Abstract
The aim of this study is to synthesize composite micro-nutrient nanoparticles (NPs) composed of four essential nutrient elements (Ni, Cu, Zn, and Fe) for plants as well as to investigate the incorporation of these micro-nutrients into the plant tissues. For these purposes, Ni0.4Cu0.2Zn0.4Nd0.05Y0.05Fe1.9O4 NPs were synthesized by sol-gel auto combustion method and characterized by using transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). The NPs were applied to barley (Hordeum vulgare L.) in varying concentrations (62.5, 125, 250, and 500 mg L−1) for 21 days. Hydroponically grown seedlings were harvested and the element content of the root and leaf parts was analyzed by using an inductively coupled plasma optical emission spectrometer (ICP-OES). Results showed that NPs have a spherical structure with an average crystallite size of 18 nm. Raising NP doses gradually increased the amount of the elements found in the composition of NPs (Ni, Cu, Zn, Nd, Y, and Fe) both in root and leaf tissues. In addition, compared with the untreated control, 500 mg L−1 of NP treatment increased the abundance of some macro-elements (K, Ca, Mg, and P) in roots, while the amount of these elements except for Ca significantly decreased in the leaves (p < 0.05). This study is the first to show that Zn, Ni, Cu, and Fe can be incorporated into the plant body by the inclusion of NPs. This finding suggests that NPs with micro-nutrients can be used to heal the plants suffering from single or multiple nutrient deficiencies. New nano-formulations can be designed and applied to plants according to their nutritional requirement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plants need 18 elements for their proper growth and development (C, H, O, N, P, K, Mg, Ca, S, Fe, Zn, Cu, Mn, B, Cl2, Mo, Co, and Ni; NRCCA 2010). These elements are classified as macro- and micro-nutrients. Micro-nutrients (Zn, Fe, Mn, Cu, B, Mo, Co, and Ni) applied in small quantities are vital for plant growth. However, they become toxic after a certain dose. The deficiency or excessive concentration of these nutrients causes negative effects on agricultural production (Lynch 2019). The countries geographically placed at tropical or subtropical climates with weathered soils are having a shortage of Ca, Mg, K, N, and P (Sanchez 1976). In addition, soils which are subject to the semi-arid and arid climates are inclined to have a deficiency of P and some other micro-nutrients that can be ranged as Fe, Cu, Zn, and Mn (Clair and Lynch 2010). As an example, most of the soils in Ethiopia suffer from fertility challenges some of which are macro- and micro-nutrient deficiencies. The soil system is simultaneously having multi-deficiencies of essential elements (Girma 2017; Atnafu 2018). Similarly, the soils in Najd plateau (Saudi Arabia) which comprises most of the arable land possess marginal levels for Fe, deficiency for Zn and Mn, and sufficient or deficient for Cu (Sallam 2002). Insufficient or deficient soil conditions result in food insecurity and malnutrition, which poses a huge risk for diseases (Sanchez and Swaminathan 2005; Bain et al. 2013).
Materials with a size range from 1 to 100 nm are called nanoparticles (NPs) (Suguna et al. 2017; Hema et al. 2016). Depending on physical and chemical characteristics (i.e., shape, size, and chemical composition), NPs have been used in many applications ranging from electronic devices (Kefeni et al. 2017) and biomedicine (McNamara and Tofail 2017) to energy-based research (Manikandan et al. 2015; Zhang et al. 2018) and environmental applications (Zou et al. 2016; Khan et al. 2017). In addition, NPs have been used in agriculture for the purposes of alleviating nutrient deficiencies, plant growth enhancement, and drug delivery (Monreal et al. 2016; Manikandan et al. 2016; Dimkpa et al. 2017; Karny et al. 2018). They are recommended as additives for their contribution to nutrition use efficiency, plant growth, and seed germination (Rui et al. 2016; Pacheco and Buzea 2018; Tombuloglu et al. 2018; Santo Pereira et al. 2019; Singh et al. 2019). New particulate designs can contribute to alleviating the nutritional deficiency problem of plants, especially in suffering soils or environments.
