Abstract
Despite increasing interest in animal emotions, jealousy has rarely been directly addressed in comparative research, except for studies of human-pet interactions. Jealous behavior emerges when a valuable social bond is threatened by a third-party, prompting aggression or intervention attempts to direct the partner’s attention away from the rival. Emotional reactions that protect relationships are expected in species in which social relationships are important for fitness, including primates. Previous primate studies have alluded to this ultimate function, but never explicitly tested predictions corresponding to a proximate jealousy mechanism. We demonstrate jealous behavior in a long-established colony of chimpanzees (N = 17) during a socially disruptive period due to group introductions, which provided an ideal experimental opportunity to test predictions of a jealousy hypothesis. Specifically, we found that negative reactions (agonism and intervention attempts) towards social closeness between two groupmates were generally more common when the aggressor/intervener had a valuable relationship to one (as compared with both or neither) of the dyad’s members, indicating that the other partner represented a potential social rival. In line with this suggestion, we found that negative reactions most often targeted dyads containing newly introduced individuals, especially when the social conditions for jealousy were met, and in particular during the socially unstable introduction period. Results underscore the potential adaptive role of jealousy in protecting fitness-enhancing relationships from social interlopers, by extension indicating that this emotion likely evolved in diverse animal societies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The past several decades have witnessed an upsurge in the scientific study of nonhuman animal (hereafter, animal) emotions (Bekoff, 2000; Dawkins, 2016; de Waal, 2019; Mendl, Burman, & Paul, 2010; Panksepp, 2004). Originally limited to basic emotions such as fear, joy, sadness, and surprise, research on animal affective states has more recently expanded to include complex socioemotional capacities such as empathy, shame, guilt, and grief (Bekoff, 2007; de Waal & Preston, 2017; King, 2003; Kujala, 2017; Morris, Doe, & Godsell, 2008). Jealousy is the latest term to enter the purview of animal emotion research. Jealousy is typically defined as an emotional response to a perceived threat posed by a social rival to an important relationship (Dillon, 2013; Hart & Legerstee, 2010; Parrot & Smith, 1993). In humans, this negative reaction may be signaled by overt behaviors to reclaim the attention of one’s partner, which can include aggression against the rival or other interventions to interrupt the rival-partner interaction (Hart, 2010). As such, jealousy is an emotion that functions to promote the retention of valuable social bonds against potential intruders. In line with this, ultimate explanatory frameworks have posited that emotions are specialized states shaped by natural selection to increase an animal’s ability to cope with situational threats and opportunities (Nesse, 1990). In gregarious animal societies, relationships are among the most valuable resources that an individual possesses—their quantity, strength, and stability directly predicting individual fitness (McFarland et al., 2017; Silk et al., 2010; Thompson, 2019).
The empirical investigation of jealousy in other species has thus far focused on interactions between domestic dogs and humans—likely prompted in part by owners’ readiness to attribute jealous emotions to their pets (Martens, Enders-Slegers, & Walker, 2016; Morris et al., 2008; Morris, Knight, & Lesley, 2012). Dogs are good candidates for jealousy, thanks to their advanced socio-cognitive abilities and long-lasting attachment relationships to humans (Hare & Woods, 2013). Yet results are mixed: whereas some studies have demonstrated dogs’ jealous reactions to their owners interacting with a “rival” dog (Abdai, Baño Terencio, Pérez Fraga, & Miklósi, 2018; Cook, Prichard, Spivak, & Berns, 2018; Harris & Prouvost, 2014), other researchers have failed to find evidence for jealousy (Prato-Previde, Nicotra, Fusar Poli, Pelosi, & Valsecchi, 2018a; Prato-Previde, Nicotra, Pelosi, & Valsecchi, 2018b). Such discrepancies may stem from the artificial nature of the experimental paradigms employed: some studies show that dogs exhibit jealous behavioral and neurological responses to fake dogs (Cook et al., 2018; Harris & Prouvost, 2014), but findings that fake dogs are not perceived as real social threats cast doubt on this interpretation (Prato-Previde, Nicotra, Pelosi, et al., 2018, 2018a, 2018b). Variation in the nature of relationships under consideration is therefore critical to both precise definitions of, and methodological approaches to, jealousy (Webb & de Waal, 2018)—underlining the need to use real social interlopers and move beyond artificial experimental contexts (Prato-Previde, Nicotra, Fusar Poli, et al., 2018).
Zoo introduction programs, wherein unfamiliar individuals are integrated into established groups, offer an ideal setting in which to observe spontaneously occurring jealousy. The arrival of new stranger conspecifics, particularly within longstanding groups, may threaten the strength of existing social and sexual bonds, and has been proposed as the ideal test of animal jealousy (Panksepp, 2010). The expression of jealousy should vary according to the quality of relationships within this social triangle (i.e., actor-partner, actor-rival, partner-rival), thus allowing for more nuanced definitions and tests of a jealousy hypothesis. Furthermore, widening the scope from human-dog interactions to study jealous interactions among conspecifics situates the emotion in the evolutionary context in which it likely emerged.
