Abstract
Melatonin (MT) and salicylic acid (SA) have important roles in coping with various abiotic stresses; however, their combined treatment effect under these stressful conditions is unknown. The current study, as a first investigation, was aimed to investigate the effect of their exogenous applications on the photosynthetic machinery in plants grown in salty soils. A pot experiment was conducted to investigate the potential effects of 70 μM MT and/or 75 mg l−1 SA applied to wheat (Triticum aestivum L. cv. Sids 14) plants grown under non-saline or saline conditions (6.0 and 12.0 dS m–1). The experimental layout was completely randomized design with four replications. Exogenously applied MT and/or SA significantly alleviated salt-induced decrease in wheat growth and productivity, and this was closely associated with the improvement of the photosynthetic pigments content, photochemical reactions of photosynthesis, net photosynthetic rate, transpiration rate, stomatal conductance, maximum quantum efficiency of PSII photochemistry, actual photochemical efficiency of PSII, electron transport rate, photochemical quenching coefficient, effective quantum yield of PSII photochemistry, photosynthetic enzymes activity, NADPH content, osmoprotectants accumulation, and grains carbohydrate content along with the reduction of the intercellular CO2 concentration, non-photochemical quenching coefficients, glycolate oxidase activity, and lipid peroxidation, as well as NADP+ and H2O2 content. Notably, the best response was registered with MT and SA combined treatment. Therefore, the co-application of melatonin and salicylic acid can be a better strategy for ameliorating salt toxicity in sustainable agricultural systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil salinity is one of the most important environmental hazards that inhibits plant growth and development, causing significant yield losses (Negrao et al. 2017). It severely affects 50% of irrigated land; moreover, 1.5 million hectares are removed from production each year as a result of high salinity levels in the soil (Yang and Guo 2018). It can adversely affect plant physiology by damaging cellular organelles, inhibiting protein synthesis and enzyme activities, uncoupling photosynthesis and respiration, altering nutrient uptake and/or transport, and reducing soil osmotic potential, as well as hindering water uptake by roots (Talaat 2019a; Hasanuzzaman et al. 2020). Photosynthesis is the most important physico-chemical process accountable for the energy production in higher plants (Talaat 2013; 2020). It is very sensitive to saline conditions (Talaat 2019b). Salinity-induced inhibition of the photosynthetic electron transport results in excessive accumulation of toxic reactive oxygen species (ROS), which promote degradation of chlorophyll, reduce photochemical efficiency of photosystem II, decline gas exchange and chlorophyll fluorescence, destruct photosynthetic organs, and cause lipid peroxidation in cellular membranes, as well as impair photosynthetic enzymes activity (Rady et al. 2019; Shen et al. 2019; Siddiqui et al. 2019; Shu et al. 2020). Reduced CO2 availability under saline conditions alters the ratios of ATP/ADP and NADPH/NADP+ (i.e., electron and proton acceptors), causing over-reduction of photosystems and producing ROS which damages photosynthetic machinery (Asrar et al. 2017; Talaat 2019c).
Melatonin (MT, N-acetyl-5-methoxytryptamine) is a natural biostimulating molecule, playing a vital role in coping with various environmental stresses. It can act as a potent-free radical scavenger and as a plant growth regulator (Nawaz et al. 2016; Khan et al. 2020; Jahan et al. 2021). Exogenous MT can be absorbed by different plant organs and plays the same function with endogenous MT (Nawaz et al. 2016). Exogenously MT treatment enhanced salt stress tolerance in few species such as watermelon (Li et al. 2017), wheat (Ke et al. 2018), rapeseed (Liu et al. 2018), melon (Castañares and Bouzo 2019), naked oat (Gao et al. 2019), tomato (Siddiqui et al. 2019), and strawberry (Zahedi et al. 2020) by regulating enzymes activity, ROS production, and stress-responsive genes expression. Moreover, MT ameliorates the photosynthetic activity by up-regulating the expression of photosystem I and photosystem II genes, facilitating the photosystem II repair, and decreasing the chlorophyll’s degradation, therefore enhancing salt stress tolerance (Shafi et al. 2021). However, the potential mechanisms of MT-mediated salinity tolerance are still largely unknown.
Salicylic acid (SA, 2-hydroxybenzoic acid) acts as a secondary messenger that increases plant tolerant to environmental stresses by inducing the expression of genes that encode defense-related compounds like jasmonic acid and proline (Wani et al. 2017). It also serves as a biostimulator that activates the biochemical pathways associated with salt tolerance mechanisms in plants (Fariduddin et al. 2018). Several studies have shown that the application of SA improved plant salt tolerance by acting as chemical messengers in numerous metabolic processes such as mineral nutrition uptake, antioxidant activity, methylglyoxal detoxification, photosynthesis, respiration, cellular signaling, senescence, and overall cellular redox homeostasis (Bukhat et al. 2020; Hoang et al. 2020; Kaya et al. 2020; Es-sbihi et al. 2021; Shamili et al. 2021). However, its possible roles in plants grown under salty soils are not fully understood.
Wheat is one of the major cereal crops worldwide. It feeds greater portion of the world population depends on its vital nourishment. Saline conditions restrict its productivity and therefore affect food security around the globe (Talaat 2019c). Improving its adaptation under this harsh environmental state is considered as the most effective economical approach. Some reports studied the impact of MT or SA on improving plant salt tolerance; however, the combined effects of MT and SA under stressful conditions remain unexplored. Therefore, this could be the first investigation conducting with an objective to determine whether MT and/or SA foliar applications could suppress salt-induced growth inhibition through mediating photosynthetic process in wheat (Triticum aestivum L.). It was hypothesized that these applications may alleviate salt-induced severe harm to plant photosynthetic system and thus may enhance plant growth and productivity under salty soils. To verify this hypothesis, the response of photosynthetic capacity including the photosynthetic pigments concentration, photochemical reactions activity, gas exchange parameters, chlorophyll fluorescence system, Rubisco, Rubisco activase, carbonic anhydrase and glycolate oxidase activities, osmoprotectants accumulation, grains carbohydrate content, and lipid peroxidation, as well as NADP+, NADPH, and H2O2 content was investigated under the impact of MT and/or SA exogenous applications in wheat plants grown under both non-saline and saline conditions. Indeed, this study provides a novel physiological basis that contributes to expand the understanding of the vital impact of MT and SA on plant salt tolerance.