In addition, the route, uptake, and incorporation of NPs into the plant tissues should be assessed before their agricultural application. Bioavailability and bioaccumulation of NPs in the plant body are also important to have an idea for their possible transfer to the end users such as animals and humans. In plants, NP application can alter nutrient uptake of plants, and this may increase the abundance of the elements found in the structure of applied NPs. For instance, in a previous work, it has been shown that NiFe2O4 NP treatment increased the leaf nickel (Ni) and iron (Fe) content of barley (Tombuloglu et al. 2019a). In the same way, the treatment of barley with SrCaMg nano-hexaferrite raised Fe, magnesium (Mg), calcium (Ca), and strontium (Sr) content of the leaf (Tombuloglu et al. 2019b). Because of potential bioaccumulation of NPs in the plant body, humans as well as animals can eat these foods with NPs as a result of the food chain. However, their effects on these organisms are not fully known yet. Therefore, before using NPs in the farm soils/ecosystems or food/plants as nano-solutions, they should be carefully investigated as to whether they are taken up by the plant.
In this study, we, for the very first time, designed and synthesized a novel nanoparticulate formulated as Ni0.4Cu0.2Zn0.4Nd0.05Y0.05Fe1.9O4. The composition of the NPs includes six metals nickel (Ni), zinc (Zn), copper (Cu), and iron (Fe) which are essential micro-nutrients for plant growth at low concentrations (Mitra 2017). In addition, two rare earth elements, namely neodymium (Nd) and yttrium (Y), were substituted to the NPs in order to track and confirm the existence of NPs in the aerial tissues. After synthesis and detailed characterizations (transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), and energy-dispersive X-ray spectroscopy (EDX)), varying concentrations of NPs (62.5, 125, 250, and 500 mg L−1) were introduced to a common crop plant, namely barley (Hordeum vulgare L.), which is ranked as the fourth in terms of annual production among the cereals. Root and leaf tissues were reaped from hydroponically grown 21-day old seedlings, and elemental analyses were conducted by an inductively coupled plasma optical emission spectrometer (ICP-OES).
2 Material and Methods
2.1 Nanoparticle Preparation
Ni0.4Cu0.2Zn0.4Nd0.05Y0.05Fe1.9O4 NPs were synthesized via the sol-gel auto combustion approach. Ni(H2O)6·(NO3)2 (nickel (II) nitrate hexahydrate), Fe(NO3)3·9H2O (iron (III) nitrate nonahydrate), Cu(NO3)2·6H2O (cupric nitrate hexahydrate), Zn(NO3)2·6H2O (zinc nitrate hexahydrate), Nd(NO3)3·6H2O (neodymium nitrate hexahydrate), Y(NO3)3·6H2O (yttrium(III) nitrate hexahydrate), and C6H8O7 (citric acid) were used as starting materials. The spinel ferrite was prepared by mixing the metal nitrate salts with citric acid in 80 mL of deionized water (DI) under 90 °C. The pH of the solution was adjusted to 7.0, then the suspension was heated up to 160 °C for 30 min. The temperature then was raised up to 350 °C until the whole solution was burned and turned to solid powder.
2.2 Characterization of the Nanoparticles
X-ray diffraction (XRD) analysis was performed by using the Miniflex benchtop XRD instrument (Rigaku) with Cu Kα radiation at room temperature and 2θ = 20–70°. The morphology of the NPs was observed with a scanning electron microscope (SEM, FEI Titan ST with energy-dispersive X-ray spectroscopy (EDX)) and a transmission electron microscope (TEM, FEI, Morgagni 268, Czech Republic).
