
Santos VP, Marques NSS, Maia PCSV, de Lima MAB, Franco LD, de Campos-Takaki GM. Seafood waste as attractive source of chitin and chitosan production and their applications. Int J Mol Sci 2020;21:4290.
Mirmajidi T, Chogan F, Rezayan AH, Sharifi AM. In vitro and in vivo evaluation of a nanofiber wound dressing loaded with melatonin. Int J Pharm 2021;596:120213.
Peluso G, Petillo O, Ranieri M, Santin M, Ambrosic L, Calabró D, Avallone B, Balsamo G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994;15:1215–20.
Feng PP, Luo Y, Ke CH, Qiu HF, Wang W, Zhu YB, Hou RX, Xu L, Wu SZ. Chitosan-based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol 2021;9:650598.
Dai TH, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther 2011;9:857–79.
Xu T, Yang H, Yang D, Yu ZZ. Polylactic acid nanofiber scaffold decorated with chitosan islandlike topography for bone tissue engineering. ACS Appl Mater Interfaces 2017;9:21094–104.
Thomas MS, Pillai PKS, Faria M, Cordeiro N, Barud H, Thomas S, Pothen LA. Electrospun polylactic acid-chitosan composite: a bio-based alternative for inorganic composites for advanced application. J Mater Sci: Mater Med 2018;29:137.
Shan XQ, Li FQ, Liu CS, Gao Q. Electrospinning of chitosan/poly(lactic acid) nanofibers: the favorable effect of nonionic surfactant. J Appl Polym Sci 2014;131:41098.
Mao DY, Li Q, Bai NN, Dong HZ, Li DK. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr Polym 2018;180:104–11.
Shen R, Xu W, Xue Y, Chen L, Ye H, Zhong E, Ye Z, Gao J, Yan Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif Cells Nanomed Biotechnol 2018;46:419–30.
Zhu HT, Qiu SS, Jiang W, Wu DX, Zhang CY. Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ Sci Technol 2011;45:4527–31.
Silva SS, Caridade SG, Mano JF, Reis RL. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr Polym 2013;98:581–8.
Yin J, Xu L. Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int J Biol Macromol 2020;160:352–63.
Li L, Gao S, Peng L, Wang X, Zhang Y, Hu Z, Gao J. Evaluation of efficacy of aloin in treating acute trauma in vitro and in vivo. Biomed Pharmacother 2017;88:1211–9.
Park MY, Kwon HJ, Sung MK. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci Biotechnol Biochem 2009;73:828–32.
Pan Q, Pan HM, Lou HZ, Xu YH, Tian L. Inhibition of the angiogenesis and growth of aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int 2013;13:69.
Xie J, Li X, Xia Y. Putting Electrospun nanofibers to work for biomedical research. Macromol Rapid Commun 2008;29:1775–92.
Sharma K, Bullock A, Ralston D, MacNeil S. Development of a one-step approach for the reconstruction of full thickness skin defects using minced split thickness skin grafts and biodegradable synthetic scaffolds as a dermal substitute. Burns 2014;40:957–65.
Mirsian S, Khodadadian A, Hedayati M, Manzour-ol-Ajdad A, Kalantarinejad R, Heitzinger C. A new method for selective functionalization of silicon nanowire sensors and Bayesian inversion for its parameters. Biosens Bioelectron 2019;142:111527.
Khodadadian A, Hosseini K, Manzour-Ol-Ajdad A, Hedayati M, Kalantarinejad R, Heitzinger C. Optimal design of nanowire field-effect troponin sensors. Comput Biol Med 2017;87:46–56.
Khodadadian A, Stadlbauer B, Heitzinger C. Bayesian inversion for nanowire field-effect sensors. J Comput Electron 2020;19:147–59.
Khodadadian A, Heitzinger C. A transport equation for confined structures applied to the OprP, Gramicidin A, and KcsA channels. J Comput Electron 2015;14:524–32.
Xiong R, Hua D, Van Hoeck J, Berdecka D, Leger L, De Munter S, Fraire JC, Raes L, Harizaj A, Sauvage F, Goetgeluk G, Pille M, Aalders J, Belza J, Van Acker T, Bolea-Fernandez E, Si T, Vanhaecke F, De Vos WH, Vandekerckhove B, van Hengel J, Raemdonck K, Huang C, De Smedt SC, Braeckmans K. Photothermal nanofibres enable safe engineering of therapeutic cells. Nat Nanotechnol 2021;16:1281–91.
Wang Y, Xu L. Preparation and characterization of porous core-shell fibers for slow release of tea polyphenols. Polymers 2018;10:144.
Thorvaldsson A, Stenhamre H, Gatenholm P, Walkenstrom P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromol 2008;9:1044–9.
Miao X, Lin J, Bian F. Utilization of discarded crop straw to produce cellulose nanofibrils and their assemblies. J Bioresour Bioprod 2020;5:26–36.
Wang CY, Wang J, Zeng LD, Qiao ZW, Liu XC, Liu H, Zhang J, Ding JX. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019;24:834.
Huang C, Thomas NL. Fabricating porous poly(lactic acid) fibres via electrospinning. Eur Polym J 2018;99:464–76.
Yin J, Ahmed A, Xu L. High-throughput free surface electrospinning using solution reservoirs with different depths and its preparation mechanism study. Adv Fiber Mater 2021;3:251–64.
Lu L, Wu D, Zhang M, Zhou W. Fabrication of polylactide/poly(ε-caprolactone) blend fibers by electrospinning: morphology and orientation. Ind Eng Chem Res 2012;51:3682–91.
Fang Y, Xu L. Four self-made free surface electrospinning devices for high-throughput preparation of high-quality nanofibers. Beilstein J Nanotechnol 2019;10:2261–74.