2 Materials and Methods
2.1 Plant Material and Growth Condition
Grains of wheat (Triticum aestivum L. cv. Sids 14) were provided by the Wheat Research Department, Agriculture Research Center, Ministry of Agriculture, Egypt. Sids 14 cultivar was selected based on its high yield productivity, and I tried to increase its salt tolerance by using melatonin and/or salicylic acid foliar applications. Grains were sown in plastic pots (30 cm × 35 cm) filled with 15 kg clay loamy soil (sand 37%, silt 28%, clay 35%). Pots were fertilized with ammonium nitrate (33.5% N), calcium superphosphate (15.5% P2O5), and potassium sulfate (48% K2O) at the rate of 2.0, 2.0, and 0.5 g pot−1, respectively. In addition, when wheat plants were at 30 days after planting, the remaining ammonium nitrate was added at the rate of 2.0 g pot−1. Soil chemical analysis is carried out following the procedures of Cottenie et al. (1982) and presented in Table 1. Pots were placed under natural light in the greenhouse of the Department of Plant Physiology, Faculty of Agriculture, Cairo University, Egypt, where the relative humidity was 65%, and the temperature was 22/16 ± 2 °C (day/night). The experiment was repeated twice, in September 10 of 2019 and 2020, with consistent results.
The experimental layout was completely randomized design with two factors: three levels of salinity [0.1 dS m–1 (non-saline), 6.0 and 12.0 dS m–1] and four spraying treatments [0.00 (distilled water; DW), 70 μM MT, 75 mg l−1 SA, and 70 μM MT + 75 mg l−1 SA]. Each treatment had four replicates.
2.2 Salt Stress Treatments
Three levels of salinity [0.1 dS m–1 (non-saline), 6.0 and 12.0 dS m–1] were used; saline conditions were obtained by adding to the soil a mixture of NaCl, CaCl2, and MgSO4 at the molar ratio of 2:2:1, respectively.
2.3 Melatonin (MT) and Salicylic Acid (SA) Treatments
The concentrations of 70 μM MT and 75 mg l−1 SA were the most effective concentrations according to preliminary experiments within a range of concentrations from 0 to 100 μM for MT and from 0 to 100 mg l−1 for SA.
Plants at 45 and 90 days old from each salt stress treatment were sprayed with 0.00 (distilled water; DW), 70 μM MT, 75 mg l−1 SA, and 70 μM MT + 75 mg l−1 SA. Melatonin and salicylic acid were purchased from Sigma (USA) and were dissolved in sufficient quantity of ethanol. Tween-20 (0.05%) was added as surfactant at the time of treatment.
2.4 Plant Growth Measurements
For analyzing the plant growth, sampling was done at 70 days old after 25 days of MT and/or SA first applications. Shoot height, root length, number of leaves plant−1, and total leaf area plant−1 (portable leaf area meter; Model LI-COR 3000, Lambda Instruments Corporation, Lincoln, NE, USA) were measured. For dry weight, plants with intact roots were dug out from the soil and washed to remove the soil particles. Plant parts (shoot and root) were separated and dried in oven (70 °C) until constant weight achieved. Dry weight was taken in g plant−1. Each treatment includes four replicates, and each replicate includes six plants gathered from the same pot.
2.5 Plant Productivity Analysis
At maturity, number of grains plant−1 and grain yield plant−1 were recorded.
2.6 Biochemical Analysis
The plants were sampled at 70 days old after 25 days of MT and/or SA first foliar applications to assess the following biochemical parameters. At maturity, seeds were collected, and extraction and determination of total carbohydrate content in wheat grains were carried out.
2.6.1 Photosynthetic Pigments Measurement
Photosynthetic pigments from fresh leaves were extracted in 80% (v/v) acetone and the concentrations of chlorophyll a, chlorophyll b, and carotenoids were determined spectrophotometrically according to the method of (Lichtenthaler and Buschmann 2001), using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan).
2.6.2 Chloroplast Isolation and Measurement of Photosynthetic Photochemical Reactions Activity
The chloroplast isolation was performed by the method described by Cerovic and Plesnicar (1984). PSII-mediated electron transport from H2O to p-benzoquinone was determined following the method of Tiwari et al. (1998). PSI-mediated electron transport was measured in terms of oxygen consumption using 2, 6-dichlorophenol indophenols as electron donor and methyl viologen as final acceptor (Allen and Holmes 1986).
2.6.3 Gas Exchange Measurement
The gas exchange of attached leaves was measured at 8:30–11:30 am using an infrared gas analyzer, Li-Cor-6400 (Li-Cor Inc., Lincoln, NE, USA). The photosynthetic photon flux density (PPFD) was set at 1000 µmol m−2 s−1. Air temperature, air relative humidity, and CO2 concentration were set at ambient conditions in the greenhouse. Net photosynthetic rate (Pn, μmol CO2 m−2 s−1), stomatal conductance (Gs, mol H2O m−2 s−1), transpiration rate (Tr, mmol H2O m−2 s−1), and intercellular CO2 concentration (Ci, μmol CO2 mol air−) were recorded simultaneously.
2.6.4 Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence parameters were measured in leaves with a portable chlorophyll fluorometer (PAM2500; Heinz Walz, Effeltrich, Germany) after a 30-min dark adaptation. Chlorophyll fluorescence in dark- and light-adapted leaves was excited and measured. The maximum quantum efficiency of PSII photochemistry (Fv/Fm), electron transport rate (ETR), actual photochemical efficiency of PSII (ΦPSII), photochemical quenching coefficient (qP), effective quantum yield of PSII photochemistry (Fv′/Fm'), and non-photochemical quenching coefficients (qN) were calculated according to Pfündel et al. (2008).