2.3 Plant Treatment
Different concentrations of NPs (62.5, 125, 250, and 500 mg L−1) were made in hydroponic media which includes 6 mM KNO3, 1 mM NH4H2PO4, 2 mM MgSO4, 4 mM Ca(NO3)2, 50 μM H3BO3, 9 μM MnCl2, 0.3 μM CuSO4, 0.8 μM ZnSO4, 0.1 μM Fe-EDTA, and 0.12 μM MoO3 (85%) (Hoagland and Arnon 1950; Tombuloglu et al. 2013, 2015). The control solution was free from NPs. Before the treatment, the sonication process was carried out for the solutions at room temperature about 30 min (Powersonic 410, Hwashin Technology, Korea). Afterwards, the suspensions were introduced to the seedlings in a hydroponic condition (Bostancioglu et al. 2018; Aydin et al. 2019). The nutrient solutions (with or without NPs) were aerated at regular periods with the help of an aquarium pump. The seedlings were grown in greenhouse conditions which are adjusted to 20/25 °C of temperature, 60–70% of humidity, and 16/8 of light/dark regime. After 3 weeks, the tissues (root and leaf) were reaped from a minimum of 10 seedlings. The sonication process for a period of 5 s was applied to roots in order to clean the absorbed NPs from the root surface (Powersonic 410, Hwashin Technology, Korea). Later, the roots were fully washed with DI water three times. Then, the tissue parts were dried inside an oven at 60 °C for a period of 4 days, and they were transformed into fine powder in a mortar using a pestle.
2.4 Elemental Analysis
0.5 g samples of roots and leaves were subjected to the digestion process inside a microwave oven (CEM MARSX, CEM) in accordance with the U.S. Environmental Protection Agency (USEPA) digestion method 3051 (USEPA 1995). For this purpose, the mixture of hydrogen peroxide (H2O2, 30% v/v) and plasma pure nitric acid (HNO3, 65%) (SCP Science, Thermo Fisher Sci.) (4:1) was discharged on the dry powder, and then they were heated up to a temperature of 180 °C. An inductively coupled plasma optical emission spectrometer (ICP-OES) (PerkinElmer AvioR 145 500 ICP-OES Scott/Cross-Flow, USA) was used to determine the elements (iron, zinc, copper, nickel, neodymium, yttrium, calcium, potassium, magnesium, and phosphate). The formula below is used in order to compute the translocation index (TI %) of the elements (Cannata et al. 2014; Tombuloglu et al. 2019c).
where NC denotes the nutrient’s concentration.
2.5 Statistical Analysis
The SPSS program v15.0 (SPSS Inc., Chicago, USA) was used to analyze the data extracted from elemental analyses. The means of data obtained from non-treated (control) and experimental groups were compared by using the t test variance analysis program (Microsoft, Excel 2010). The significance (p < 0.05, p < 0.01, and p < 0.005) shown was specified using an asterisk symbol.
3 Results
3.1 Synthesis and Characterization of Nanoparticles
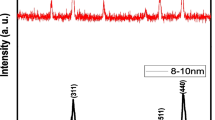
Figure 1 a depicts the XRD pattern of Ni0.4Cu0.2Zn0.4Nd0.05Y0.05Fe1.9O4 NPs. The pattern exhibited the characteristic peaks of Ni-Cu-Zn spinel ferrite with an average crystallite size of 18 nm. The SEM images (Fig. 2c) of the NPs showed the aggregation of spherical particles with an average size of less than 20 nm. Similarly, the TEM image confirmed the structure and size of Ni-Cu-Zn spinel ferrite (Fig. 2b). The EDX presents the existence of elements (Cu, Zn, Ni, Nd, Y, and Fe) in the structure of the NPs as revealed in Fig. 2d.
The graph represents the concentration of elements existing in the composition of NPs analyzed in barley tissues upon 3 weeks of NP treatment. a Iron (Fe), b zinc (Zn), c copper (Cu), d and nickel (Ni) contents in the leaf and root tissues. The units of the value, which was obtained from the samples of dried plant (DW), were stated in milligrams per kilogram. The wavelength number (nm) specific for each element was indicated on the graphs. Error bars represent standard errors computed from three technical replicates of pooled samples (n = 5). t test was used to analyze data statistically (***p < 0.005). nd, detected
3.2 Fe, Cu, Zn, and Ni Content of Plant Tissues
In order to evaluate NP uptake together with their translocations and the inclusion of micro-elements to the plant tissues, the elements found in the composition of NPs were determined by using an ICP-OES. In Fig. 2, the root and leaf content of iron (Fe), zinc (Zn), copper (Co), and nickel (Ni) are shown. In the root control, Fe, Zn, and Cu level were, respectively, 117, 94, and 12 mg kg−1 in dry weight (DW), while they were 19, 79, and 4 mg/kg in the non-treated leaves. The addition of NPs in the nutrient solution significantly raised the quantity of those elements in the tissues (both roots and the leaves) (at least p < 0.05). The increase was proportional to the increasing NP concentrations. As was expected, the content of elements was the highest in 500 mg L−1 of the treatment which raised Fe, Zn, and Cu contents in the leaf at about 5, 3, and 18 times than the control, respectively.