Lastly, extending the study of jealous behavior from canids to other socially complex species will better illuminate the selection pressures that give rise to this socioemotional response. In the case of nonhuman primates, such research can strengthen or weaken the case for an emotion homologous to human jealousy. Prior primate affective studies have examined the neural substrates of jealousy in experimental contexts (Maninger et al., 2017; Rilling, Winslow, & Kilts, 2004) but not conducted observations of spontaneous jealous behaviors. Previous primate behavioral studies have reported bystander interference in affiliative interactions (e.g., Mondragón-Ceballos, 2001; Schino & Lasio, 2018; especially in the “political” context of male power struggles in chimpanzees: de Waal, 1982; Nishida & Hosaka, 1996) and occasionally alluded to jealousy’s ultimate function (Baniel, Cowlishaw, & Huchard, 2018; Mielke et al., 2017), but never explicitly tested predictions corresponding to a proximate jealousy hypothesis.
We studied the occurrence of jealousy behaviorsFootnote 1 in a colony of chimpanzees with long-established social relationships upon the introduction of three new group members. Jealousy was defined as a negative behavioral response from one individual to social closeness between two partners (Abdai et al., 2018). Specifically, we tested whether variability in the quality of social bonds between all three individuals predicted the occurrence of jealous behavior. Consistent with a jealousy-based explanation for the behavior, we expected directed aggression and intervention attempts towards an affiliating dyad to be more common when the actor had a valuable relationship with one (compared with both or neither) partner (prediction: p1). Furthermore, we predicted this jealous behavioral pattern to be higher during the introduction period compared with before or after (p2), given that newcomers could threaten existing social bonds upon arrival but ultimately become integrated into the group. Accordingly, we also predicted jealous behaviors to target newly introduced individuals more than established group members (p3).
Methods
Subjects were 17 adult chimpanzees (Pan troglodytes) housed at Royal Burgers’ Zoo in Arnhem, the Netherlands. The group comprised 4 males and 13 females—3 of whom arrived at the zoo amid the study period (additional subject demographics are available in Table S1). Subjects’ housing consisted of an indoor (~ 386 m2) and outdoor (~ 7000 m2) enclosure, both equipped with climbing structures and nesting material, as well as adjacent off-exhibit cages for routine zoo management purposes. The group was fed approximately three times per day and had access to water ad libitum.
Behavioral observations were conducted between Sep 16, 2015, and Jun 01, 2018. The new females arrived at the zoo on Nov 18, 2015, but were housed off-exhibit together until Feb 12, 2016, at which point they were temporarily transferred to a group with 5 females from the original colony. Observations conducted on the original, intact colony preceding this date are thus considered the pre-introduction period (~ 5 months). A series of subsequent 1-to-1 introductions (which took place in the off-exhibit cages) between the new females and 2 of the colony’s males resulted in the formation of 2 subgroups on Jul 05, 2016 (see Tables S1 and S2 for details): the introduction subgroup, which included the new females, and the established subgroup, which comprised the rest of the colony. This period is referred to as the introduction period (~ 15 months). These two subgroups merged on Oct 18, 2017, with observations after Nov 20, 2017, representing the post-introduction period (~ 8 months).
Observations typically occurred 3–5 days per week, between 9:00 and 17:00 h, using the Time Stamped Fieldnotes iPad program (https://www.neukadye.com/mobile-applications/timestamped-field-notes/). On each observation day, at least one 90-min group observation recorded all occurrences of dyadic affiliative and sexual interactions (see Table S3) as well as any potential “triadic” jealous behaviors—i.e., negative reactions from one subject (A) towards social closeness between two groupmates (subjects B and C). Social closeness was operationally defined as B and C engaged in grooming, contact-sitting (sitting in close proximity with body contact), or playing. Negative reactions comprised both agonistic behaviors directed towards the affiliating dyad (i.e., hitting, bluff-displaying, grabbing, charging, vocalizing, or throwing tantrums) and intervention attempts (i.e., A attempting to physically interfere with B-C’s affiliation, either via sitting in between them or otherwise trying to disrupt their grooming or play bout). When agonistic and intervention interactions co-occurred within the same affiliative bout between B and C, we counted only one behavior by selecting the encounter that occurred last in the sequence. In total, we collected 890.4 h of observation across the study period (pre-introduction period: 190.4 h; introduction period: 373.6 h, i.e., 189.5 h for the introduction subgroup and 184.1 h for the established subgroup; post-introduction period: 326.4 h), which were counterbalanced across time of day.
During group observations, scan samples of state behaviors—grooming, mutual grooming, contact-sitting, and sitting in marginal contact (i.e., within arm’s reach)—were also recorded in 10-min intervals. Scan data were used to calculate dyadic affiliation rates (between A-B, A-C, and B-C, respectively) from the total number of scan points in which partners were grooming or in proximity, divided by the total number of scan points taken. Affiliation level was determined via the quartile points of these dyadic scores, where only dyads with scores higher than the top quartile were considered to have a strong affiliative relationship. Dyadic agonistic interactions were recorded ad libitum, and used in calculating dyadic agonistic rates (the frequency of agonism directed from one subject to another, divided by the total number of observation hours) and constructing the group’s dominance hierarchy (see below). Two observers concurrently collected data (one observing, the other recording) following a training period in which a Cohen’s kappa value > 0.70 was achieved for all observational protocols. A total of six observers (2 every ~ 9 months) collected data across the study period and overlapped such that inter-rater reliability between existing and incoming observers was always ensured.