2.6.5 Assay of the Activity of Enzymes Involved in Photosynthetic Process
Carbonic anhydrase (CA; EC 4.2.1.1) activity was assayed using the method of Dwivedi and Randhawa (1974). To fresh leaf tissues, cystein hydrochloride solution was added, and samples were incubated at 4 °C for 20 min. Then, phosphate buffer (pH 6.8), alkaline bicarbonate solution, and bromothymol blue were added to the leaf tissues, and they were incubated at 5 °C for 20 min. Finally, titration was done against 0.05 N HCl.
Glycolate oxidase (GO; EC 1.1.3.15) activity was determined by following glyoxylate phenylhydrazone formation at 324 nm for 3 min after an initial lag phase of 1 min as described by Blasco et al. (2010). For this, fresh leaf tissues were extracted by PVPP, and 1 mL of 50 mM Tris–HCl buffer (pH 7.8) with 0.01% Triton X-100 and 5 mM dithiotreithol (DTT) were added and then centrifuged at 30,000 g for 20 min. The GO assay was performed in a reaction medium containing 50 μL plant extract, 50 mM Tris–HCl buffer (pH 7.8), 3.3 mM phenylhydrazine HCl (pH 6.8), 0.009% Triton X-100, and 5 mM glycolic acid. Protein concentration was quantified according to Lowry et al. (1951).
Estimation of Rubisco activity was done as described by Jiang et al. (2012). The Rubisco activation state (%) was calculated using the ratio initial activity/total activity. The RCA activity was estimated using a Rubisco Activase Assay Kit (Genmed Scientifics Inc., Wilmington, DE, USA).
2.6.6 Determination of Compatible Solutes Accumulation
Total soluble sugars and glycinebetaine concentration were determined in dried ground leaves by the anthrone reagents method (Irigoyen et al. 1992) and the method of Grieve and Grattan (1983), respectively. Proline was determined in fresh leaf samples according to Bates et al. (1973).
2.6.7 Estimation of Grains Carbohydrate Content
Extraction and determination of total carbohydrate content in dried ground wheat grains were carried out according to Yih and Clark (1965) and Dubois et al. (1956). Dried ground seeds was extracted with 1.5 N H2SO4 and then centrifuged at 4000 g for 10 min. For 1 mL of the extract, 1 mL of 5% distilled phenol was added, and absorbance was taken at 490 nm using spectrophotometer.
2.6.8 Measurement of Hydrogen Peroxide (H2 O 2 ), Malondialdehyde (MDA), Nicotinamide Adenine Dinucleotide Phosphate Oxidized (NADP +), and Nicotinamide Adenine Dinucleotide Phosphate Reduced (NADPH) Contents
For the estimation of H2O2 and MDA, 0.1 g of fresh wheat leaves was ground in a mortar with 900 µL buffer, following the instructions described in H2O2 and MDA kits, according to the method described by Nawaz et al. (2018). H2O2 and MDA contents were recorded at a wavelength of 405 nm and 532 nm, respectively. The NADP+, NADPH contents in wheat leaves were measured according to the instructions of the kit using a spectrophotometric assay (Liu et al. 2019).
2.7 Statistical Analysis
A completely randomized design was used with four replicates per treatment. Combined analysis was made for the two growing seasons, since the results of the two seasons followed a similar trend. All measured parameters were statistically analyzed by the two-way ANOVA test, where the first factor was the salt treatments, and the second was the foliar application treatments. Differences between the treatments were tested by the least significant difference (LSD) test at a level of significance p < 0.05. The data are presented as means ± standard error (SE). The SAS software (SAS Inc., Cary, NC, USA) was used for the statistical analysis.
3 Results
3.1 Exogenously Applied MT and/or SA Alleviate Salt-Induced Decreases in Plant Growth and Productivity
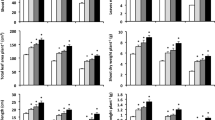
Salt stress had strong inhibitory effects on overall plant growth and production, leading to significant (p < 0.05) decreases in values of shoot height, leaves number plant−1, total leaf area plant−1, dry weight of shoot plant−1, root length, dry weight of root plant−1, number of grains plant−1, and grain yield plant−1 (Table S). On the contrary, foliar applications of MT and/or SA significantly alleviated those declines when compared with salt-stressed plants that had not received any supplementations (Fig. 1a, b, c, d, e, and f as well as Fig. 2a and b). The highest protective effect was achieved by MT and SA combined treatment. It significantly improved the shoot height by 37.7%, 46.9%, and 65.8%; leaves number plant−1 by 38.6%, 45.0%, and 75.0%; total leaf area plant−1 by 48.8%, 61.2%, and 80.0%; shoot dry weight plant−1 by 53.4%, 73.3%, and 107.7%; root length by 40.5%, 53.9%, and 88.9%; root dry weight plant−1 by 64.8%, 80.3%, and 115.0%; number of grains plant−1 by 51.2%, 75.4%, and 110.9%; and that of grain yield plant–1 by 57.2%, 83.8%, and 130.8% at 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively, when compared with untreated plants.
Variations in the (a) shoot height (cm), (b) leaves number plant−1, (c) total leaf area plant−1 (cm2), (d) shoot dry weight plant−1 (g), (e) root length (cm), and (f) root dry weight plant−1 (g) of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
Variations in the (a) grains number plant−1 and (b) grain yield plant−1 (g) of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.2 Foliar Application of MT and/or SA Alleviate the Inhibition of Photosynthetic Pigments Content in Salt-Stressed Plants
In response to salt stress, the photosynthetic pigments content in wheat leaves showed considerable variations in comparison with those of the unstressed plants. Generally, chlorophyll a, chlorophyll b, carotenoids, and total pigments concentration were significantly (p < 0.05) decreased with increasing saline treatments; however, these decreases were alleviated by foliar application of MT and/or SA (Fig. 3a, b, c, and d). The best response was registered with MT and SA combined treatment. It significantly elevated the content of chlorophyll a by 20.3%, 31.0%, and 60.0%; that of chlorophyll b by 23.8%, 55.2%, and 104.9%; that of carotenoids by 29.0%, 61.9%, and 123.1%; and that of total pigments by 22.4%, 41.9%, and 79.3% at 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively, compared with control treatment.
