Abstract
All-solid-state Li-SeS2 batteries (ASSLSs) are more attractive than traditional liquid Li-ion batteries due to superior thermal stability and higher energy density. However, various factors limit the practical application of all-solid-state Li-SeS2 batteries, such as the low ionic conductivity of the solid-state electrolyte and the poor kinetic property of the cathode composite, resulting in unsatisfactory rate capability. Here, we employed a traditional ball milling method to design a Li7P2.9W0.05S10.85 glass–ceramic electrolyte with high conductivity of 2.0 mS cm− 1 at room temperature. In order to improve the kinetic property, an interpenetrating network strategy is proposed for rational cathode composite design. Significantly, the disordered cathode composite with an interpenetrating network could promote electronic and ionic conduction and intimate contacts between the electrolyte–electrode particles. Moreover, the tortuosity factor of the carrier transport channel is considerably reduced in electrode architectures, leading to superior kinetic performance. Thus, assembled ASSLS exhibited higher capacity and better rate capability than its counterpart. This work demonstrates that an interpenetrating network is essential for improving carrier transport in cathode composite for high rate all-solid-state Li-SeS2 batteries.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Li-SeS2 batteries are attracting marvelous research interest for electric vehicles and large-scale energy storage in the future due to their lower cost and higher energy density than the traditional lithium-ion battery [1,2,3,4,5]. SeS2 has the advantage of having a high gravimetric capacity of 1124 mAh g−1; it exists as an eight-membered ring molecule with Se-S bonds and is being explored as a cathode material due to Se (10−5 S cm−1) better electric conductivity than S (10−30 S cm−1) [6,7,8,9]. At the same time, Li2Se demonstrated identical chemical properties and larger Li-ion migration ability than Li2S [10, 11]. Moreover, it's possible that using Se instead of S in the cathode composite will result in superior active materials consumption and rate capability.
The employment of solid-state electrolytes (SSEs) in Li-SeS2 batteries is supposed to alleviate liquid battery safety problems and the shuttling effect [12,13,14,15,16]. Sulfide-based electrolytes have a higher ionic conductivity advantage over other solid electrolyte classes [17, 18]. However, all-solid-state Li-SeS2 batteries have poor rate capability, owing to non-ideal carrier transportation inside cathode composites and poor kinetic characteristics. Exceeding the limits necessitates developing new electrode materials and electrode architectures capable of eliciting chemistries for outstanding rate performance and high columbic efficiency. These materials must also be enriched with ionic and electronic conducting networks at the electrode level and optimized transportation parameters to improve electrode kinetics. Also, the efficient carrier migration, i.e., the ionic and electronic conduction within cathode composites, is still a challenge due to its complex microstructure and three components (electrolyte, active material, and conductive agent). The ideal composite cathode provides sufficient charge transport paths for electrons and Li-ions and restricted use of electronic insulating components and tailored transportation parameters. Also, in-depth knowledge of the kinetics of cathode composites in ASSLSs is still indistinct.
The high content of carbon-based materials (CNT, Super P, and VGCF) are consistently implemented in the cathode composites to enhance the kinetic performance of all-solid-state lithium batteries (ASSLBs) [19,20,21,22]. However, the inclusion of carbon conductive agents would severely degrade sulfide-based solid-state electrolytes and lead to the poor efficiency of ASSLBs. Meanwhile, incorporating conductive polymers into the cathode composites would lead to large voltage polarization in the cell due to interfacial side reactions between the conductive polymer and sulfide electrolytes [23, 24]. Moreover, the practical applications of ASSLSs are achieved by increasing the areal loading of cathode materials and decreasing the volume fractions of inactive components such as conductive agents [25]. Thus, the electrode architectures and their related tortuosity factors within the cathode composite significantly impact the electrochemical performance of ASSLBs.
Herein, Li7P2.9W0.05S10.85 glass–ceramic electrolyte with high lithium-ion conductivity is successfully synthesized by traditional ball milling and heat treatment. The carrier transport properties of cathode composites are well studied in ASSLSs. More important, an interpenetrating network is designed in cathode composite based on VGCF conductive agent. The interpenetrating network builds the low tortuosity of electrode architecture as well as enhances the cathode composites of electronic/ionic conduction. The assembled all-solid-state Li-SeS2 batteries based on interpenetrating network cathode composite exhibit a high specific capacity of 512 mAh g−1 at the current density of 0.06 mA cm−1. Surprisingly, it retains a reversible specific capacity of 506 mAh g−1 with 98.8% capacity retention after the 90th cycle. Besides, it also presents discharge capacities of 515, 430, 364, 329, 307 and 285 mAh g−1 at the current density of 0.06, 0.09, 0.12, 0.15, 0.18 and 0.21 mA cm−1, respectively. As a result, the designed all-solid-state Li-SeS2 battery demonstrates superior cycling stability and better rate capability.
Results and Discussion
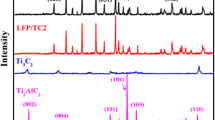
Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes are prepared by traditional ball milling method and following crystallization process. Figure S1 displays the XRD pattern of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass, obtained via high energy ball-milling. No diffraction peaks are displayed in Figure S1, showing that a glassy phase is generated after the ball milling process. Thus, high-quality glass–ceramic electrolytes could be obtained by precipitation of glass samples using high-temperature processing. Figure 1a presents the differential thermal analysis (DTA) curves of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass samples. These DTA curves exhibited the same characteristics and a strong peak between 260 and 280 °C, corresponding to the exothermic effect of crystal growth [26, 27]. In order to attain the crystallization of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass electrolytes more sufficiently, the temperature at the exothermic peak is chosen as the optimal condition. As a result, the glass electrolyte's heat treatment temperature is set to 270 °C. After appropriate heat treatment, the Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes were obtained successfully. The X-ray diffraction (XRD) technique is performed to analyze further the glass–ceramic electrolyte of phase and crystal structure. Figure 1b is the XRD pattern of the Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes. As shown in Fig. 1b, the characteristic peak of Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramic are identical to Li7P3S11 glass–ceramics sample, indicating that Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramics deliver a similar Li7P3S11 fast ionic conductor [28]. It is worth noting that the characteristic peak of Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 have slightly deviated under the basis in comparison to Li7P3S11glass ceramics. The differences at marked (Fig. 1b) and magnified zone (Figure S2) suggest that W has been successfully doped into the glass–ceramic matrix of Li7P3S11 after high-energy ball milling and heat treatment. Moreover, the minor deviation of the characteristic peak of Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 samples is due to W doping into Li7P3S11-type electrolyte, generating lattice distortion.