3.3 Incorporation of Rare Earth Elements (Nd and Y) into the Plant Body
Supplementation of barley seedlings with Nd- and Y-incorporated NPs increased the concentration of these elements both in the tissues of the root and leaf, depending on the dose applied (Fig. 3). In the control leaves, the quantity of Nd and Y elements are about 18.6 and 42.8 μg g−1 in DW, while in the roots, they were about 29 and 84 μg g−1 in DW, respectively. In the leaves, Nd and Y elements were at the highest levels in the case of NP concentration of 500 mg L−1, and these levels, respectively, were approximately 3.5 and 2.5 times more than those of the control. However, the average change of those elements between the control and the 500-mg L−1-treated sample in the roots was higher than that in the leaves. For instance, Nd content was about 100 times and the Y content was about 30 times more in the 500-mg L−1-treated roots compared with those in controls.
The concentration of the rare earth elements a Nd (neodymium) and b Y (yttrium), into the plant leaf and root. The values were determined from the samples of dried plant (DW) stated in micrograms per gram. The wavelength number (nm) specific for every element was indicated on the graphs. Error bars represent standard errors computed from three technical replicates of pooled samples (n = 5). t test was used to analyze the data statistically (*p < 0.05, **p < 0.01, ***p < 0.005)
3.4 Macro-nutrient (Mg, Ca, P, and K) Level in Plant Tissues
Figure 4 shows the content of Mg, Ca, P, and K macro-elements in plant tissues upon NP treatment. Compared with the untreated leaf control, lower doses (i.e., 62.6–250 mg L−1) did not significantly change the abundance of Ca, Mg, and P. Only in the treatment of 500 mg L−1, K and P levels in the leaf significantly decreased (p < 0.05). However, 500 mg L−1 of the NP treatment increased the Ca, Mg, and P levels of the roots compared with those of controls (p < 0.05). The content of Ca and Mg both in the roots and leaves did not alter by the inclusion of lower NP doses (i.e., 62.6–250 mg L−1).
Elemental analysis of the tissues of the roots and the leaves for barley seedlings upon 3 weeks of NP treatment. a Potassium (K), b magnesium (Mg), c calcium (Ca), and d phosphate (P). The values which were obtained from samples of dried plant (DW) were stated in milligrams per kilogram. Error bars represent standard errors computed from three technical replicates of pooled samples (n = 5). t test was used to analyze data statistically (*p < 0.05)
3.5 Root-to-Leaf Translocation of Nutrients
TI (%) of the nutrients found in the composition of NPs (Cu, Zn, Y, Fe, and Nd) as a function of applied NP concentration is shown in Fig. 5a–e. Starting from 62.5 mg L−1, the TI of the elements logarithmically decreased by the inclusion of increasing NP doses. In parallel to this, the TI of the macro-elements (Ca, Mg, P, and K) showed a similar pattern (Fig. 6). The 500-mg L−1 NP application dramatically decreased the TI of these elements. However, no significant change was observed at lower NP concentrations.
4 Discussion
There are 18 essential nutrient elements required for plants to grow and develop properly. The insufficiency of an essential nutrient(s) affects the normal functioning and growth of plants (Park et al. 2019), which led to a reduction in global agricultural production (Lynch 2019). The idea of using nanoparticles as a nutritional supplement in agriculture has emerged in recent years. In some studies, it has been suggested that NPs promote plant growth and also provide the essential elements for the plant (Rui et al. 2016; Tombuloglu et al. 2018; Santo Pereira et al. 2019). Therefore, this study aimed to synthesize composite micro-nutrient nanoparticle (NP) composed of four essential nutrient elements (Ni, Cu, Zn, and Fe) and assess its incorporation into the plant tissues.