Variations in the concentrations of (a) chlorophyll a, (b) chlorophyll b, (c) carotenoids, and (d) total pigments (mg g−1 FW) in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.3 Spraying of MT and/or SA Mitigate Salinity-Induced Reduction in PSI and PSII Activities
The activities of PSI and PSII were significantly (p < 0.05) decreased with increasing saline treatments. Spraying of MT and/or SA sharply increased their activities in comparison with that of the un-sprayed plants (Fig. 4a and b). The maximum activity was detected by MT and SA co-application. It significantly enhanced the PSI activity by 23.2%, 32.3%, and 73.9% and that of PSII activity by 26.9%, 76.0%, and 177.8% under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively, compared with control treatment.
Variations in the (a) PSI and (b) PSII electron transport activities (µmol O2 mg−1 Chl h−1) in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.4 Exogenous Treatments of MT and/or SA Improve Gas Exchange Traits in Salt-Stressed Plants
Salt stress treatments resulted in significant (p < 0.05) decreases in gas exchange attributes (net photosynthetic rate, transpiration rate, and stomatal conductance), while it increased the intercellular CO2 concentration. Interestingly, MT and/or SA exogenous applications significantly ameliorated salt stress-induced reduction in leaf Pn, Gs, and Tr, whereas MT and SA co-application appeared to be the most effective treatment in alleviating salt injures and almost neutralized the toxicity effects (Table 2). It significantly enhanced the net photosynthetic rate by 31.9%, 50.0%, and 104.4%; that of stomatal conductance by 49.5%, 69.7%, and 175.5%; and that of the transpiration rate by 38.9%, 51.4%, and 122.0% compared to values of control plants under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively. On the other hand, combined treatment significantly decreased the intercellular CO2 concentration value by 20.7%, 27.2%, and 34.1% under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively, in comparison to control treatment.
3.5 Exogenously Applied MT and/or SA Meliorate Chlorophyll Fluorescence Attributes Under Salt Stress
Soil salinization significantly (p < 0.05) impaired the maximum quantum efficiency of PSII photochemistry, the actual photochemical efficiency of PSII, the effective quantum yield of PSII photochemistry, the electron transport rate, and the photochemical quenching coefficient values of salt-stressed plants compared to that of unstressed ones. Salt stress-induced decreases in these attributes were significantly alleviated by MT and/or SA applications (Table 3). The non-photochemical quenching coefficients value was significantly (p < 0.05) induced by salt stress, while this induction was attenuated by the exogenous treatments (Table 3). Co-application of MT and SA was more effective in ameliorating the deleterious effects of salt stress, particularly at high saline condition. It significantly provoked positive effects and improved the maximum quantum efficiency of PSII photochemistry by 22.6%, 45.1%, and 137.8%; that of the effective quantum yield of PSII photochemistry by 21.3%, 43.8%, and 145.8%; that of the actual photochemical efficiency of PSII by 18.8%, 51.5%, and 175.0%; that of the electron transport rate by 16.9%, 43.2%, and 132.7%; and that of the photochemical quenching coefficient by 18.3%, 36.7%, and 87.1% compared to values of control plants under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively.
3.6 Foliar Application of MT and/or SA Ameliorate the Activity of Enzymes Involved in Photosynthetic Process Under Saline Conditions
Salt stress significantly (p < 0.05) decreased the initial and total Rubisco activity, Rubisco activation state, and RCA activity in wheat leaves, while exogenous treatments of MT and/or SA significantly improved their activities under saline conditions (Fig. 5a, b, c, and d). The greatest enzymes activity was obtained by MT and SA combined treatment. It significantly elevated the initial Rubisco activity by 37.5% and 128.4%; that of total Rubisco activity by 23.1% and 77.4%; that of Rubisco activation state by 11.5% and 28.7%; and that of RCA activity by 40.0% and 105.0% under 6.0 and 12.0 dS m–1 salinity levels, respectively, compared with control treatment.
Variations in the (a) initial Rubisco activity (μmol m−2 s−1), (b) total Rubisco activity (μmol m−2 s−1), (c) Rubisco activation state (%), and (d) Rubisco activase activity (μmol ECM min−1) in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
Salt-induced reduction in carbonic anhydrase activity was significantly (p < 0.05) alleviated by exogenously applied MT and/or SA. Highest value for CAase activity was recorded in plants supplemented with MT and SA combined treatment (Fig. 6a). It significantly enhanced its activity by 25.0%, 45.5%, and 85.7% under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively, compared with control treatment. Glycolate oxidase activity increased significantly in wheat leaves with increasing salts concentration (Fig. 6b). However, combined application decreased its value by 10.0%, 16.7%, and 35.3% compared to value of control plants under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively.
Variations in the activities of (a) carbonic anhydrase (CA, mol CO2 kg−1 leaf FM s−1) and (b) glycolate oxidase (GO, nmol min−1 mg−1 protein) in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.7 Exogenous MT and/or SA Applications Enhance Osmoprotectants Accumulation in Salt-Stressed Plants
Total soluble sugars, proline, and glycinebetaine concentrations were increased under salt stress and increased further upon exposing plants to MT and/or SA applications (Fig. 7a, b and c). The MT and SA combined treatment resulted in the highest values for all of them. It significantly enhanced the total soluble sugars level by 41.9% and 69.7%; that of proline by 51.4% and 80.9%; and that of glycinebetaine by 59.2% and 92.1% under 6.0 and 12.0 dS m–1 salinity levels, respectively, compared with control treatment.