Three peaks are detected at 417, 406, and 387 cm−1, which belong to the stretching vibration of the P-S bonds in PS43−, P2S74− and P2S64− conductive units, respectively as presents in Fig. 1c. Thus, the heteroatom doping pattern does not generate any new structural moieties in Li7P3S11-type glass–ceramic electrolytes. Nonetheless, the relative peak intensity of the P2S74− the conductive unit of Li7P2.9W0.05S10.85 sample is a little higher as compared to Li7P3S11 and Li7P2.8W0.1S10.7 glass–ceramic samples. A small amount of the P2S64− (less ionic conductive) units exist in the Li7P2.9W0.05S10.85 as compared to other glass–ceramic samples. The results deliver appropriate W doping tend to form additional P2S74− (higher ionic conductive) units. Moreover, the P2S74− ions contribute to improving ionic conductivity [29]. It is demonstrated that as-prepared Li7P2.9W0.05S10.85 glass–ceramic sample would present the highest ionic conductivity among other samples.
XPS technique is used to explore chemical elements and structure for the Li7P2.9W0.05S10.85 glass–ceramic electrolyte. Figure S3a reveals all existent elements and their valence states found in the Li7P2.9W0.05S10.85 sample and Figure S3b displays the XPS spectrum of S 2p. According to spin-orbital splitting, the S 2p peaks spectrum has two components, which could be assigned to S 2p3/2 and S 2p1/2. Furthermore, the Lorentzian-Gaussian function fits S 2p spectrum into different peaks. The coexistence of non-bridging sulfur bond (P-S-Li) and bridging sulfur bond (P-S-P) are evidenced by the presence of a peak at 161 eV and 163 eV, respectively [30]. Meanwhile, the peak of S 2p3/2 at 162 eV is ascribed to the double phosphorus-sulfur bond of P = S. Besides, the fitting peak at 162 eV also involves the W-S bond, indicating that W atoms successfully doped into the Li7P3S11 crystal [31].
Figure 2a shows the EIS spectra of the as-prepared Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes at room temperatures. The resistance could be determined using the intersection coordinates of the EIS spectrum line and the horizontal axis. The resistance of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes are 50, 35, and 62 Ω, respectively. The ionic conductivity (бion) of sulfide-based electrolyte is obtained from the thickness, area, and resistance of the sample according to the following Eq. (1):

where d presents the sample thickness, A is the sample area, and R is sample resistance at room temperature. Figure 2b exhibits that the ionic conductivity of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes are 1.4, 2.0, and 1.1 mS cm−1, respectively. The ionic conductivity of Li7P2.9W0.05S10.85 is higher than the previously reported sulfide electrolytes [32, 33]. According to the doping mechanism, the larger ionic radius of W would widen the lithium-ion channel and reduce the activation energy of the lithium-ion transition. As a result, the W-doped strategy contributes to improving the conduction of lithium-ion in the lattice distortion.
a The impedance spectra of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes at room temperature, b the lithium-ion conductivities of Li7P3S11, Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramic electrolytes, c Arrhenius plots of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolyte, d electrochemical stability window of Li7P2.9W0.05S10.85 glass–ceramic electrolyte
Activation energy is the most critical parameter to evaluate sulfide solid-state electrolyte performance. The activation energy (Ea) of sulfide electrolyte is calculated by Arrhenius Eq. (2):

where R denotes the gas constant, T is the absolute temperature, б0 is the pre-exponential factor, and б is the ionic conductivity. Figure 2c depicts a relationship between temperature and ionic conductivity that follows the Arrhenius formula. Thus, the activation energies of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes are calculated to be 25.8, 23.2, and 26.6 kJ mol−1, respectively. The results indicate that appropriate doping with aliovalent ions would reduce the activation energy and promotes fast transport of carrier in an inorganic conductor system.
In order to measure the electrochemical stability of Li7P2.9W0.05S10.85 glass–ceramic electrolyte, the non-blocking cell is constructed with lithium as a reference electrode and stainless steel (SS) as a working electrode in the glove box. The Li/Li7P2.9W0.05S10.85/SS cell is tested for CV with a scan rate of 1 mV S−1. As revealed in Fig. 2d, the whole scanning potential range is from − 0.5 to 5 V. Except for lithium deposition peak (Li+ + e− → Li) and lithium dissolution peak (Li → Li+ + e−), no other peaks are observed, indicating that no side reactions occurred [34, 35]. Thus, the Li7P2.9W0.05S10.85 glass–ceramic electrolyte with lithium metal has good stability and an electrochemical window of up to 5 V.
Figure 3a displays 7Li MAS NMR spectra of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolyte samples. A strong peak at 1.7 ppm in 7Li MAS NMR spectra, indicating the Li environment [36]. Therefore, the WS2 doped strategy for the Li7P3S11 system does not involve the alteration of intrinsic structural units. Figure 3b presents the 31P MAS NMR spectra of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic samples. 31P MAS NMR spectrum consists of three obvious peaks with different integral intensities, revealing the sulfur environments around the P atom [37]. These NMR peaks of 106 ppm, 90.8 ppm, and 86 ppm correspond to P2S64−, P2S74− and PS43− structural units, respectively [38]. The analysis of 31P MAS NMR spectra is in agreement with the analytical findings of Raman spectroscopy. These results suggest that the WS2 doped strategy for Li7P3S11-type electrolyte does not generate new structural units (such as POS33−).
a 7Li MAS NMR spectra of Li7P3S11, Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramic samples, b 31P MAS NMR spectra of Li7P3S11, Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramic samples, c, d and e peak-deconvolution results of the 31P MAS NMR spectra, f the crystallization degree for Li7P3S11, Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glass–ceramic samples
It is well-known that Li7P3S11-type glass–ceramic electrolytes are composed of a crystalline and amorphous phase, where the crystalline phase plays an essential role in lithium-ion conduction. The crystalline phase is a high lithium-ion conductor as compared to the amorphous phase. Consequently, the proportion of the conductive crystalline phase determines the ionic conductivity of glass–ceramic electrolytes. The crystalline and amorphous portion ratio is evaluated by the degree of crystallization from the 31P MAS NMR spectrum. As shown in Fig. 3c–e, they display peak deconvolution results of Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic samples, respectively. The degree of crystallization (XC) of sulfide electrolytes is calculated according to Eq. (3):
In this equation, the intensity of ФPS4 and ФP2S7 is the crystalline phase, and Фall is the total intensity of 31P MAS NMR peaks. Consequently, based on Eq. (3), Li7P3S11, Li7P2.9W0.05S10.85, and Li7P2.8W0.1S10.7 glass–ceramic electrolytes present the degree of crystallization of 38.5%, 45.4%, and 36.2%, as shown in Fig. 3f, respectively. It is evidence that heteroatom doping for Li7P3S11-type glass–ceramic samples would alter the ratio of crystalline and amorphous phases [39, 40]. The newly designed Li7P2.9W0.05S10.85 glass–ceramic electrolyte exhibits the highest value of crystallinity, signifying the highest ionic conductivity as compared to Li7P3S11 and Li7W0.1P2.8S10.7. In addition, compared to Li7P3S11 glass–ceramic electrolyte, the amplified lithium transmission channels contribute to the transport of lithium ions, which helps to improve the lithium-ion conductivity of Li7P2.9W0.05S10.85 glass–ceramic electrolyte.