As shown in Fig. 2, the root and leaf content of Ni, Cu, Zn, and Fe is proportionally increased by the inclusion of NPs, which indicates the uptake and translocation of NPs into the plant body. The Ni element was not present in the root and leaf tissues of the control. However, it started to appear in the leaves of the 125-mg L−1-treated seedlings. In the same concentration, a significant amount of Ni was determined in the roots, and this shows the uptake of NPs by the roots although they were not translocated to the leaves yet. The NPs with increasing doses raised the Ni content in the leaves, and of course, this increase was dose-dependent. Overall, these results showed that barley took up NPs with roots, and then translocated them to the aerial parts. Since infertile soils suffer from multi-element deficiencies (Girma 2017; Atnafu 2018), the inclusion of NPs to the plant growth environment may alleviate the nutrient deficiency problem of the plants.
Neodymium (Nd) and yttrium (Y) are rare earth elements found in the earth’s crust. They have no known biological function (Ramos et al. 2016). In the current study, Nd and Y elements were substituted in the NP composition in order to verify the migration of NPs. The content of both elements was gradually increased by the applied NP concentration (Fig. 3). The Nd concentration in the leaves increased from 18.6 mg kg−1 in the control to 72.2 mg kg−1 in 500 mg L−1 of NP treatment. On average, the natural level of Nd is 40 mg kg−1 in soil (Greenwood and Earnshaw 1997; Carpenter et al. 2015). Depending on the soil type, Nd concentration ranged between 1.2 and 52.5 mg kg−1 dry soils (Ichihashi et al. 1992; Patnaik 2003). The Y content of the leaves was 42.6 mg kg−1 in the control and 108 mg kg−1 in 500 mg L−1 of NP treatment. The soil range of Y is 6.48–27.93 mg kg−1 (Jeske and Gworek 2013). According to Ichihashi et al. (1992), the soil range of Y is 10 to 150 mg kg−1. The Y content of edible plants ranged from 20 to 100 ppm (Shacklette et al. 1978; Kastori et al. 2010). Compared with that in the root, bioaccumulation of the elements is low in the leaves, which indicates a limited translocation of NPs from the root to the leaves. In accordance with this study, Maksimovic et al. (2014) found that root accumulation of Y was much more intensive than the translocation of Y to the leaves of maize. Overall, these results indicated that, as rare earth elements, Nd and Y are incorporated into the plant body. They can be used to track the bioavailability of NPs in plants.
The content of macro-elements was determined in the tissues of the roots and the leaves for both NP-treated (62.5–500 mg L−1) and NP-non-treated barley seedlings (Fig. 4). Potassium (K) and phosphorus (P) are a relatively large amount of primary nutrients required for metabolism and plant growth. In addition, magnesium (Mg) and calcium (Ca) are the secondary nutrients applied in larger quantities (Mitra 2017). According to the results, the amount of macro-elements did not show a significant change at lower NP doses in the leaves (62.5–250 mg L−1). However, at the highest dose (500 mg L−1), the nutrient content of the tissues obviously altered. This could be attributed to a possible root injury due to a high concentration of NPs (i.e., 500 mg L−1 in this study), which eventually may affect the nutrient uptake and transfer (Al-amri et al. 2020; Tombuloglu et al. 2019d).