Variations in the concentrations of (a) total soluble sugars (mg glucose g−1 DW), (b) proline (mg g−1 FW), and (c) glycinebetaine (µmol g−1 DW) in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.8 Spraying of MT and/or SA Meliorate the Grains Carbohydrate Content Under Salinity
Salt stress treatments caused a significant (p < 0.05) decrease in grains carbohydrate content. Exogenous application of MT and/or SA alleviated the deleterious impact of salinity and enhanced its content. Higher carbohydrate accumulation was observed in plants treated with the combined treatment (Fig. 8). It significantly improved its content by 15.4%, 40.7%, and 82.2% compared to value of control plants under 0.1, 6.0, and 12.0 dS m–1 salinity levels, respectively.
Variations in the grains carbohydrate content (mg g−1 DW) of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.9 Exogenous Application of MT and/or SA Relive Salt-Induced Stress by Reducing MDA, H 2 O 2 , and NADP + Production
Soil salinization significantly (p < 0.05) increased MDA, H2O2, and NADP+ values in wheat leaves compared with that of unstressed ones. On the contrary, MT and/or SA foliar applications significantly promoted the recovery of the salt-stressed plants, and the MT + SA treatment is the most effective in mitigating salt-related toxicity symptoms (Fig. 9a, b and c). It significantly reduced the H2O2 content by 21.7% and 33.5%; that of MDA content by 21.0% and 31.5%; and that of NADP+ content by 20.3% and 32.3% compared to values of control plants at 6.0 and 12.0 dS m−1 salinity levels, respectively. Furthermore, salt stress was associated with a significant reduction in NADPH content. Interestingly, stressed MT- and/or SA-treated plants showed higher NADPH content when compared with untreated stressed ones (Fig. 9d). Co-application of MT and SA yielded the best response and significantly elevated NADPH content by 50.0% and 132.0% at 6.0 and 12.0 dS m–1 salinity levels, respectively, compared with control treatment.
Variations in the contents (a) H2O2, (b) lipid peroxidation, (c) NADP+, and (d) NADPH in leaves of wheat plants grown under 0.1, 6, and 12 dS m−1 salinity levels and exposure to foliar applications of salicylic acid (SA, 75 mg l−1) and/or melatonin (MT, 70 μM). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
4 Discussion
Salinity is considered as a major environmental stress that seriously affecting the growth and development of plants via interrupting a series of physiological, biochemical, and molecular mechanisms (Talaat 2019c; Hasanuzzaman et al. 2020). Photosynthesis is the most severely physiological process that is affected by saline conditions. Using plant growth regulators with natural antioxidant property under this harsh environmental condition is becoming of greater interest. Melatonin and salicylic acid can serve as plant master regulators and/or potent antioxidants that improve plant tolerance to both abiotic and biotic stresses (Wani et al. 2017; Bukhat et al. 2020; Hoang et al. 2020; Khan et al. 2020). Due to their special properties, they may boost the plant physiological activity against saline conditions. Indeed, this study provides a novel physiological basis that contributes to expand the understanding of the vital impact of MT and SA on plant salt tolerance.
Exposure to saline conditions triggered biochemical and physiological changes in wheat plants and significantly (p < 0.05) reduced their growth and productivity. In contrast, exogenous MT and/or SA applications alleviated the deleterious effects of saline conditions on wheat production, which is consistent with the findings of Fariduddin et al. (2018), Liu et al. (2018), Gao et al. (2019), Siddiqui et al. (2019), Bukhat et al. (2020), and Kaya et al. (2020). Indeed, foliar-applied MT and/or SA increased wheat salt tolerance which led to a better performance of the photosynthetic apparatus via alleviating photosynthetic inhibition and improving the photosynthetic carbon assimilation as well as via alleviating oxidative damage induced by salinity. These findings reveal that MT and/or SA can act as active growth regulators involving in wheat salt tolerance.
Chlorophyll is the most important pigment for capturing light energy, and its reduction severely affected photosynthetic process (Talaat 2019b). In the present study, exogenous supplementation with MT and/or SA significantly (p < 0.05) inhibited chlorophyll degradation resulted from salt stress (Fig. 3a and b) implying that these applications may be able to repair chlorophyll destruction by reducing salt-induced oxidative damage which was facilitated by lower H2O2 formation (Fig. 9a). This result is in agreement with those of Fariduddin et al. (2018), Ke et al. (2018), Castañares and Bouzo (2019), Gao et al. (2019), Siddiqui et al. (2019), Bukhat et al. (2020), and Kaya et al. (2020). Exogenous MT and/or SA applications significantly mitigate such damage and protect chlorophyll from degradation by regulating antioxidant molecules synthesis, suppressing genes involved in the senescence process, up-regulating chlorophyll biosynthesis genes, and/or down-regulating chlorophyll degradation genes (Nawaz et al. 2018; Gao et al. 2019; Bukhat et al. 2020; Altaf et al. 2021). Surprisingly, foliar applications of MT and/or SA significantly (p < 0.05) neutralized the salt injuries and maintained high concentration of carotenoids in salt-stressed plants (Fig. 3c). Similar observation was also reported by Arnao and Hernández-Ruiz (2019), Bukhat et al. (2020), and Faraz et al. (2020), who said that MT and SA can regulate steps in carotenoid biosynthesis and/or influence cellular signaling and trigger redox-sensitive regulatory pathways. It is worth to mention that the growth amelioration caused by MT and/or SA treatments might be due to the up-regulation of photosynthetic pigments content.
Salt treatments significantly (p < 0.05) impaired the photochemical reactions of photosynthesis (Fig. 4a and b), and this inhibition results in imbalance between the generation and utilization of electrons. These electrons transport to molecular oxygen, thus generating different types of ROS (Hasanuzzaman et al. 2020). On the contrary, MT treatment maintained better PSI and PSII activities in salt-stressed plants, and this could be achieved by (a) up-regulating the expression of genes in both PSI and PSII (Wei et al. 2015), (b) facilitating the repair of PSII via maintaining the availability of D1 protein (Zhou et al. 2016), and (c) increasing the accumulation of organic osmolytes such as proline and glycinebetaine (Fig. 7b and c) that correlated strongly with the activity of PSII. Similarly, the protective role of foliar SA application was also observed on PSI and PSII activities, which could be induced by increasing ΦPSII that protect the function of PSII (Khalid et al. 2015). Hence, it appears that MT and/or SA applications alleviate salt-induced growth reduction by maintaining PSI and PSII functions.