As given in Fig. 4a, the morphology and microstructure of the Li7P2.9W0.05S10.85 glass–ceramic particles are investigated by SEM. It may be seen intuitively that the contact between particles is very close. This phenomenon is mainly due to the heat treatment temperature being above the crystallization temperature. Simultaneously, the EDX mapping results of the Li7P2.9W0.05S10.85 glass–ceramic sample show that P, W, and S elements in Fig. 4b–d are uniformly distributed throughout the sample’s surface. It also verifies the uniform doping of WS2 without any local concentration at one place.
The Li7P3S11 glass–ceramic electrolyte also delivers the uniform distribution of P and S elements in Figure S4a–c. The high-resolution TEM image is used to investigate the impacts of W-doping on the crystal structure. Figure 4e and f represent the microstructure as well as the crystal structure of the as-prepared Li7P2.9W0.05S10.85 glass–ceramic sample. In Fig. 4e, the microstructure is displayed by an irregular block. Figure 4f, the HRTEM image, demonstrates that the glass–ceramic electrolyte contains both crystalline and amorphous phases, consistent with the SEM observations. The measured lattice distance of the Li7P2.9W0.05S10.85 glass–ceramic sample is 0.579 nm, which corresponds to the lattice distance of the (311) plane [41, 42]. According to the HRTEM result in Figure S5a, b, the lattice distance of the (311) plane of Li7P3S11 glass–ceramic electrolyte is 0.576 nm. As a result of the doping of WS2, the lattice distance is increased.
The as-prepared Li7P2.9W0.05S10.85 solid electrolytes with high ionic conductivity are achieved successfully, which contributes significantly to the outstanding rate performance of the all-solid-state Li-SeS2 battery. However, the rate capability of the all-solid-state Li-SeS2 battery is influenced not only by the ionic conductivity of solid-state electrolyte but also depends on the carrier transport path of the composite cathode. Our study aims to improve kinetics by designing a cathode composite enriched with ionic and electronic conducting networks and optimizing transportation factors such as effective conductivity and tortuosity. The tortuosity of the carrier transport path directly determines the ionic and electronic conductivity of composite cathode, which also affects the kinetics feature. The diverse microstructures offer different carrier transport pathways in composite cathodes. By designing the two cathode microstructure architectures, we tailor the tortuosity of the carrier transport path. Common cathode composite (CCC) and interpenetrating network composite cathode (INCC) are designed via extra steps to add conductive agent and softness of as-prepared electrolyte. The detailed concept and preparation process are shown in Figure S6.
The electronic conductivity of the cathode composite can be determined using a sandwich structure. Figure 5a, b exhibits the current–time plot of composite cathodes for the measurement of electronic conductivity. The electronic conductivity (бele) of cathode composite is calculated by the formula (4):

where L is the thickness of the cathode composite, I is the steady-state current, S is the area of the composite cathode, and V is the applied voltage. The electronic conductivity of interpenetrating network composite cathode (INCC) and common cathode composite (CCC) is 1.18 and 0.116 S cm−1. The electronic conductivity of INCC is 10.2 times higher than that of CCC. It is evident that the tailoring interpenetrating network significantly improves the electronic conductivity of cathode composite due to the different dispersion forms of conductive agent. Lithium-ion migration and electrical transport are critical challenges for ideal electrode design.
Furthermore, the ionic conductivities of cathode composites are also explored by a Penta-layer cell via the AC impedance method [43]. The cell consists of a composite cathode, sulfide electrolyte, and lithium foil in Fig. 5c. As shown in Fig. 5d, the resistance of INCC and CCC is 194 and 416 Ω, respectively. According to formula (2), the ionic conductivity of INCC was 1.83 × 10–4 S cm−1 and the ionic conductivity of CCC 0.852 × 10–4 S cm−1 at room temperature. As a result, the ionic tortuosity factor of INCC and CCC is 6.55 and 14.08 based on the formula (5), respectively. Thus, INCC delivers higher ionic conductivity than CCC. Furthermore, it indicates that ionic transportation in the cathode composite is more significant than the electron transfer. Also, the little fraction of the conductive agent affects the ionic conductivity of composite cathode due to the altered the carrier transport path.
Moreover, Janek et al. explore carrier transport toward optimized cathode composites in all-solid-state batteries [44, 45]. They conclude that complex cathode composite hinders carrier transport and results in tortuous conduction pathways. Therefore, the tortuosity of the electron (or ionic) carrier transport path determines the electron (or ionic) conductivity of the cathode composite. For further quantification and comparison of the electron (or ionic) transport between different systems, the tortuosity factor (t) of the transport path is often considered and calculated by the formula (5):
where \({\sigma }^{pri}\) is the electron (or ionic) conductivity of pristine component, \({\sigma }^{mea}\) denotes the experimentally measured electron (or ionic) conductivity, and φ is the volume fraction of conductive agent (or sulfide electrolyte). This formula quantifies the effect of a heterogeneous microstructure on the electron transport in the composite cathode. The concept of tortuosity in terms of homogenization enables us to evaluate the hindrance of the charge transport in the complex microstructure. A high tortuosity factor indicates tortuous and hampered transport, corresponding to low electron-transport property [45]. Based on formula (5), the electron tortuosity factor of CCC is 5.49, and the electron tortuosity factor of INCC is 0.54. It indicates that INCC has a significant advantage on electron transport due to the lower tortuosity factor. As a result, interpenetrating network tactics could effectively reduce the tortuosity factor of the electron transport path and lead to rapid transport of electrons in cathode composite. Notably, the reduced tortuosity factor improves the electronic conduction and ion migration resulting in dynamic characteristics of all-solid-state batteries.