The translocation index (TI) shows the transfer ratio of macro- and micro-nutrients from the roots to the shoots (Paiva et al. 2002). The TI of the elements logarithmically decreased by the inclusion of increasing NP doses (Fig. 5). The reduction of TI by the inclusion of increasing NP doses could be related to the accumulation of those elements or the NPs in the root tissue. However, as shown in Fig. 2, the abundance of these elements in the leaves is limited compared with that in the root tissue. This result suggested that the elements found in the structure of NPs were translocated from the root to leaf; however, it was limited. The majority of elements seem to have accumulated in the roots. In parallel, the TI of the macro-elements (Ca, Mg, P, and K) showed a similar pattern in the increasing NPs (Fig. 6). These findings pointed out the presence of some biological barriers that limit the migration of macro- and micro-elements at high concentrations. For example, in plants, some sort of barriers such as the Casparian strip, cell wall, and the cell membrane might restrict the mobility of nutrients. Depending on the plant type, their pore sizes are varying from 1 to100 nm. Besides, the size of plant cell wall pores changes from 3.5 to 20 nm (Chichiriccò and Poma 2015). These potential barriers can stop or let NPs reach cells or tissues (Wang et al. 2016). In this study, the average particular size of the NPs was determined as 18 nm (Fig. 1), which is ideal for NPs to penetrate the cell wall pores. However, due to their magnetic character, they can attract each other and form agglomerates, which in turn may increase their sizes. Therefore, the translocation of NPs might have been prevented, or only a limited quantity of the NPs was allowed to migrate.
5 Conclusions
This study for the first time investigates the uptake and translocation of iron oxide nanoparticles composed of four micro-elements (Zn, Fe, Cu, and Ni) in a crop species. The results showed that the inclusion of nanoparticles raised the amount of these elements in the leaves of barley. It shows that applied nanoparticles, with an average size of 18 nm, are taken up by the roots, and they are also translocated to the leaves. Multi-element deficiency is a common problem in soils where plants suffer from this problem and influence agricultural production globally. The findings of this study have suggested that new nano-formulations can be applied to the plants that suffer from multi-element deficiencies. New nanoparticles can be built according to plant or soil requirement. Overall, the results of this study showed that nanoparticles harboring multiple elements could be incorporated into the plant’s body but may change the nutritional status of the plant at higher concentrations. Further studies should demonstrate the effect of nanoparticles on plant growth by conducting comparative experiments in deficient conditions.
References
Al-Amri N, Tombuloglu H, Slimani Y, Akhtar S, Barghouthi M, Almessiere M, Alshammari T, Baykal A, Sabit H, Ercan I, Ozcelik S (2020) Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol Environ Saf 194:110377
Atnafu O (2018) Response of maize (Zea mays L.) to omission of nutrients at Kersa district, Jimma zone, South Western Ethiopia. Doctoral dissertation, Jimma University
Aydin M, Tombuloglu G, Sakcali MS, Hakeem KR, Tombuloglu H (2019) Boron alleviates drought stress by enhancing gene expression and antioxidant enzyme activity. J Soil Sci Plant Nutr 19:545–555
Bain LE, Awah PK, Geraldine N, Kindong NP, Siga Y, Bernard N, Tanjeko AT (2013) Malnutrition in Sub–Saharan Africa: burden, causes and prospects. Pan Afr Med J 15(1)
Bostancioglu SM, Tombuloglu G, Tombuloglu H (2018) Genome-wide identification of barley MCs (metacaspases) and their possible roles in boron-induced programmed cell death. Mol Biol Rep 45(3):211–225
Cannata MG, Bertoli AC, Carvalho R, Bastos ARR, Freitas MP, Augusto AS (2014) Effects of cadmium on the content, accumulation, and translocation of nutrients in bean plant cultivated in nutritive solution. Commun Soil Sci Plant Anal 45(2):223–235