Statistical Analyses
We analyzed data from a total of 173 triadic interactions (111 agonisms, 62 interventions) in which A exhibited a negative behavioral reaction to social closeness between B and C. To test predictions of the jealousy hypothesis, we structured data according to each potential triad per period, where for every subject (A), we tallied the number of negative behavioral reactions towards social closeness between every B and C dyad (note that the occurrence of agonism significantly positively predicted the occurrence of intervention attempts; see Fig. S1). The same dyad was not represented twice—i.e., as B-C and C-B—given that, for interventions, A’s attempts were by definition directed to both dyad members, and for agonistic interactions, A’s specific target was indiscernible in the majority (> 60%) of cases. We then ran generalized linear mixed models with a Poisson error distribution and a logit-link function (hereafter, Poisson GLMM), using the function glmer of the R package “lme4” (Bates, Maechler, Bolker, & Walker, 2014) in R version 3.5.0 (R Core Team, 2018). To validate all statistical models, we examined the distribution of scaled residuals (via the function simulateResiduals) and confirmed the absence of overdispersion and zero-inflation using the “DHARMa” package (Hartig, 2018). The alpha level was set at P < 0.05 for all analyses.
In order to test whether negative reactions were more common when the actor had a valuable relationship with one (compared with both or neither) partner (p1), our first Poisson GLMM (Model 1) incorporated a categorical fixed effect (affABC) representing the affiliation level between A and B-C—coded as 0 when A had a strong relationship to neither B nor C, 1 when A had a strong relationship to either B or C, and 2 when A had a strong relationship to both B and C. As negative reactions were expected to covary with the frequency of B-C’s affiliation, we also incorporated the affiliation rate between B and C (affrateBC) as a fixed effect. Negative reactions could also be an artifact of agonistic tendencies more generally, so A’s agonistic rate towards B and C, respectively (aggrateAB and aggrateAC), comprised additional fixed effects. Further fixed effects included kinship (kinABC)—reserved for matrilineal relationships, where only (grand)mother-offspring and maternal siblings were considered kin, again modeled categorically (0 = A kin to neither B nor C; 1 = A kin to B or C; 2 = A kin to both B and C). Dominance (calculated via the direction of submissive signals like pant-grunts and non-agonistic approach/retreat interactions, combined with zookeepers’ knowledge) was also included as a fixed factor (domABC; 0 = A dominant to neither B nor C; 1 = A dominant to B or C; 2 = A dominant to both B and C). Given that female reproductive status could influence aggression and intervention attempts directed from males, we incorporated whether the target dyad contained an estrous female (estrousBC, 0 = no, 1 = yes) for the majority of the triadic interactions noted within a given observation period (determined a posteriori from keeper records), as well as A’s sex (maleA, 0 = female, 1 = male) and B-C’s sex-class (sexclassBC: “female-female,” “male-female,” “male-male”). Finally, observation period (“pre-introduction,” “introduction,” “post-introduction”) was included as an additional fixed factor. Random effects comprised the identities of A, B, and C, respectively. The total number of observation hours (log-transformed) for the group per period was incorporated as an offset variable.
To test whether the jealous behavioral pattern was more pronounced during the introduction period compared with that during either the pre- or post-introduction periods (p2), we adopted a two-pronged approach. In the first approach, we assessed whether observation period significantly predicted negative reactions in Model 1. In the second approach, we added the interaction between period and a binary variable (affABC1) corresponding to whether/not the social conditions for jealousy were met (i.e., when A had a close relationship to only one member of the dyad B-C, i.e., when affABC = 1). This allowed us to test not only whether negative reactions were generally more likely during the introductions compared with before/after (p2a) but also whether changes in the likelihood of negative reactions across periods were more pronounced when the social conditions for jealousy were met (p2b). To avoid overfitting our models, we only included those fixed effects that were significant in Model 1 (see Table 1).
To test whether jealous behaviors targeted newly introduced individuals more often than established group members (p3), we subsetted the data to the observation periods in which the newly introduced females were present (i.e., the introduction and post-introduction periods) and incorporated whether the target dyad contained a newly introduced female (introBC: 0 = no, 1 = yes) as a fixed factor. Again, we first determined whether introBC predicted negative reactions as a main effect (p3a), and subsequently added the introBC × affABC1 interaction term to determine whether the effect of a target dyad containing a newly introduced female on the likelihood of negative reactions was most pronounced when the social conditions for jealousy were met (p3b). We used the prior Model 1 specifications (including significant fixed effects), but also incorporated the subgroup (0 = established group, 1 = introduction group) as a fixed factor whenever the analysis was limited to the introduction period.
Results
Negative behavioral reactions to dyadic social closeness were significantly more likely when A had a strong relationship to either B or C, compared with neither (Z = − 3.99, b = − 0.79, P < 0.001) or both (Z = − 2.21, b = − 0.65, P = 0.029) of the dyad’s members (Table 1). There was no significant difference depending on whether A had a strong relationship to neither versus both of the dyad’s members (Z = − 0.39, b = − 0.13, P = 0.699). In other words, subjects were most likely to attempt interventions or direct agonism towards social closeness between two groupmates when they had a strong affiliative bond with one but not the other individual, which supports the jealousy hypothesis (p1).