As presented in Fig. 6a, reveals the morphology of the CCC. Due to the soft nature of sulfide electrolytes, it is unavoidable to coat the conductive agent within the electrolyte system after ball milling. The ability of carrier transport is significantly reduced. More importantly, the conductive agent is covered inside the electrolyte, and the carrier transport between the electrolytes lacks the necessary channel. Time-of-flight secondary ion mass spectrometry (TOF–SIMS) is employed to study the dispersion of the electrode composite due to ultra-high sensitivity for all chemical elements [46, 47]. Figure 6b displays TOF–SIMS secondary ion images of the CCC and indicates homogeneous dispersion for all the components. Thus, the ball milling method is an excellent choice to obtain a homogeneous composite cathode. In Fig. 6c, we observe some VGCF uncoated with sulfide electrolyte due to lower ball milling speed. These uncovered VGCF conductive agent bridges charge carrier transfer channels among sulfide electrolytes. Thus, we successfully prepare the INCC electrode. As a result, it enhances carrier conductivity in cathode composite and improves rate capability. Moreover, Fig. 6d presents TOF–SIMS secondary ion images of INCC and reveals better dispersion for all the components, particularly with the lithium image being more intense, indicating their high concentration.
Figure 7 presents the electrochemical properties of all-solid-state Li-SeS2 batteries based on different cathode composites, where the thickness of solid-state electrolyte is about 0.67 mm. Figure 7a exhibits a series of CV curves for INCC /Li7P2.9W0.05S10.85/Li-In battery at different scan rates ranging from 0.2 to 0.6 mV s−1. In an all-solid-state system, there is only one reduction could be observed at 1.25 V with the scan rate of 0.2 mV s−1, attributed to the formation of Li2Se/Li2S instead of long-chain lithium polysulfides/polyselenides [48, 49]. The subsequent anodic scan shows a strong oxidation peak at 1.8 V at 0.2 mV s−1, corresponding to the reversible conversion of SeS2. Besides, the current peaks of every CV curve gradually increase with the amplifying of scan rate. The all-solid-state Li-SeS2 batteries display similar redox processes at different sweep rates as well as exhibited superior reversibility at the highest scan rate of 0.6 mV s−1, suggesting the lower polarization of interpenetrating network cathode composite. It evidences that the all-solid-state Li-Se batteries would possess excellent rate capability. On the other hand, compared with CCC/Li7P2.9W0.05S10.85/Li-In battery, the CV curves present unsatisfactory behavior in Fig. 7e. The oxidation/reduction peaks are asymmetrical and gradually become widen as the scanning speed increases. It demonstrates that the greater polarization appears in CCC/Li7P2.9W0.05S10.85/Li-In system.
Electrochemical properties of all-solid-state Li-SeS2 batteries based on interpenetrating network cathode composite (a–d) and common cathode composite (e–h): a, e cyclic voltammetry curves with different scanning rates in the voltage window of 0.5–3 V; b, f AC impedance spectra in the frequency range of 1 MHz to 100 Hz; c, g cycling stability of Li-SeS2 battery at room temperature; d, h rate capability of Li-SeS2 batteries at a different current density at room temperature
As shown in Fig. 7b and f, the EIS spectra of all-solid-state Li-SeS2 batteries have typical characteristics of ionic conductors, with a circular arc in the high-frequency region representing the interface resistance and an inclined line in the low-frequency region exhibiting the ion diffusion. The measured resistance is the point at which the ion-diffusion line intersects the transverse axis. Thus, the resistance of the INCC /Li7P2.9W0.05S10.85/Li-In battery is 60 Ω, and the CCC /Li7P2.9W0.05S10.85/Li-In battery is 100 Ω. Overall, both batteries reveal a relatively small resistance (< 200 Ω). All-solid-state batteries are pressurized under high pressure, which significantly reduces interface and grain boundary resistance. However, under the same condition, INCC/Li7P2.9W0.05S10.85/Li-In battery has a low charge transfer interface resistance of 30 Ω. The value was about half of the CCC /Li7P2.9W0.05S10.85/Li-In battery (about 60 Ω). It proves that a unique interpenetrating network in cathode composite reduces battery impedance and charge transfer impedance simultaneously.
All-solid-state Li-SeS2 batteries are tested at room temperature in a voltage range of 1–3 V to determine practical capacity, cycling stability, and rate capability. The INCC /Li7P2.9W0.05S10.85/Li-In battery exhibits a high specific capacity of 512 mAh g−1 at the first cycle, as shown in Fig. 7c. It also has a high coulombic efficiency of 99% at the current density of 0.06 mA cm−1. The excellent electron transport systems could improve the active material utilization and present much higher capacity and coulombic efficiency. As exhibited in Fig. 7c, the specific capacity of the INCC /Li7P2.9W0.05S10.85/Li-In battery gradually increases as the loop measurement progresses, owing to the activation process of electrode materials. Surprisingly, after the 90th cycle, the INCC /Li7P2.9W0.05S10.85/Li-In battery retains a reversible specific capacity of 506 mAh g−1. Based on the first discharge specific capacity, the capacity retention can reach up to 98.8%. Therefore, INCC /Li7P2.9W0.05S10.85/Li-In battery has an excellent kinetic characteristic. In comparison, the CCC /Li7P2.9W0.05S10.85/Li-In battery only delivers a relatively low capacity of 336 mAh g−1 with a current density of 0.06 mA cm−1 at the first cycle presented in Fig. 7g. It only offers a relatively low capacity of 223 mAh g−1 after 50 cycles with capacity retention of 66.4% cycles due to poor electronic conductivity. As a result, it has a significant impact on active material utilization and electrochemical reaction kinetics.
Figure 7d depicts the rate capability of INCC/Li7P2.9W0.05S10.85/Li-In battery. In detail, the all-solid-state battery presents discharge capacities of 515, 430, 364, 329, 307 and 285 mAh g−1 at various current density of 0.06, 0.09, 0.12, 0.15, 0.18 and 0.21 mA cm−1, respectively. While recovering current density to 0.06 mA cm−1, the capacity is partially restored. This result suggests that INCC/Li7P2.9W0.05S10.85/Li-In battery has superior rate performance due to superior electrochemical kinetic characteristics. In contrast, the rate capability of the CCC/Li7P2.9W0.05S10.85/Li-In battery could not be performed in the manner described above due to poor cycling stability. Only one measurement is performed at each current density to obtain intrinsic rate capability. As presented in the Fig. 6h, CCC/Li7P2.9W0.05S10.85/Li-In battery displays lower capacities of 339, 295, 174 and 65 mAh g−1 at different current density of 0.06, 0.09, 0.12 and 0.15 mA cm−1, respectively. It indicates that the reaction kinetic of CCC/Li7P2.9W0.05S10.85/Li-In is inadequate.