Miguel SP, Ribeiro MP, Coutinho P, Correia IJ. Electrospun polycaprolactone/aloe vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017;9:183.
Li CW, Fu RQ, Yu CP, Li ZH, Guan HY, Hu DQ, Zhao DH, Lu LC. Silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressings: a preclinical study. Int J Nanomed 2013;8:4131–45.
Wang Y, Xiao D, Zhong Y, Zhang L, Chen Z, Sui X, Wang B, Feng X, Xu H, Mao Z. Facile fabrication of carboxymethyl chitosan/paraffin coated carboxymethylated cotton fabric with asymmetric wettability for hemostatic wound dressing. Cellulose 2020;27:3443–53.
Sun FL, Zheng M. Antibacterial activity of polyester fabric treated with nano-TiO2 via one-bath process. Adv Mater Res 2013;843:58–65.
Shin YC, Lee JH, Kim MJ, Park JH, Kim SE, Kim JS, Oh JW, Han DW. Biomimetic hybrid nanofiber sheets composed of RGD peptide-decorated PLGA as cell-adhesive substrates. J Funct Biomater 2015;6:367–78.
Yin J, Fang Y, Xu L, Ahmed A. High-throughput fabrication of silk fibroin/hydroxypropyl methylcellulose (SF/HPMC) nanofibrous scaffolds for skin tissue engineering. Int J Biol Macromol 2021;183:1210–21.
Mohammadian M, Haghi AK. Systematic parameter study for nano-fiber fabrication via electrospinning process. Bulg Chem Commun 2014;46:545–55.
Yang T, Wu DF, Lu LL, Zhou WD, Zhang M. Electrospinning of polylactide and its composites with carbon nanotubes. Polym Compos 2011;32:1280–8.
Hang TA, Lan NP, Thu HTV, Park JS. Fabrication of an antibacterial non-woven mat of a poly(lactic acid)/chitosan blend by electrospinning. Macromol Res 2012;20:51–8.
Casasola R, Thomas NL, Georgiadou S. Electrospinning of poly(lactic acid): theoretical approach for the solvent selection to produce defect-free nanofibers. J Polym Sci Part B Polym Phys 2016;54:1483–98.
Wang FF, Sun ZY, Yin J, Xu L. Preparation, characterization and properties of porous PLA/PEG/Curcumin composite nanofibers for antibacterial application. Nanomaterials 2019;9:508.
Singh S, Anjum S, Joy J, Gupta B. Polysaccharide-aloe vera bioactive hydrogels as wound care system. In: Mondal M, editors. Cellulose-based superabsorbent hydrogels. Polymers and Polymeric Composites: A Reference Series. 2019;48:1473–90.
Esyanti R, Zaskia H, Amalia A, Nugrahapraja H. Chitosan nanoparticle-based coating as postharvest technology in banana. J Phys Conf Ser 2019;1204:012109.
Xu R, Xia HS, He WF, Li ZC, Zhao J, Liu B, Wang YZ, Lei Q, Kong Y, Bai Y, Yao ZH, Yan RS, Li HS, Zhan RX, Yang SS, Luo GX, Wu J. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci Rep 2016;6:24596.
Dutta J, Mohini P. Effect of degree of deacetylation and molecular weight on physicochemical properties of chitosan films. J Indian Chem Soc 2020;97:731–5.
Dong YL, Kong JH, Phua SL, Zhao CY, Thomas NL, Lu XH. Tailoring surface hydrophilicity of porous electrospun nanofibers to enhance capillary and push-pull effects for moisture wicking. ACS Appl Mater Interfaces 2014;6:14087–95.
Zou PF, Lee WH, Gao Z, Qin D, Wang Y, Liu J, Sun T, Gao Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr Polym 2020;232:115786.
Zakaria Z, Islam MS, Hassan A, Mohamad Haafiz MK, Arjmandi R, Inuwa IM, Hasan M. Mechanical properties and morphological characterization of PLA/Chitosan/Epoxidized natural rubber composites. Adv Mater Sci Eng 2013;3:10413–20.
Chan KYT, Yong ASM, Wang X, Ringgold KM, St John AE, Baylis JR, White NJ, Kastrup CJ. The adhesion of clots in wounds contributes to hemostasis and can be enhanced by coagulation factor XIII. Sci Rep 2020;10:20116.
Wang CH, Cherng JH, Liu CC, Fang TJ, Hong ZJ, Chang SJ, Fan GY, Hsu SD. Procoagulant and antimicrobial effects of chitosan in wound healing. Int J Mol Sci 2021;22:7067.
Filmon R, Basle MF, Atmani H, Chappard D. Adherence of osteoblast-like cells on calcospherites developed on a biomaterial combining poly(2-hydroxyethyl) methacrylate and alkaline phosphatase. Bone 2002;30:152–8.
Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res Part A 2003;67:531–7.
Meng FL, Chian KS, Mhaisalkar PS, Ong WF, Ratner BD. Effect of electrospun poly(d, l-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res Part A 2009;89:1040–8.
Acknowledgements
The work is supported financially by National Natural Science Foundation of China (Grant no. 11672198), Jiangsu Higher Education Institutions of China (Grant no. 20KJA130001), Six Talent Peaks Project of Jiangsu Province (Grant no. GDZB-050), and PAPD (A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions).
Author information
Authors and Affiliations
Contributions
JY and LX designed the experiments. JY performed the experiments and characterization, analyzed the data, and wrote the paper. LX supervised the data analysis and revised the paper. AA revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, J., Xu, L. & Ahmed, A. Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing. Adv. Fiber Mater. 4, 832–844 (2022). https://doi.org/10.1007/s42765-022-00141-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00141-y