Stressed plants close their stomata to reduce water loss that block CO2 diffusion into the chloroplasts, thereby decreasing the stomatal conductance and limiting the photosynthetic rate (Talaat 2019b). Data in Table 2 show that MT and/or SA treatments significantly alleviated the disturbance in the gas exchange attributes, thus reversing the adverse effect of salt stress, which is supported by the results of Fariduddin et al. (2018), Bukhat et al. (2020), and Faraz et al. (2020). The follow-up treatment with MT can alleviate the inhibition in gas exchange parameters by enhancing PSII efficiency and thus maintaining a higher capacity for CO2 assimilation (Wang et al. 2013), improving the stomata function via enabling plants to reopen their stomata (Ye et al. 2016), controlling the stomatal closure through the CAND2/PMTR1-mediated signaling pathway (Wei et al. 2018), regulating the excitation energy dissipation via changing the level of PSII proteins (Chen et al. 2018), and acting as an enhancer or protector of photosynthesis (Arnao and Hernández-Ruiz 2019). Similarly, SA treatment can mitigate the inhibitory effect of stressful conditions on gas exchange attributes by regulating certain metabolic factors associated with carbon uptake and/or fixation in the chloroplast (Nazar et al. 2015), stimulating the stomatal activity (Faraz et al. 2020), and enhancing the PSII functional activity (Bukhat et al. 2020). Furthermore, one of the major injuries due to salinity stress is the decreases in chlorophyll fluorescence attributes in salt-stressed wheat plants (Table 3) that may be attributed to severe damage in the PSII reaction center. However, these decreases were significantly improved by MT and/or SA treatments through protecting PSII against over excitation and enhancing thylakoid membranes flexibility (Arnao and Hernández-Ruiz 2019; Kaya et al. 2020).
Salt-stressed wheat plants exhibited less activity of Rubisco (Fig. 5a, b, c, and d) and CA (Fig. 6a) enzymes than the unstressed ones. This may be due to the destabilization in the folding configuration of many native proteins, causing an inhibition in enzymes activity (Talaat 2019a; Hasanuzzaman et al. 2020). However, MT and/or SA foliar applications significantly (p < 0.05) enhanced their activities under saline conditions, as was also reported by Fariduddin et al. (2018) and Siddiqui et al. (2019). Indeed, both CA and Rubisco enzymes are involved in carbon fixation during photosynthesis. Therefore, enhancing their activities by MT and/or SA might be responsible for maintaining a constant supply of CO2 to wheat leaves and consequently improving the photosynthetic rate. It is worth noting that the enhancement in CA activity along with higher net photosynthetic rate, transpiration rate, and stomatal conductance values (Table 2) due to MT and/or SA treatments improves the CO2 availability for Rubisco and consequently facilitates the utilization of light energy for carbon assimilation that enhances the photosynthetic capacity under saline conditions. Furthermore, as shown in Fig. 6b, salt stress induces a significant increase in GO activity, but this response is significantly suppressed by MT and/or SA applications that may be due to their possible involvement in the enhancement of stomatal conductance (Table 2).
Osmoprotectants played crucial roles in plant salt tolerance. Total soluble sugars, proline, and glycinebetaine concentrations were higher in MT- and/or SA-treated plants compared to that in untreated ones (Fig. 7a, b, and c), which contributed in enhancing cell membranes stability, alleviating oxidative damage, and protecting photosynthetic system from damage. In this concern, Szabados and Savouré (2010) and Bukhat et al. (2020) pointed out that protecting PSII activity and stabilizing cell membrane can be linked to osmoprotectants accumulation.
The grains carbohydrate content was found to be significantly improved by MT and/or SA foliar applications (Fig. 8) that could be due to the role played by MT and SA on pigments content (Fig. 3a, b, c, and d), PSI and PSII performance (Fig. 4a and b), gas exchange (Table 2), chlorophyll fluorescence (Table 3), and Rubisco activity (Fig. 5a, b, c, and d). Therefore, it is worth noting that these exogenous treatments can induce photosynthetic efficiency and enhance sink strength and phloem uploading that stimulate assimilate flow from source to sink organs and resulted in higher grains carbohydrate content.
Soil salinization significantly (p < 0.05) increased H2O2 accumulation and subsequently enhanced lipid peroxidation in wheat leaves. On the contrary, MT and/or SA as potent-free radical scavengers and antioxidants significantly decreased ROS level and protected membrane lipids against free radical damage by decreasing MDA level (Fig. 9a and b), which is in strong agreement with the findings of Ke et al. (2018), Castañares and Bouzo (2019), and Bukhat et al. (2020). Additionally, the function of photosynthetic electron transport in the thylakoid membrane of chloroplasts is to generate NADPH from NADP+, and at the same time, to pump protons from stroma to lumen in order to form a proton gradient, driving ATP synthesis (Yamori and Shikanai 2016). Results in Fig. 9c and d reveal that NADP+ content in wheat leaves were significantly increased, while NADPH content was decreased in salt-stressed plants, indicating that the process of NADPH production in photosynthesis might be disturbed by salt stress. Exogenously applied MT and/or SA significantly increased NADPH content, whereas they decreased NADP+ content, indicating that these applications could alleviate negative impacts of salt stress on the process of NADPH production. It is interesting to mention that these applications may be able to repair the disrupted cellular membrane and reduce salt-induced oxidative damage and thus protect the photosynthetic apparatus from salt injuries.
On the basis of these results, it is apparent that salt stress severely affected the physiological and biochemical properties of wheat plant causing yield inhibition and photosynthetic machinery destruction. Interestingly, the exogenous applications of melatonin and/or salicylic acid alleviated these adverse impacts of saline conditions. Obviously, this mitigation effect is closely related to the maintenance of cell structural integrity, the activation of photosynthetic enzymes, the stabilization of osmotic regulation, and the maintenance of normal photosynthetic efficiency under salt stress as melatonin and/or salicylic acid treatments capable to prevent the destructive effect of ROS. Additionally, although melatonin or salicylic acid alone was able to ameliorate salt-induced toxicity in wheat plants, their co-application was the most effective treatment in nullifying salt-induced damage; perhaps, it could be due to their synergistic or additive effects. In this concern, Albacete (2020) reported that both melatonin and salicylic acid play multiple functions in plant physiological processes under biotic and abiotic stresses as these regulatory molecules share a common biosynthetic precursor and have similar physiological actions and stress regulation signals.