In comparison, all-solid-state Li-Se batteries with an interpenetrating electron network cathode have a higher specific capacity, better cycling stability, and superior rate capability. Thus, the interpenetrating network could prevent ineffective contact with no effective conducting agent, avoiding significant capability degradation. Due to weak electronic/ionic conductivity and low active material usage, the common cathode composite (CCC) exhibits low capacity, poor cycling stability, and rate capability.
It is well known that the diffusion coefficient (DLi+) is one of the essential factors that evaluate the kinetics of the electrochemical energy storage devices [50,51,52]. The galvanostatic intermittent titration technique (GITT) is utilized to explore the diffusion coefficient of all-solid-state Li-Se battery. The diffusion coefficient (DLi+) can be calculated according to equation (6):
where m and M are mass and molar mass of the SeS2 (g, g mol−1), respectively. Vm represents the molar volume of SeS2. A is contact area (cm2). τ is pulse duration (s). ΔEs and ΔEτ can be obtained from the single potential profile before, during, and after a constant current pulse. Figure 8a reveals the GITT curves of CCC /Li7P2.9W0.05S10.85/Li-In and INCC/Li7P2.9W0.05S10.85/Li-In battery systems. The all-solid-state Li-Se battery only exhibits one plateau during the charging process between 1.0 and 3.0 V. Evidently, the SeS2 electrode in an all-solid-state battery presents a one-step solid–solid phase transition between Li2Se and SeS2, which coincides with CV results of the Se cathode. In addition, Fig. 8b and Figure S11 reveal the single potential profile before, during, and after a constant current pulse, contributing to acquiring ΔEs and ΔEτ at a constant current pulse. As given in Fig. 8c, it exhibits a straight line behavior between the responsible potential and time period τ1/2. Thus, the diffusion coefficients for both composite cathodes can be calculated according to the simplified Eq. (6). Figure 8d delivers the calculated DLi+ for composite cathodes from the GITT profiles during the charging process. Overall, DLi+ values for two cathodes show a similar variation trend during charging (from 1 to 3 V) with the different order, where the DLi+ values decrease first and then increase. The minimum DLi+ values for INCC/Li7P2.9W0.05S10.85/Li-In battery are observed at around 1.75 V in Fig. 8c, ascribing to the voltage plateaus during the charging process. It can be seen that the DLi+ value ranges from 10–9 to 10–12 cm2 s−1 during the charging process. By comparison, the CCC/Li7P2.9W0.05S10.85/Li-In battery presents less DLi+ value range from 10–9 to 10–14 cm2 s−1 during the charging process. Thus, the diffusion coefficient of INCC/Li7P2.9W0.05S10.85/Li-In battery is higher on average than that of CCC/Li7P2.9W0.05S10.85/Li-In battery. One of the most important reasons that ionic/electronic diffusion path is shortened with the help of an interpenetrating network, generating superior kinetic property. Moreover, the Li7P2.9W0.05S10.85 sulfide electrolyte with high ionic conductivity also improves the kinetic property of all-solid-state batteries. Thus, the enhancing rate capability of an all-solid-state battery mainly depends on the design of an excellent composite cathode and the development of high ionic conductivity electrolytes.
a GITT curves of CCC/Li7P2.9W0.05S10.85/Li-In and INCC/Li7P2.9W0.05S10.85/Li-In system at the process of charge, b the potential profile before, during, and after a constant current pulse with schematic labeling, c the corresponding linear behavior of E against τ1/2, d the calculated Li+ diffusion coefficients at the process of charge
Conclusion
In conclusion, the Li7P2.9W0.05S10.85 sulfide electrolyte is prepared by W doping, which delivers a high ionic conductivity of 2.0 mS cm−1 at room temperature. In addition, the cathode composite with an interpenetrating network is also tailored by three-step ball grinding, whose electronic conductivity and ionic conductivity are 1.18 S cm−1 and 0.183 mS cm−1, respectively. The electronic conductivity of ICCC is 10.2 times, and the ionic conductivity of INCC is 2.1 times that of CCC. The reason is that interpenetrating network tactics could effectively reduce the tortuosity factor of the carrier transport path, leading to the rapid transport of electrons and ions in the composite cathode. The cathode composite with an interpenetrating network is applied to all-solid-state Li-SeS2 batteries, which shows a high specific capacity of 512 mAh g−1 at the current density of 0.06 mA cm−1. Significantly, it also reveals excellent rate capability due to enhanced electrochemical dynamics. This work provides a feasible way to tailor the high ionic conductivity electrolyte and interpenetrating ion/electron network composite for high rate all-solid-state Li-SeS2 batteries.
Experimental Section
Preparation of Sulfide Solid-State Electrolytes
70Li2S-(30-x)P2S5-xWS2 (x = 0, 1, 2) sulfide glass–ceramic electrolytes were prepared using a high-energy ball milling method and appropriate heat treatment. Li2S (Alfa Aesar, 99.9%), P2S5 (Macklin, 99%), and WS2 (Aladdin, 99.9%) were used as precursors without further treatment. Firstly, appropriate precursors with an exact molar ratio were weighed in the argon glove box (O2 < 0.1 ppm, H2O < 0.1 ppm) due to the reaction with oxygen and moisture in the air. The above precursors were placed into a zirconia pot filled with zirconia balls and then ground by the high-energy ball-milling machine (Fritsch, Germany). After high energy ball milling (45 h, 510 rpm), the obtained glass sample was heated at a suitable temperature. Finally, Li7P3S11 (70Li2S-30P2S5), Li7P2.9W0.05S10.85 (70Li2S-29P2S5-1WS2), and Li7P2.8W0.1S10.7 (70Li2S-28P2S5-2WS2) glass–ceramic electrolyte were obtained successfully.
Preparation of SeS2/CNT composite
Commercial carbon nanotubes (CNT) power (purity > 95%, diameter 10–20 nm, length 10–30) was purchased from Nanjing XFNANO technology Co., Ltd. The CNT power was treated with concentrated hydrochloric acid stirring for 10 h to wash away any metal ions, suction-washed, and then dried at 90 °C in an oven. The commercial SeS2 power was purchased from (Alfa Aesar, 99.9%). SeS2/CNT composite was prepared as to the reported melt-diffusion method. Typically, SeS2 and CNT power with the weight ratio of 7:3 were mixed uniformly by grinding. Then they were heated at 155 °C for 12 h with a heating rate of 5 °C min−1 in a sealed vessel. After cooling to room temperature, the SeS2/CNT composite was prepared successfully. Figure S5 displays the morphology feature SeS2/CNT composite, indicating better dispersion for SeS2 and CNT. The XRD patterns of pristine SeS2 and SeS2/CNT samples are exhibited in Figure S6. It demonstrates that the melt-diffusion method does not generate new materials and change the crystal structure of SeS2.