As shown in Table 1, A’s agonism and intervention attempts towards B-C also varied according to kinship patterns. Subjects were significantly more likely to respond negatively when they were kin to one (Z = 7.44, b = 1.46, P < 0.001) or both (Z = 5.34, b = 4.46, P < 0.001) as compared with neither of the dyad’s members, though the latter result should be interpreted with considerable caution given that there was only one triad in which all members were related (i.e., where kinABC = 2). Subject A’s dominance relationship to B-C did not significantly predict negative reactions. Consistent with p2a, negative reactions to dyadic social closeness were overall significantly more common during the introduction period compared with those during the period before (Z = 4.59, b = 1.19, P < 0.001) and after (Z = 5.34, b = 1.38, P < 0.001), whereas the difference between the pre- and the post-introduction periods was non-significant (Table 1). Unweighted rates calculated from the raw data corroborated this pattern and revealed that such reactions were overall relatively infrequent (pre-introduction period: 0.19 negative reactions/h; introduction period: 0.33 negative reactions/h; post-introduction period: 0.23 negative reactions/h).
Whether B or C was an estrous female significantly positively predicted the occurrence of negative reactions from A (Z = 2.28, b = 0.65, P = 0.023). Negative reactions were also significantly more likely when A was a male compared with a female (Z = 2.43, b = 0.98, P = 0.015), though note that there was no significant estrousBC × maleA interaction (Z = − 1.52, b = − 0.56, P = 0.129). The sex-class composition of B-C did not significantly predict A’s negative reactions, though a significant sexclassBC × maleA interaction revealed that the effect of A being male (vs. female) changed as a function of B-C’s sex-class. The aforementioned sex difference (males > females) was most pronounced when A’s behavior was direct towards male-male dyads as compared with other dyad types (female-female: Z = 2.97, b = 2.17, P = 0.003; male-female: Z = 5.00, b = 3.73, P < 0.001). This difference was also more pronounced when A’s behavior was directed towards female-female dyads compared with male-female dyads (Z = 4.18, b = 1.55, P = 0.129), suggesting that males were not primarily driven to disrupt mixed-sex dyads; in fact, females exhibited this tendency more than males (see Fig. S2). B and C’s affiliative rate significantly positively predicted negative reactions from A (Z = 5.75, b = 0.36, P < 0.001), which is perhaps unsurprising given that more frequent dyadic affiliation provides more opportunities for A to interrupt. A’s rate of aggression to both B and C (respectively) did not significantly predict A’s negative reactions towards B-C’s social closeness.
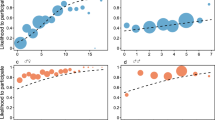
In a subsequent model incorporating the interaction between period × affABC1, we also found some support for p2b. As shown in Fig. 1, changes in the likelihood of negative reactions across the three periods depended on when the social conditions for jealousy were met. Increases in negative responses in the introduction period compared with those in the periods before (Z = 2.87, b = 1.32, P = 0.004) and after (Z = 4.05, b = 1.73, P < 0.001) were more pronounced when the actor was strongly bonded to only one partner (vs. both/neither partner). In other words, upon the arrival of unfamiliar conspecifics, chimpanzees were more likely to interfere in affiliative interactions when they had a strong relationship with one but not the other individual.
Interaction (3 × 2) between observation period (pre-introduction, introduction, post-introduction) and whether the social conditions for jealousy were met (when the actor had a valuable relationship to only one member of the dyad, denoted by a solid line) or not (when the actor had valuable relationship to neither or both member(s) of the dyad, denoted by a dashed line), on the occurrence of negative reactions. Large black points indicate predicted values, bars represent standard errors, and shading denotes the 95% confidence interval from the GLMM. ***P < 0.001; **P < 0.01; *P < 0.05
During the introduction period, there was a significant main effect of introBC on the occurrence of negative reactions (Z = 2.09, b = 0.69, P = 0.037), indicating that subjects were more likely to target dyads that contained a newly introduced female as compared with those that did not (p3a). Moreover, we found a significant introBC × affABC1 interaction (Z = 2.78, b = 2.04, P = 0.006), revealing that this pattern was upheld only when the social conditions for jealousy were met (p3b; see Fig. 2). In other words, the increase in agonism and intervention attempts towards dyads containing newly introduced group members was only true when the actor had a strong relationship to one of the dyad’s members. Consistent with a jealousy-based explanation, this suggests that such behaviors may be driven to protect valuable relationships from potential social interlopers. Introduction subgroup was a significant predictor of negative reactions (Z = 3.48, b = 1.96, P < 0.001), but only when introBC was excluded from the model, suggesting that the presence of newly introduced females drove subgroup main effects.