5 Conclusions
Results in this study demonstrate that co-application of melatonin and salicylic acid restored tolerance against salt stress by recovering the photosynthetic machinery. Advantageously, this photosynthetic acclimation strategy maximized the carbon assimilation rate and allowed wheat plants to withstand saline conditions. These applications help to maintain a better performance of the photosynthesis process, with optimal growth rates under saline conditions. This positive impact of this combined treatment offers new opportunity in agriculture field by enhancing plant tolerance to different environmental challenges. Therefore, it could be of great importance to utilize this combined treatment as a biostimulator for sustainable crop production without affecting the external environment.
References
Albacete A (2020) Get together: the interaction between melatonin and salicylic acid as a strategy to improve plant stress tolerance. Agronomy 10:1486. https://doi.org/10.3390/agronomy10101486
Allen JF, Holmes NG (1986) Electron transport and redox titrations. In: Hipkins MF, Baker NR (eds) Photosynthesis — Energy Transduction — a Practical Approach. IRL Press, Oxford, pp 103–141
Altaf MA, Shahid R, Ren M, Altaf MM, Khan LU, Shahid S, Jahan MS (2021) Melatonin alleviates salt damage in tomato seedling: a root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci Hortic 285:110145. https://doi.org/10.1016/j.scienta.2021.110145
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48. https://doi.org/10.1016/j.tplants.2018.10.010
Asrar H, Hussain T, Hadi SMS, Gul B, Nielsen BL, Khan MA (2017) Salinity induced changes in light harvesting and carbon assimilating complexes of Desmostachya bipinnata (L.) Staph. Environ Exp Bot 135:86–95. https://doi.org/10.1016/j.envexpbot.2016.12.008
Bates LS, Aldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Blasco B, Ríos JJ, Cervilla LM, Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Rosales MA, Ruiz JM, Romero L (2010) Photorespiration process and nitrogen metabolism in lettuce plants (Lactuca sativa L.): induced changes in response to iodine biofortification. J Plant Growth Regul 29:477–486. https://doi.org/10.1007/s00344-010-9159-7
Bukhat S, Manzoor H, Athar H, Zafar ZU, Azeem F, Rasul S (2020) Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J Plant Growth Regul 39:809–822. https://doi.org/10.1007/s00344-019-10024-z
Castañares JL, Bouzo CA (2019) Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic Plant J 5:79–87. https://doi.org/10.1016/j.hpj.2019.01.002
Cerovic ZG, Plesnicar M (1984) An improved procedure for the isolation of intact chloroplasts of high photosynthetic activity. Biochem J 223:543–545. https://doi.org/10.1042/bj2230543
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S, Yuan M (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 164:349–363. https://doi.org/10.1111/ppl.12737
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. In: Laboratory of analytical and agrochemistry. State University, Ghent, pp 14–24. https://lib.ugent.be/catalog/rug01:000239299
Dubois M, Gills K, Hamilton JK, Robers PA, Smith F (1956) Colorimeter method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Dwivedi RS, Randhawa NS (1974) Evaluation of a rapid test for hidden hunger of zinc in plants. Plant Soil 40:445–451. https://doi.org/10.1007/BF00011531
Es-sbihi FZ, Hazzoumi Z, Aasfar A, Joutei KA (2021) Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem Biol Technol Agric 8:25. https://doi.org/10.1186/s40538-021-00221-y
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 39:641–655. https://doi.org/10.1007/s00344-019-10007-0
Fariduddin Q, Khan TA, Yusuf M, Aafaqee ST, Khalil RRAE (2018) Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica 56:750–762. https://doi.org/10.1007/s11099-017-0727-y
Gao W, Feng Z, Bai Q, He J, Wang Y (2019) Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int J Mol Sci 20:1176. https://doi.org/10.3390/ijms20051176
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Hoang HL, de Guzman CC, Cadiz NM, Hoang TTH, Tran DH, Rehman H (2020) Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). J Soil Sci Plant Nutr 20:1759–1769. https://doi.org/10.1007/s42729-020-00248-4
Irigoyen JJ, Emerich DW, Sanchez-Dıaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugar in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Jahan MS, Shu S, Wang Y, Hasan MM, El-Yazied AA, Alabdallah NM, Hajjar D, Altaf MA, Sun J, Guo S (2021) Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA and GA-mediated pathways. Front Plant Sci 12:650955. https://doi.org/10.3389/fpls.2021.650955
Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ (2012) Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol 194:932–943. https://doi.org/10.1111/j.1469-8137.2012.04111.x
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol Biochem 147:10–20. https://doi.org/10.1016/j.plaphy.2019.11.040
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:914. https://doi.org/10.3389/fpls.2018.00914
Khalid A, Zafar ZU, Akram A, Hussain K, Manzoor H, Al- Qurainy F, Ashraf MA (2015) Photosynthetic capacity of canola (Brassicanapus L.) plants as affected by glycinebetaine under the salt stress. J Appl Bot Food Qual 88:78–86. https://doi.org/10.5073/JABFQ.2015.088.011
Khan A, Numan M, Khan AL, Lee I, Imran M, Asaf S, Al-Harrasi AA (2020) Melatonin: awakening the defense mechanisms during plant oxidative stress. Plants 9:407. https://doi.org/10.3390/plants9040407
Li H, Chang J, Chen H, Wang Z, Gu X, Wei C, Zhang Y, Ma J, Yang J, Zhang X (2017) Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci 8:295. https://doi.org/10.3389/fpls.2017.00295
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1 F4. 3.1-F4. 3.8. https://doi.org/10.1002/0471142913.faf0403s01