Preparation of Cathode Composites
Vapor-grown carbon fiber (VGCF) power was purchased from Japan Showa Denko. Before using as a conductive agent in all-solid-state batteries, VGCF was heat-treated at 80 °C for 12 h to remove the moisture. A grinding pattern of two steps prepared the CCC electrode. SeS2/CNT and VGCF were ground with a weight ratio of 3: 1: at a rotating speed of 360 rpm. Then, the above mixture and sulfide electrolyte were smoothed with a weight ratio of 4: 6 at the same rate.
The interpenetrating network composite cathode (INCC) was designed by a ball milling pattern of three steps. In detail, a mixture of SeS2/CNT and VGCF with a weight ratio of 3:0.5 was grinded by a rotating speed of 360 rpm. Following, the above mixture and sulfide electrolyte with a weight ratio of 3.5: 6 was milled at the same speed. Last, the above mixture and VGCF with a weight ratio of 3.5: 6 was milled at a rotating speed of 100 rpm. In addition, all the above processes were performed in an argon atmosphere.
Electronic Conductivity Test of Cathode Composites
Cathode Composite (100 mg) or VGCF (100 mg) was placed into a mould and pressed into a sandwich structure with stainless steel under huge pressure (about 240 MPa). The electrochemical workstation was used to perform potentiostatic measurement at a bias voltage of 5 mV, and the current changes were recorded.
Ion Conductivity Test of Cathode Composites
Cathode Composite (100 mg) was placed into a mould and pressed into a table under a pressure of 240 MPa. Then, sulfide electrolyte powder (100 mg) was added to one side of the composite cathode table and pressed into the table. Next, sulfide electrolyte powder (100 mg) was again added to another side of the composite cathode table and pressed into a table under about 240 MPa pressure. Last, Li/sulfide electrolyte/composite cathode/sulfide electrolyte/Li battery was assembled by placing lithium sheets on both sides of the composite layer. The electrochemical workstation was employed to record the voltage response of a Penta-layer cell to a charging current of 0.1 mA.
Structure and Morphology Characterization
X-ray diffraction (XRD) measurements were performed on the Bruker D8 Advance using Cu-Kα radiation with a scanning step of 5° min−1. Solid-state nuclear magnetic resonance (NMR) spectra were conducted on Bruker Ascend 700 M spectrometer to explore 31P and 7Li. Scanning electron microscope (SEM) and elemental mapping were used using S-4800 field-emission scanning electron microscopy (Hitachi, Japan). Transmission electron microscopy (TEM) and high-resolution transmission electron microscope (HRTEM) were measured on an S-4800 transmission electron microscope (Hitachi, Japan) operated at 400 kV. X-ray photoelectron spectroscopy (XPS) measurements were performed with the Thermo Fischer (ESCALAB 250Xi) instrument using Al Kα radiation source. The differential thermal analysis (DTA) of the sulfide glass sample was determined at a temperature range from 30 to 300 °C (Hitachi, TG–DTA 6200) with a heating rate of 10 °C min−1 under Ar atmosphere. Raman spectra were measured at an excitation laser beam wavelength of 532 nm using a Renishaw in Via Raman microscope. The TOF–SIMS measurements were performed on an ION-TOF (GmbH, Germany) TOF–SIMS IV with a bismuth liquid metal ion source.
Fabrication of All-Solid-State Battery
All-solid-state Li-Se batteries were fabricated by powder compression pattern in glove box (O2 < 0.1 ppm, H2O < 0.1 ppm). These batteries have consisted of as-prepared Li7P2.9W0.05S10.85 glass–ceramic electrolyte, SeS2/CNT composite cathode, and Li-In alloy anode. The detailed steps were as follows. Initially, solid-state electrolyte (150 mg) was assembled firstly in the mold under great pressure. Then, the cold pressing method was employed to construct SeS2/CNT cathode composite. Finally, Li-In alloy (10 wt.% Li) was pressed on the bottom of the interlayer electrolyte.
Electrochemical Characterization
A blocking electrode system was employed to explore the lithium ionic conductivity of sulfide glass–ceramic electrolytes. The electrochemical impedance spectroscopy (EIS) of the blocking electrode system, including sulfide glass–ceramic electrolyte, was performed by the electrochemical workstation (PARSTAT 2273, USA) with an amplitude of 15 mV at frequencies ranging from 1 MHz to 100 Hz. The non-blocking system tested the electrochemical window of glass–ceramic electrolyte. The cyclic voltammetry (CV) curve of the non-blocking electrode system was characterized at the voltage from − 0.5 to 5 V with a scan rate of 1 mV s−1.
The electrochemical workstation measured the cyclic voltammetry and Nyquist plots of assembled all-solid-state Li-Se batteries. Specifically, the CV curves were recorded with the voltage range from 0 to 3.5 V (vs Li-In) at different scan rates. The Nyquist plots were performed at frequencies ranging from 1 MHz to 100 Hz by applying an alternating current voltage of 15 mV amplitude. The galvanostatic charge/discharge behavior and rate capability of assembled cells were examined by using an advanced battery test system (LAND, China) at room temperature. The specific capacity of the assembled cell was calculated according to the weight of SeS2 in the composite cathode. All electrochemical measurements were performed in an argon-filled atmosphere. Galvanostatic intermittent titration technique (GITT) experiments were conducted at the Land battery testing system. The two cells were charged at a constant current of 0.015 mA cm−1 with an interval of 30 min followed by an open-circuit stand for 120 min to allow the cell potential to relax to its steady-state values. The procedure was repeated for the full electrochemical window.
References
Yang CP, Xin S, Yin YX, Ye H, Zhang J, Guo YG. An advanced selenium-carbon cathode for rechargeable lithium-selenium batteries. Angew Chem Int Ed 2013;52:8363.
Armand M, Tarascon J-M. Building better batteries. Nature 2008;451:652.
Li X, Liang J, Li X, Wang C, Luo J, Li R, Sun X. High-performance all-solid-state Li-Se batteries induced by sulfide electrolytes. Energy Environ Sci 2018;11:2828.