Carpenter D, Boutin C, Allison JE, Parsons JL, Ellis DM (2015) Uptake and effects of six rare earth elements (REEs) on selected native and crop species growing in contaminated soils. PLoS One 10(6):e0129936
Chichiriccò G, Poma A (2015) Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 5(2):851–873
Clair SBS, Lynch JP (2010) The opening of Pandora’s Box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 335(1-2):101-115
Dimkpa CO, Bindraban PS, Fugice J, Agyin-Birikorang S, Singh U, Hellums D (2017) Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron Sustain Dev 37(1):5
Girma T, (2017) Potato productivity, nutrient uptake and use efficiency as influenced by organic and inorganic amendments in Arbegona district, Southern Ethiopia (Doctoral Dissertation). Hawassa University
Greenwood NN, Earnshaw A (1997) Chemistry of the elements. Elsevier
Hema E, Manikandan A, Karthika P, Durka M, Antony SA, Venkatraman BR (2016) Magneto-optical properties of reusable spinel NixMg1− xFe2O4 (0.0≤ x≤ 1.0) nano-catalysts. J Nanosci Nanotechnol 16(7):7325–7336
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular California agricultural experiment station 347(2nd edit)
Ichihashi H, Morita H, Tatsukawa R (1992) Rare earth elements (REEs) in naturally grown plants in relation to their variation in soils. Environ Pollut 76:157–162
Jeske A, Gworek B (2013) Distribution and mobility of scandium and yttrium in selected types of soils in Poland. Chem Speciat Bioavailab 25(3):216–222
Karny A, Zinger A, Kajal A, Shainsky-Roitman J, Schroeder A (2018) Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci Rep 8(1):1–10
Kastori RR, Maksimović IV, Zeremski-Škorić TM, Putnik-Delić MI (2010) Rare earth elements: yttrium and higher plants. Zbornik Matice Srpske za Prirodne Nauke 118:87–98
Kefeni KK, Msagati TA, Mamba BB (2017) Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mat Sci Eng B Adv 215:37–55
Khan MN, Mobin M, Abbas ZK, AlMutairi KA, Siddiqui ZH (2017) Role of nanomaterials in plants under challenging environments. Plant Physiol Biochem 110:194–209
Lynch JP (2019) Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol 223(2):548–564
Maksimovic I, Kastori R, Putnik-Delic M, Borišev M (2014) Effect of yttrium on photosynthesis and water relations in young maize plants. J Rare Earths 32(4):372–378
Manikandan A, Saravanan A, Antony SA, Bououdina M (2015) One-pot low temperature synthesis and characterization studies of nanocrystalline α-Fe2O3 based dye sensitized solar cells. J Nanosci Nanotechnol 15(6):4358–4366
Manikandan A, Durka M, Amutha Selvi M, Arul Antony S (2016) Sesamum indicum plant extracted microwave combustion synthesis and opto-magnetic properties of spinel MnxCo1-xAl2O4 nano-catalysts. J Nanosci Nanotechnol 16(1):448–456
McNamara K, Tofail SA (2017) Nanoparticles in biomedical applications. Adv Phys X 2(1):54–88
Mitra G (2017) Essential plant nutrients and recent concepts about their uptake. In: Naeem M, Ansari AA, Gill SS (eds) Essential Plant Nutrients. Springer, Cham, pp 3–36
Monreal CM, DeRosa M, Mallubhotla SC, Bindraban PS, Dimkpa CO (2016) Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol Fertil Soils 52:423–437
NRCCA (2010) North east region certified crop adviser study resources. Cornell University, USA
Pacheco I, Buzea C (2018) Nanoparticle uptake by plants: beneficial or detrimental? In: Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA (eds) Phytotoxicity of nanoparticles. Springer, Cham, pp 1–61
Paiva HN, Carvalho JG, Siqueira JO (2002) Índice de translocação de nutrientes em mudas de cedro (Cedrela fissilis Vell.) e de ipê-roxo (Tabebuia impetiginosa Mart Standl.) submetidas a doses crescentes de cádmio, níquel e chumbo. Revista Árvore 26:467–473
Park JW, Melgar JC, Kunta M (2019) Plant nutritional deficiency and its impact on crop production. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense. Springer, Cham, pp 231–258
Patnaik P (2003) Handbook of inorganic chemicals. McGraw-Hill, USA