Liu CL, Dong HG, Zhan J, Liu X, Yang Y (2019) Multi-modular engineering for renewable production of isoprene via mevalonate pathway in Escherichia coli. J Appl Microbiol 126:1128–1139. https://doi.org/10.1111/jam.14204
Liu Z, Jun-song C, Jing-jing L, Guang-yuan L, Chun-sheng L, Gui-ping F, Xue-kun Z, Hai-qing M, Qing-yun L, Xi-ling Z, Yong C (2018) Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J Integr Agric 17:328–335. https://doi.org/10.1016/S2095-3119(17)61757-X
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, Hameed S (2016) Melatonin: current status and future perspectives in plant science. Front Plant Sci 6:1230. https://doi.org/10.3389/fpls.2015.01230
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtia M, Bie Z, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon on seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127. https://doi.org/10.1016/j.jplph.2017.11.003
Nazar R, Umar S, Khan NA, Sareer O (2015) Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South Afr J Bot 98:84–94. https://doi.org/10.1016/j.sajb.2015.02.005
Negrao S, Schmockel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Pfündel E, Klughammer C, Schreiber U (2008) Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl Notes 1:21–24
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky EM (2019) Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J Hortic Sci Biotech 94:777–789. https://doi.org/10.1080/14620316.2019.1626773
Shafi A, Singh AK, Zahoor I (2021) Melatonin: role in abiotic stress resistance and tolerance. In: Aftab T, Hakeem KR (eds) Plant growth regulators. Springer Cham, pp 239–273. https://doi.org/10.1007/978-3-030-61153-8_12
Shamili M, Ghalati RE, Samari F (2021) The impact of foliar salicylic acid in salt-exposed guava (Psidium Guajava L.) seedlings. Int J Fruit Sci 21:323–333. https://doi.org/10.1080/15538362.2021.1887050
Shen J, Wang Y, Shu S, Jahan MS, Zhong M, Wu J, Sun J, Guo S (2019) Exogenous putrescine regulates leaf starch overaccumulation in cucumber under salt stress. Sci Hortic 253:99–110. https://doi.org/10.1016/j.scienta.2019.04.010
Shu S, Tang Y, Zhou X, Jahan MS, Sun J, Wang Y, Guo S (2020) Physiological mechanism of transglutaminase-mediated improvement in salt tolerance of cucumber seedlings. Plant Physiol Biochem 156:333–344. https://doi.org/10.1016/j.plaphy.2020.09.010
Siddiqui MH, Alamri S, Al-Khaishany MY, Khan MN, Al-Amri A, Ali HM, Alaraidh IA, Alsahli AA (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci 20:353. https://doi.org/10.3390/ijms20020353
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Talaat NB (2013) RNAi based simultaneous silencing of all forms of light-dependent NADPH:protochlorophyllide oxidoreductase genes result in the accumulation of protochlorophyllide in tobacco (Nicotiana tabacum). Plant Physiol Biochem 71:31–36. https://doi.org/10.1016/j.plaphy.2013.06.025
Talaat NB (2019a) Role of reactive oxygen species signaling in plant growth and development. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. John Wiley & Sons, Ltd, UK, pp 225–266. https://doi.org/10.1002/9781119468677.ch10
Talaat NB (2019b) Effective Microorganisms: an innovative tool for inducing common bean (Phaseolus vulgaris L.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci Hortic 250:254–265. https://doi.org/10.1016/j.scienta.2019.02.052
Talaat NB (2019c) Abiotic stresses-induced physiological alteration in wheat. In: Hasanuzzaman M, Nahar K, Hossain A (eds) Wheat production in changing environments—responses, adaptation and tolerance. Springer, Singapore, pp 1–30. https://doi.org/10.1007/978-981-13-6883-7_1
Talaat NB (2020) 24-Epibrassinolide and spermine combined treatment sustains maize (Zea mays L.) drought-tolerance by improving photosynthetic efficiency and altering phytohormones profile. J Soil Sci Plant Nutr 20:516–529. https://doi.org/10.1007/s42729-019-00138-4
Tiwari BS, Bose A, Ghosh B (1998) Photosynthesis in rice under salt stress. Photosynthetica 34:303–306. https://doi.org/10.1023/A:1006857027398
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54:292–302. https://doi.org/10.1111/jpi.12017
Wani AB, Chadar H, Wani AH, Singh S, Upadhyay N (2017) Salicylic acid to decrease plant stress. Environ Chem Lett 15:101–123. https://doi.org/10.1007/s10311-016-0584-0
Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, Chen Q (2018) Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J Pineal Res 65:e12500. https://doi.org/10.1111/jpi.12500
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707. https://doi.org/10.1093/jxb/eru392
Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67:81–106. https://doi.org/10.1146/annurev-arplant-043015-112002
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217:523–539. https://doi.org/10.1111/nph.14920
Ye J, Wang S, Deng X, Yin L, Xiong B, Wang X (2016) Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol Plant 38:48. https://doi.org/10.1007/s11738-015-2045-y
Yih RY, Clark HE (1965) Carbohydrate and protein content of boron deficient tomato root tips in relation to anatomy and growth. Plant Physiol 40:312–315. https://doi.org/10.1104/pp.40.2.312
Zahedi SM, Hosseini MS, Abadia J, Marjani M (2020) Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol Biochem 149:313–323. https://doi.org/10.1016/j.plaphy.2020.02.021
Zhou X, Zhao H, Cao K, Hu L, Du T, Baluska F, Zou Z (2016) Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front Plant Sci 7:1823. https://doi.org/10.3389/fpls.2016.01823
Acknowledgements
This research was supported by the Academy of Scientific Research and Technology in Egypt and the Bulgaria-Egypt Joint Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Talaat, N.B. Co-application of Melatonin and Salicylic Acid Counteracts Salt Stress-Induced Damage in Wheat (Triticum aestivum L.) Photosynthetic Machinery. J Soil Sci Plant Nutr 21, 2893–2906 (2021). https://doi.org/10.1007/s42729-021-00576-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00576-z