Cai W, Yao Y-X, Zhu G-L, Yan C, Jiang L-L, He C, Huang J-Q, Zhang Q. A review on energy chemistry of fast-charging anodes. Chem Soc Rev 2020;49:3806.
Eftekhari A. The rise of lithium-selenium batteries. Sustain Energy Fuels 2017;1:14.
Zeng L-C, Li W-H, Jiang Y, Yu Y. Recent progress in Li-S and Li-Se batteries. Rare Met 2017;36:339.
Abouimrane A, Dambournet D, Chapman KW, Chupas PJ, Weng W, Amine K. A new class of lithium and sodium rechargeable batteries based on selenium and selenium-sulfur as a positive electrode. J Am Chem Soc 2012;134:4505.
Lee JT, Kim H, Oschatz M, Lee DC, Wu F, Lin HT, Zdyrko B, Cho WI, Kaskel S, Yushin G. Micro-and mesoporous carbide-derived carbon-selenium cathodes for high-performance lithium selenium batteries. Adv Energy Mater 2015;5:1400981.
Wang WP, Zhang J, Yin YX, Duan H, Chou J, Li SY, Yan M, Xin S, Guo YG. A rational reconfiguration of electrolyte for high-energy and long-life lithium-chalcogen batteries. Adv Mater 2020;32:2000302.
Liu F, Wang L, Zhang Z, Shi P, Feng Y, Yao Y, Ye S, Wang H, Wu X, Yu Y. A mixed lithium-ion conductive Li2S/Li2Se protection layer for stable lithium metal anode. Adv Func Mater 2020;30:2001607.
Yi Z, Yuan L, Sun D, Li Z, Wu C, Yang W, Wen Y, Shan B, Huang Y. High-performance lithium-selenium batteries promoted by heteroatom-doped microporous carbon. J Mater Chem A 2015;3:3059.
Yang X, Luo J, Sun X. Towards high-performance solid-state Li-S batteries: from fundamental understanding to engineering design. Chem Soc Rev 2020;49:2140.
Chen S, Xie D, Liu G, Mwizerwa JP, Zhang Q, Zhao Y, Xu X, Yao X. Sulfide solid electrolytes for all-solid-state lithium batteries: structure, conductivity, stability and application. Energy Storage Mater 2018;14:58.
Zheng F, Kotobuki M, Song S, Lai M, Lu L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 2018;389:198.
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017;33:363.
Manthiram A, Yu X, Wang S. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater 2017;2:1.
Ren H-T, Zhang Z-Q, Zhang J-Z, Peng L-F, He Z-Y, Yu M, Yu C, Zhang L, Xie J, Cheng S-J. Improvement of stability and solid-state battery performances of annealed 70Li2S-30P2S5 electrolytes by additives. Rare Met 2022;41:106.
Wu J, Liu S, Han F, Yao X, Wang C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv Mater 2021;33:2000751.
Deng Z, Zhang Z, Lai Y, Liu J, Liu Y, Li J. A sulfur-carbon composite for lithium/sulfur battery based on activated vapor-grown carbon fiber. Solid State Ionics 2013;238:44.
Yoon K, Kim J-J, Seong WM, Lee MH, Kang K. Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in all-solid-state lithium battery. Sci Rep 2018;8:1.
Zheng H, Yang R, Liu G, Song X, Battaglia VS. Cooperation between active material, polymeric binder and conductive carbon additive in lithium ion battery cathode. J Phys Chem C 2012;116:4875.
Sun D, Tan Z, Tian X, Ke F, Wu Y, Zhang J. Graphene: A promising candidate for charge regulation in high-performance lithium-ion batteries. Nano Res 2021;14:1.
Zhang W, Leichtweiß T, Culver SP, Koerver R, Das D, Weber DA, Zeier WG, Janek J. The detrimental effects of carbon additives in Li10GeP2S12-based solid-state batteries. ACS Appl Mater Interfaces 2017;9:35888.
Hu Y, Zhong Y, Qi L, Wang H. Inorganic/polymer hybrid layer stabilizing anode/electrolyte interfaces in solid-state Li metal batteries. Nano Res 2020;13:3230.
Deng S, Sun Y, Li X, Ren Z, Liang J, Doyle-Davis K, Liang J, Li W, Norouzi Banis M, Sun Q. Eliminating the detrimental effects of conductive agents in sulfide-based solid-state batteries. ACS Energy Lett 2020;5:1243.
Xu R, Xia X, Li S, Zhang S, Wang X, Tu J. All-solid-state lithium–sulfur batteries based on a newly designed Li7P2.9Mn0.1S10.7I0.3 superionic conductor. J Mater Chem A 2017;5:6310.
Zhang Y, Chen R, Liu T, Shen Y, Lin Y, Nan C-W. High capacity, superior cyclic performances in all-solid-state lithium-ion batteries based on 78Li2S-22P2S5 glass-ceramic electrolytes prepared via simple heat treatment. ACS Appl Mater Interfaces 2017;9:28542.
Busche MR, Weber DA, Schneider Y, Dietrich C, Wenzel S, Leichtweiss T, Schröder D, Zhang W, Weigand H, Walter D. In situ monitoring of fast Li-ion conductor Li7P3S11 crystallization inside a hot-press setup. Chem Mater 2016;28:6152.
Park M, Jung H-G, Jung WD, Cho SY, Yun B-N, Lee YS, Choi S, Ahn J, Lim J, Sung JY. Chemically evolved composite lithium-ion conductors with lithium thiophosphates and nickel sulfides. ACS Energy Lett 2017;2:1740.
Wenzel S, Weber DA, Leichtweiss T, Busche MR, Sann J, Janek J. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ionics 2016;286:24.
Altavilla C, Sarno M, Ciambelli P. A novel wet chemistry approach for the synthesis of hybrid 2D free-floating single or multilayer nanosheets of MS2@ oleylamine (M=Mo, W). Chem Mater 2011;23:3879.
Rajagopal R, Ryu K-S. Evaluation of Li6P2S8I solid electrolyte for all solid-state lithium battery applications. Chem Eng J 2020;402:126179.
Wang Z, Jiang Y, Wu J, Jiang Y, Huang S, Zhao B, Chen Z, Zhang J. Reaction mechanism of Li2S-P2S5 system in acetonitrile based on wet chemical synthesis of Li7P3S11 solid electrolyte. Chem Eng J 2020;393:124706.
Wu Z, Xie Z, Yoshida A, An X, Wang Z, Hao X, Abudula A, Guan G. Novel SeS2 doped Li2S-P2S5 solid electrolyte with high ionic conductivity for all-solid-state lithium sulfur batteries. Chem Eng J 2020;380:122419.