Interaction (2 × 2) between whether or not the target dyad contained a newly introduced female and whether the social conditions for jealousy were met (when the actor had a valuable relationship to only one member of the dyad, denoted by a solid line) or not (when the actor had valuable relationship to neither or both member(s) of the dyad, denoted by a dashed line), on the occurrence of negative reactions during the introduction period. Large black points indicate predicted values, bars represent standard errors, and shading denotes the 95% confidence interval from the GLMM. ***P < 0.001; **P < 0.01; *P < 0.05
Discussion
We found evidence for jealous behavior in chimpanzees during a socially disruptive period due to group introductions, which provided a natural experimental opportunity to test predictions of a jealousy hypothesis. Namely, we found that negative reactions (agonism and intervention attempts) towards social closeness between two groupmates increased markedly during the introduction period as compared with those during the periods before or after. Consistent with a jealousy-based explanation, we found that these reactions were most likely when the actor had a close relationship to only one of dyad’s members (compared with both or neither), indicating that the other partner could represent a potential interloper. In line with this suggestion, we found that negative reactions most often targeted dyads containing newly introduced individuals, especially when the social conditions for jealousy were met, and in particular during the socially unstable introduction period.
Jealousy’s proposed function in protecting valuable bonds from external social threats was the impetus for the present study’s emphasis on how reactions to social closeness between other groupmates varied according to relationship quality, further premised on a social context that might threaten long-established bonds (i.e., introductions). The extent to which agonism and interventions towards others’ affiliation actually succeeded in preserving valuable social bonds (not to mention disrupting the real-time interaction) remains open to investigation, and it is worth considering the additional possibility that jealous behaviors might be maladaptive in certain social contexts, particularly when dispersal is limited by captivity. Unlike primates living in stable social groups, wild chimpanzee social relationships are characterized by a high degree of fission-fusion (Aureli et al., 2008), which may influence jealous behavioral strategies and their relative importance for maintaining intersexual and intrasexual bonds (Buunk, Massar, Dijkstra, & Fernández, 2019).
It is noteworthy that agonism and intervention attempts were more frequent from males and more likely to target dyads containing estrous females; however, the lack of a significant interaction between actor sex and targets’ reproductive state suggests that sexual coercion did not drive the observed behavioral patterns (Muller, Kahlenberg, & Wrangham, 2009; Smuts & Smuts, 1993). In fact, males were more likely to intervene in same-sex (especially male-male) as opposed to mixed-sex dyads, further suggesting that their behavior was not driven by sexual jealousy. The likely explanation is the “political” one offered by de Waal (1982) and Nishida and Hosaka (1996) as the prevention or undermining of hostile coalitions (see below). On the contrary, although their rates of jealous behavior were overall lower, females were more likely to respond negatively to social closeness between mixed-sex dyads than other dyad types. However, descriptively, this pattern appears to be the result of mothers disrupting affiliative interactions between their children and opposite-sex partners, rather than sexual jealousy per se. Although our dataset lacked sufficient power to test these patterns systematically, they highlight the various strategic ends that jealous behavior may serve.
Under a functional approach to emotions, jealousy may be advantageous across a number of social contexts in which there are perceived threats to fitness-enhancing social relationships (e.g., mate-guarding, sibling and parental competition, friendship), but sexual jealousy is the scenario most often invoked (Buss, 2000). The present study broadens this scope to highlight jealousy’s additional role in the protection of non-sexual social bonds, which adds to a literature that has thus far only alluded to this ultimate explanation in primates (Mielke et al., 2017), or explicitly tested corresponding predictions in the context of interspecific bonds between dogs and their human owners (Abdai et al., 2018; Cook et al., 2018; Harris & Prouvost, 2014; Prato-Previde, Nicotra, Fusar Poli, et al., 2018; Prato-Previde, Nicotra, Pelosi, et al., 2018).
The conspecific context analyzed here illuminates intraspecific selection pressures that may also give rise to jealous behavior in various animal societies. For instance, chimpanzees and mangabeys monitor others’ grooming interactions and intervene when the alliance between groomers could negatively impact their social relationships and rank (Mielke et al., 2017). In both species, bystanders intervened more when they had a strong affiliative relationship with one or both groomers, contrasting with the current study’s finding that negative reactions peaked when bystanders were strongly affiliated to just one of the target dyad’s members. Although we would not discount that other relationship configurations prompt jealousy (indeed, this was one reason for examining main effects on negative reactions overall, in addition to interactions with relationship quality), the present work assumes that a proximate jealousy mechanism would motivate bystanders to deter newly introduced group members (who have yet to form stable social bonds) from disrupting existing strong relationships. Nonetheless, it is certainly conceivable that an individual would be jealous when two valuable social partners affiliate, presuming this affiliation could threaten the individual’s existing bond with one or both of those partners. The disparity between our results and those reported in Mielke et al. (2017) could further be attributable to the study setting (captivity vs. wild) or to the present study’s inclusion of a wider repertoire of triadic interactions (i.e., not just grooming interventions). Although we found no effect of dominance on negative reactions to dyadic social closeness (cf Mielke et al.’s (2017) result that rank-related patterns predicted bystanders’ intervention tendencies), our finding that males were especially likely to disrupt male-male affiliation could reflect similar strategic motivations. Such triadic interactions among males have been previously described in chimpanzees as “separating interventions” (de Waal, 1982; Nishida & Hosaka, 1996), which function to deter individuals with similar rank from forming alliances. Although we did not test the efficacy of these behaviors in disrupting immediate affiliation or future hostile coalitions, the general notion that interventions generally function to prevent future alliances and competition has received additional support in ravens (Massen, Szipl, Spreafico, & Bugnyar, 2014) and horses (Schneider & Krueger, 2012).