Ramos SJ, Dinali GS, Oliveira C, Martins GC, Moreira CG, Siqueira JO, Guilherme LR (2016) Rare earth elements in the soil environment. Curr Pollut Rep 2(1):28–50
Rui M, Ma C, Hao Y, Guo J, Rui Y, Tang X et al (2016) Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci 7:815
Sallam AS (2002) Evaluation of some soils in Najd Plateau, central region, Saudi Arabia. J Saudi Soc Agric Sci 1:21–40
Sanchez PA (1976) Properties and mangement of soils in the tropics. J. Wiley and Sons Inc. New York, 618pp
Sanchez PA, Swaminathan MS (2005) Hunger in Africa: the link between unhealthy people and unhealthy soils. Lancet 365:442–444
Santo Pereira ADE, Oliveira HC, Fraceto LF (2019) Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: a field study. Sci Rep 9(1):1–10
Shacklette HT, Erdman JA, Harms TF (1978) Trace elements in plant foodstuffs. In: Oehme FW (ed) Toxicity of heavy metals in the environments, part I. Marcel Dekker, New York
Singh J, Kumar S, Alok A, Upadhyay SK, Rawat M, Tsang DC et al (2019) The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J Clean Prod 214:1061–1070
Suguna S, Shankar S, Jaganathan SK, Manikandan A (2017) Novel synthesis of spinel MnxCo1−xAl2O4 (x= 0.0 to 1.0) nanocatalysts: effect of Mn2+ doping on structural, morphological, and opto-magnetic properties. J Supercond Nov Magn 30(3):691–699
Tombuloglu H, Kekec G, Sakcali MS, Unver T (2013) Transcriptome-wide identification of R2R3-MYB transcription factors in barley with their boron responsive expression analysis. Mol Gen Genomics 288(3–4):141–155
Tombuloglu G, Tombuloglu H, Sakcali MS, Unver T (2015) High-throughput transcriptome analysis of barley (Hordeum vulgare) exposed to excessive boron. Gene 557(1):71–81
Tombuloglu H, Tombuloglu G, Slimani Y, Ercan I, Sozeri H, Baykal A (2018) Impact of manganese ferrite (MnFe2O4) nanoparticles on growth and magnetic character of barley (Hordeum vulgare L.). Environ Pollut 243:872–881
Tombuloglu H, Slimani Y, Tombuloglu G, Almessiere M, Baykal A, Ercan I, Sozeri H (2019a) Tracking of NiFe2O4 nanoparticles in barley (Hordeum vulgare L.) and their impact on plant growth, biomass, pigmentation, catalase activity, and mineral uptake. Environ Nanotechnol Monit Manag 11:100223
Tombuloglu H, Slimani Y, Tombuloglu G, Almessiere M, Sozeri H et al (2019b) Impact of calcium and magnesium substituted strontium nano-hexaferrite on mineral uptake, magnetic character, and physiology of barley (Hordeum vulgare L.). Ecotoxicol Environ Saf 186:109751
Tombuloglu H, Slimani Y, Alshammari T, Tombuloglu G, Almessiere M et al (2019c) Magnetic behavior and nutrient content analyses of barley (Hordeum vulgare L.) tissues upon CoNd0.2Fe1.8O4 magnetic nanoparticle treatment. J Soil Sci Plant Nutr 1–10. https://doi.org/10.1007/s42729-019-00115-x
Tombuloglu H, Slimani Y, Tombuloglu G, Almessiere M, Baykal A (2019d) Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 226:110–122
USEPA (United States Environmental Protection Agency) (1995) Method 3051: microwave assisted acid digestion of sediments, sludges, soils, and oils. Test methods for evaluating solid waste 1–30
Wang P, Lombi E, Zhao FJ, Kopittke PM (2016) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21(8):699–712
Zhang X, Gao W, Su X, Wang F, Liu B, Wang JJ, Liu H, Sang Y (2018) Conversion of solar power to chemical energy based on carbon nanoparticle modified photo-thermoelectric generator and electrochemical water splitting system. Nano Energy 48:481–488
Zou Y, Wang X, Khan A, Wang P, Liu Y, Alsaedi A, Hayat T, Wang X (2016) Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ Sci Technol 50(14):7290–7304
Funding
The authors received funding from the Deanship of Scientific Research (DSR) of Imam Abdulrahman Bin Faisal University (IAU) provided via the 2018-139-IRMC project number.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tombuloglu, H., Ercan, I., Alshammari, T. et al. Incorporation of Micro-nutrients (Nickel, Copper, Zinc, and Iron) into Plant Body Through Nanoparticles. J Soil Sci Plant Nutr 20, 1872–1881 (2020). https://doi.org/10.1007/s42729-020-00258-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00258-2