Ge Q, Zhou L, Lian Y-m, Zhang X, Chen R, Yang W. Metal-phosphide-doped Li7P3S11 glass-ceramic electrolyte with high ionic conductivity for all-solid-state lithium-sulfur batteries. Electrochem Commun 2018;97:100.
Kaus M, Stöffler H, Yavuz M, Zinkevich T, Knapp M, Ehrenberg H, Indris S. Local structures and Li ion dynamics in a Li10SnP2S12-based composite observed by multinuclear solid-state nmr spectroscopy. J Phys Chem C 2017;121:23370.
Hirai K, Tatsumisago M, Takahashi M, Minami T. 29Si and 31P MAS-NMR spectra of Li2S-SiS2-Li3PO4 rapidly quenched glasses. J Am Ceram Soc 1996;79:349.
Ahmad N, Zhou L, Faheem M, Tufail MK, Yang L, Chen R, Zhou Y, Yang W. Enhanced air stability and high Li-Ion conductivity of Li6.988P2.994Nb0.2S10.934O0.6 glass-ceramic electrolyte for all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces 2020;12:21548.
Zhou L, Tufail MK, Yang L, Ahmad N, Chen R, Yang W. Cathode-doped sulfide electrolyte strategy for boosting all-solid-state lithium batteries. Chem Eng J 2020;391:123529.
Tufail MK, Zhou L, Ahmad N, Chen R, Faheem M, Yang L, Yang W. A novel air-stable Li7Sb0.05P2.95S10.5I0.5 superionic conductor glass-ceramics electrolyte for all-solid-state lithium-sulfur batteries. Chem Eng J 2021;407:127149.
Eckert H, Zhang Z, Kennedy JH. Structural transformation of non-oxide chalcogenide glasses. The short-range order of lithium sulfide (Li2S)-phosphorus pentasulfide (P2S5) glasses studied by quantitative phosphorus-31, lithium-6, and lithium-7 high-resolution solid-state NMR. Chem Mater 1990;2:273.
Seino Y, Nakagawa M, Senga M, Higuchi H, Takada K, Sasaki T. Analysis of the structure and degree of crystallisation of 70Li2S-30P2S5 glass ceramic. J Mater Chem A 2015;3:2756.
Kaiser N, Spannenberger S, Schmitt M, Cronau M, Kato Y, Roling B. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte. J Power Sources 2018;396:175.
Bielefeld A, Weber DA, Janek Jr. Modeling effective ionic conductivity and binder influence in composite cathodes for all-solid-state batteries. ACS Applied Mater Interfaces 2020;12:12821.
Dewald GF, Ohno S, Hering JG, Janek J, Zeier WG. Analysis of charge carrier transport toward optimized cathode composites for all-solid-state Li-S batteries. Batteries Supercaps 2021;4:183.
Song W, Scholtis ES, Sherrell PC, Tsang DK, Ngiam J, Lischner J, Fearn S, Bemmer V, Mattevi C, Klein N. Electronic structure influences on the formation of the solid electrolyte interphase. Energy Environ Sci 2020;13:4977.
Ryu J, Kang J, Kim H, Lee JH, Lee H, Park S. Electrolyte-mediated nanograin intermetallic formation enables superionic conduction and electrode stability in rechargeable batteries. Energy Storage Materials 2020;33:164.
Li Z, Zhang J, Wu HB, Lou XW. An improved Li-SeS2 battery with high energy density and long cycle life. Adv Energy Mater 2017;7:1700281.
Cui Y, Abouimrane A, Lu J, Bolin T, Ren Y, Weng W, Sun C, Maroni VA, Heald SM, Amine K. (De) Lithiation mechanism of Li/SeSx (x=0–7) batteries determined by in situ synchrotron X-ray diffraction and X-ray absorption spectroscopy. J Am Chem Soc 2013;135:8047.
Jiang M, Liu G, Zhang Q, Zhou D, Yao X. Ultrasmall Li2S-carbon nanotube nanocomposites for high-rate all-solid-state lithium-sulfur batteries. ACS Appl Mater Interfaces 2021;13:18666.
Li P, Zheng X, Yu H, Zhao G, Shu J, Xu X, Sun W, Dou SX. Electrochemical potassium/lithium-ion intercalation into TiSe2: kinetics and mechanism. Energy Storage Mater 2019;16:512.
Srout M, Kwon NH, Ben Youcef H, Semlal N, Fromm KM, Saadoune I. Li0.5Ni0.5Ti1.5Fe0.5(PO4)3/C electrode material for lithium ion batteries exhibiting faster kinetics and enhanced stability. ACS Appl Mater Interfaces 2020;12:18496.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21975025, 21203008, 51772030), the National Key Research and Development Program of China “New Energy Project for Electric Vehicle” (No. 2016YFB0100204), and the Nature Science Foundation of Beijing Municipality (No. 2172051). State Key Laboratory also funds the project for Modification of Chemical Fibers and Polymer Materials, Donghua University. DTA, XRD, XPS, and NMR measurements were performed in the Analysis & Testing Center, Beijing Institute of Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
42765_2021_123_MOESM1_ESM.docx
Supplementary XRD pattern of Li7P3S11, Li7P2.9W0.05S10.85 and Li7P2.8W0.1S10.7 glasses (Figure S1); Magnified XRD pattern of glass–ceramic samples (Figure S2); XPS spectra of Li7P2.9W0.05S10.85 glass–ceramic electrolyte (Figure S3); SEM image of Li7P3S11 glass–ceramic electrolyte (Figure S4); TEM image of Li7P3S11 glass–ceramic electrolyte (Figure S5); Schematic diagram of the preparation of composite cathode (Figure S6); SEM image of SeS2 and SeS2/CNT samples (Figure S7); XRD pattern of pristine SeS2 and SeS2/CNT samples (Figure S8); schematic diagram of all-solid-state Li-SeS2 battery (Figure S9); the first galvanostatic charge–discharge curve of the INCC/Li7P2.9W0.05S10.85/Li-In battery (Figure S10); the potential profile before, during, and after a constant current pulse with schematic labeling for CCC/Li7P2.9W0.05S10.85/Li-In (Figure S11) (DOCX 7323 KB)
Rights and permissions
About this article
Cite this article
Zhou, L., Tufail, M.K., Liao, Y. et al. Tailored Carrier Transport Path by Interpenetrating Networks in Cathode Composite for High Performance All-Solid-State Li-SeS2 Batteries. Adv. Fiber Mater. 4, 487–502 (2022). https://doi.org/10.1007/s42765-021-00123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-021-00123-6