Interestingly, a recent study in humans reported that jealousy is calibrated according to the amount of time a friend and interloper spend together (i.e., it increases with the frequency of friend-interloper interactions: Krems, Williams, Kenrick, & Aktipis, in press). Although not one of our study’s original predictions, that chimpanzees’ negative reactions to social closeness increased with the rate of affiliation between B and C suggests that individuals felt more threatened (and behaved accordingly) when potential interlopers consumed more of their partners’ time. This contrasts with chimpanzee and raven interventions wherein bystanders interrupted more when interactants exhibited weaker bonds (Massen et al., 2014; Mielke et al., 2017). Nonetheless, in the present work, B-C’s affiliative rate could have also merely provided more opportunities for A to interrupt, a confound that future research could attempt to disentangle.
Despite its recent momentum, the topic of animal emotions continues to raise a number of challenging questions and controversies (de Waal, 2019). One persistent debate centers on the extent to which emotions are private experiences, which cannot be directly measured in others. This explains why many affective researchers advocate for a science of emotions separate from that of feelings—the latter referring to private states that cannot be known in others, and the former being manifested in behavior, physiology, and other measurable phenomena (Adolphs & Anderson, 2018; Allen & Bekoff, 1997; Damasio, 2004; de Waal, 2011; Prinz, 2005). Another common feature of contemporary debates about animal emotions is the extent to which animals even have the capacity for secondary (non-basic) emotions, compounded by a reluctance to impute human mental and emotional states to other species. Historically, it is noteworthy that the study of other social emotions such as empathy has gone through similar iterations—originally considered mere anthropomorphic projection, empathy is now a term widely used to describe behavior across the animal kingdom (de Waal & Preston, 2017; Pérez-Manrique & Gomila, 2018). Similar discussions are unfolding about animal jealousy (see commentary around (Cook et al., 2018)), which will benefit (as did the study of empathy) from considering the social relationships and contexts most likely to elicit the emotion.
Lastly, chimpanzee introductions are notoriously difficult, often involving protracted aggression towards newcomers and resulting injuries (Brent, Kessel, & Barrera, 1997); and yet, the social emotions underlying this process remain largely overlooked. As waitlists at zoos densify and research chimpanzees move to sanctuaries (de Waal, 2012), staff will increasingly face the complex task of planning and managing introductions. Ensuring adequate animal welfare will require a richer consideration of socioemotional capacities like jealousy. For instance, aggression towards new group members may stem from a desire to protect existing social bonds as much as an aggressive tendency towards strangers in and of themselves. Moreover, although not a focus of this paper, evidence for individual differences in jealousy in humans (Collibee & Furman, 2016; Parker, Low, Walker, & Gamm, 2005) as well as in chimpanzees from personality trait rating approaches (Freeman et al., 2013) highlights individual-level considerations that future research could explore, ideally with a more longitudinal focus. Although jealous individuals may encounter difficulty adapting to the introduction and socialization of new group members, less jealous individuals may incur costs if they lose valuable relationships to social intruders. Either way, the study of animal jealousy has ethical implications for an often-unacknowledged consequence of captivity—the artificial nature of the social situation and its concomitant emotional ramifications.
Conclusion
Situating animal research on jealousy in the context of social relationships represents a key step forward in the development of hypotheses, theoretical frameworks, and novel methodological approaches to study this socioemotional capacity. Identifying homologous human-animal emotional processes, keeping the science of emotions separate from that of feelings, considering the ethical implications of animal emotional complexity, and pursuing these questions in the widest range of study systems possible are all central to this endeavor.
Notes
As advocated by others (Abdai et al., 2018; Abdai & Miklósi, 2018), here we deliberately refer to jealous behavior so as to be clear we refer to the emotion rather than the felt component of jealousy. We consider this issue in further detail in the “Discussion” section.
References
Abdai, J., & Miklósi, Á. (2018). Displaying jealous behavior versus experiencing jealousy. Commentary on Cook et al. on dog jealousy. Animal Sentience, 152, 1–4.
Abdai, J., Baño Terencio, C., Pérez Fraga, P., & Miklósi, Á. (2018). Investigating jealous behaviour in dogs. Scientific Reports, 8(1), 1–8.
Adolphs, R., & Anderson, D. J. (2018). The neuroscience of emotion: A new synthesis. Princeton: Princeton University Press.
Allen, C., & Bekoff, M. (1997). Species of mind: The philosophy and biology of cognitive ethology. Cambridge: MIT Press.
Aureli, F., Schaf, C. M., Boesch, C., Simon, K., Call, J., Chapman, C. A., et al. (2008). Fission-fusion dynamics new research frameworks. Current Anthropology, 49(4), 627–654.
Baniel, A., Cowlishaw, G., & Huchard, E. (2018). Jealous females? Female competition and reproductive suppression in a wild promiscuous primate. Proceedings of the Royal Society B: Biological Sciences, 285(1886).
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2014). lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. R Package Version, 1, 1–7.
Bekoff, M. (2000). Animal emotions: Exploring passionate natures. BioScience, 50(10), 861–867.
Bekoff, M. (2007). The emotional lives of animals. Novato: New World Library.
Brent, L., Kessel, A. L., & Barrera, H. (1997). Evaluation of introduction procedures in captive chimpanzees. Zoo Biology, 16(4), 335–342.
Buss, D. M. (2000). The dangerous passion: Why jealousy is as necessary as love and sex. The Free Press.
Buunk, A. P., Massar, K., Dijkstra, P., & Fernández, A. M. (2019). Intersexual and intrasexual competition and their relation to jealousy. In L. M. Welling & T. Shackelford (Eds.), The Oxford handbook of evolutionary psychology and behavioral endocrinology (pp. 215–236). Oxford University Press.
Collibee, C., & Furman, W. (2016). Chronic and acute relational risk factors for dating aggression in adolescence and young adulthood. Journal of Youth and Adolescence, 45(4), 763–776.
Cook, P., Prichard, A., Spivak, M., & Berns, G. S. (2018). Jealousy in dogs? Evidence from brain imaging. Animal Sentience, 117, 1–14.
Damasio, A. R. (2004). Emotions and feelings: A neurobiological perspective. In A. S. R. Manstead, N. Fridja, & A. Fischer (Eds.), Feelings and emotions: The Amsterdam symposium. Cambridge: Cambridge University Press.
Dawkins, M. S. (2016). Animal minds and animal emotions. American Zoologist, 40(6), 883–888.
de Waal, F. B. M. (1982). Chimpanzee politics. Johns Hopkins University Press.
de Waal, F. B. M. (2011). What is an animal emotion? Annals of the New York Academy of Sciences, 1224, 191–206.
de Waal, F. B. M. (2012). Research chimpanzees may get a break. PLoS Biology, 10(3), 1–4.
de Waal, F. B. M. (2019). Mama’s last hug. New York: Norton.
de Waal, F. B. M., & Preston, S. D. (2017). Mammalian empathy: Behavioral manifestations and neural basis. Nature Reviews Neuroscience, 18, 498–509.
Dillon, L. (2013). Functional aspects of jealousy across the lifespan. Human Ethology Bulletin, 28(2), 13–26.
Freeman, H. D., Brosnan, S. F., Hopper, L. M., Lambeth, S. P., Schapiro, S. J., & Gosling, S. D. (2013). Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. American Journal of Primatology, 75(10), 1042–1053.
Hare, B., & Woods, V. (2013). The genius of dogs. Penguin.
Harris, C. R., & Prouvost, C. (2014). Jealousy in dogs. PLoS One, 9(7), e94597.
Hart, S. L. (2010). The ontogenesis of jealousy in the first year of life: A theory of jealousy as a biologically-based dimension of temperament. In S. L. Hart & M. Legerstee (Eds.), Handbook of jealousy: Theory, research, and multidisciplinary approaches (pp. 57–82). West Sussex: Wiley-Blackwell.
Hart, S. L., & Legerstee, M. (2010). Handbook of jealousy: Theory, research, and multidisciplinary approaches. West Sussex: Wiley-Blackwell.
Hartig, F. (2018). DHARMa: Residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.2.0. https://CRAN.R-project.org/package=DHARMa.
King, B. J. (2003). How animals grieve. Chicago: University of Chicago Press.
Krems, J., Williams, K., Kenrick, D., & Aktipis, A. (in press). Friendship jealousy: One tool of friendship maintenance? Journal of Personality and Social Psychology. https://doi.org/10.1037/pspi0000311.
Kujala, M. V. (2017). Canine emotions as seen through human social cognition. Animal Sentience, 14(1), 1–34.
Maninger, N., Mendoza, S. P., Williams, D. R., Mason, W. A., Cherry, S. R., Rowland, D. J., et al. (2017). Imaging, behavior and endocrine analysis of “jealousy” in a monogamous primate. Frontiers in Ecology and Evolution, 5(119), 1–14.
Martens, P., Enders-Slegers, M., & Walker, J. K. (2016). The emotional lives of companion animals: Attachment and subjective claims by owners of cats and dogs. Anthrozoös, 29(1), 73–88.
Massen, J. J. M. M., Szipl, G., Spreafico, M., & Bugnyar, T. (2014). Ravens intervene in others’ bonding attempts. Current Biology, 24(22), 2733–2736.
McFarland, R., Murphy, D., Lusseau, D., Henzi, S. P., Parker, J. L., Pollet, T. V., & Barrett, L. (2017). The ‘strength of weak ties’ among female baboons: Fitness-related benefits of social bonds. Animal Behaviour, 126, 101–106.
Mendl, M., Burman, O. H. P., & Paul, E. S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proceedings of the Royal Society B: Biological Sciences, 277(1696), 2895–2904.
Mielke, A., Samuni, L., Preis, A., Gogarten, J. F., Crockford, C., & Wittig, R. M. (2017). Bystanders intervene to impede grooming in Western chimpanzees and sooty mangabeys. Royal Society Open Science, 4(11), 171296.
Mondragón-Ceballos, R. (2001). Interfering in affiliations: Sabotaging by stumptailed macaques, Macaca arctoides. Animal Behaviour, 62(6), 1179–1187.
Morris, P., Doe, C., & Godsell, E. (2008). Secondary emotions in non-primate species? Behavioural reports and subjective claims by animal owners. Cognition and Emotion, 22(1), 3–20.
Morris, P., Knight, S., & Lesley, S. (2012). Belief in animal mind: Does familiarity with animals influence beliefs about animal emotions? Society and Animals, 20(3), 211–224.
Muller, M. N., Kahlenberg, S. M., & Wrangham, R. W. (2009). Male aggression and sexual coercion of females in primates. In M. N. Muller & R. W. Wrangham (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 3–22). Harvard University Press.
Nesse, R. M. (1990). Evolutionary explanations of emotions. Human Nature, 1(3), 281–305.
Nishida, T., & Hosaka, K. (1996). Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In W. C. McGrew, L. F. Marchant, & T. Nishida (Eds.), Great ape societies (pp. 114–134). Cambridge University Press.
Panksepp, J. (2004). Affective neuroscience: The foundations of human and animal emotions. New York: Oxford University Press.
Panksepp, J. (2010). The evolutionary sources of jealousy: Cross-species approaches to fundamental issues. In S. L. Hart & M. Legerstee (Eds.), Handbook of jealousy: Theory, research, and multidisciplinary approaches (pp. 101–120). Malden: Wiley-Blackwell.
Parker, J. G., Low, C. M., Walker, A. R., & Gamm, B. K. (2005). Friendship jealousy in young adolescents: Individual differences and links to sex, self-esteem, aggression, and social adjustment. Developmental Psychology, 41(1), 235–250.
Parrot, W. G., & Smith, R. H. (1993). Distinguishing the experiences of envy and jealousy. Journal of Personality and Social Psychology, 64(6), 906–920.
Pérez-Manrique, A., & Gomila, A. (2018). The comparative study of empathy: Sympathetic concern and empathic perspective-taking in non-human animals. Biological Reviews, 93(1), 248–269.
Prato-Previde, E., Nicotra, V., Fusar Poli, S., Pelosi, A., & Valsecchi, P. (2018a). Do dogs exhibit jealous behaviors when their owner attends to their companion dog? Animal Cognition, 21(21), 703–713.
Prato-Previde, E., Nicotra, V., Pelosi, A., & Valsecchi, P. (2018b). Pet dogs’ behavior when the owner and an unfamiliar person attend to a faux rival. PLoS One, 13(4), 1–17.
Prinz, J. (2005). Are emotions feelings? Journal of Consciousness Studies, 12, 9–25.
R Core Team. (2018). R: A language and environment for statistical computing. Retrieved from https://www.r-project.org
Rilling, J. K., Winslow, J. T., & Kilts, C. D. (2004). The neural correlates of mate competition in dominant male rhesus macaques. Biological Psychiatry, 56(5), 364–375.
Schino, G., & Lasio, F. (2018). Competition for grooming partners and interference in affiliation among female mandrills. Ethology, 124(8), 600–608.
Schneider, G., & Krueger, K. (2012). Third-party interventions keep social partners from exchanging affiliative interactions with others. Animal Behaviour, 83(2), 377–387.
Silk, J. B., Beehner, J. C., Bergman, T. J., Crockford, C., Engh, A. L., Moscovice, L. R., Wittig, R. M., Seyfarth, R. M., & Cheney, D. L. (2010). Strong and consistent social bonds enhance the longevity of female baboons. Current Biology, 20(15), 1359–1361.
Smuts, B. B., & Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Advances in the Study of Behavior, 22, 1–63.
Thompson, N. A. (2019). Understanding the links between social ties and fitness over the life cycle in primates. Behaviour, 156(9), 859–908.
Webb, C. E., & de Waal, F. B. M. (2018). Situating the study of jealousy in the context of social relationships. Commentary on Cook et al. on dog jealousy. Animal Sentience, 3(22), 1–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgments
We would like to thank Marta Bertini, Chiara Bresciani, Zoë Goldsborough, and Tenzin Tsomo for their assistance in collecting data for this study. We would also like to thank Han de Vries for his statistical advice at an earlier stage of this project. Further gratitude goes to the Royal Burgers’ Zoo for facilitating this work and especially the chimpanzee caretakers for their dedication to the animals and assistance throughout the observations. Above all, thank you to the chimpanzees—Gaby, Geisha, Ghineau, Giambo, Erika, Fons, Jimmie, Jing, Mama, Marlene, Moni, Moniek, Morami, Roosje, Raimee, Tesua, and Tushi.
Data Availability
Data are available through the public repository GitHub at: https://github.com/cewebb/jealousy.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
N/A.
Informed Consent
N/A.
Consent to Publish
All listed authors consent to their names on the manuscript.
Additional information
Handling Editor: Karen Bales
Electronic Supplementary Material
ESM 1
(DOCX 298 kb)
Rights and permissions
About this article
Cite this article
Webb, C.E., Kolff, K., Du, X. et al. Jealous Behavior in Chimpanzees Elicited by Social Intruders. Affec Sci 1, 199–207 (2020). https://doi.org/10.1007/s42761-020-00019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42761-020-00019-5