Abstract
The aim of this research was to investigate the effect of applying sewage sludge combined with wheat crop residue as an organic amendment on the dissipation rate of simazine spiked at 2 and 20 mg kg−1 in an Andisol soil from southern Chile. Changes in some soil enzymes related to soil quality were measured by spectrophotometry, simazine dissipation rates were measured by gas chromatography, and biomass production in the contaminated soil was evaluated. Results of this study indicated that application of the organic amendment inoculated with Trametes versicolor enabled a decrease in negative effects of the herbicide on soil enzymatic activities and a reduction in final concentrations of simazine (~ 80% at both doses). The simazine half-life time was reduced from 14 to 10 days and from 36 to 15 days for doses of 2 and 20 mg kg−1, respectively. These findings prove that the combined strategy of biostimulation and bioaugmentation using these residues can be effectively used to reduce residue pesticides in soils, mainly by increasing the microbiological activity, thus improving simazine dissipation in an Andisol soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agriculture is one of the most important productive activities in the world, being vital for solving the global food demand, which increases annually due to a sustained increase in population. To improve crop yield and productivity, the addition of compounds like herbicides is a normal practice for crop development (Salem et al. 2017). However, the constant and excessive addition of herbicides can negatively impact the environment on different levels, mainly through the progressive accumulation and persistence of xenobiotics in natural environments (Hashmi et al. 2017; Pinochet et al. 2018). The herbicide simazine (2-chloro-4,6-bis (ethylamino)-s-triazine) is a widely used selective, systemic s-triazine, which has been broadly applied to control broad-leaved weeds and annual grasses that affect various crops (Cheng et al. 2017). Such widespread use affects non-target terrestrial and aquatic environments (Martínez-Iñigo et al. 2010). Arias-Estévez et al. (2008) pointed out that only a minor fraction of an applied herbicide reaches the target (< 0.1%), whereas the remaining fraction contributes to environmental pollution. At these microsites, the mobility of herbicides in bulk soils depends on processes such as retention, transport, and degradation (Jensen et al. 2018).
The use of soil microorganisms for soil bioremediation is a convenient and promising tool that has been applied to reduce the adverse effects of xenobiotics in soils (Gao et al. 2014). In this context, white-rot fungi are strong candidates to be applied in soil environmental cleanup due to their ability to synthesize nonspecific selective enzymes (laccases, lignin peroxidase, and manganese peroxidase) that could be useful in dissipating soil contaminants (Camacho-Morales et al. 2017; Coelho-Moreira et al. 2018). Hence, bioaugmentation strategies using these fungi have been reported to accelerate the onset of degradation, improve soil health in heavy metal–contaminated soils and protect the native microbiological communities against adverse effects (Arriagada et al. 2010).
Several organic residues are used in agroforestry systems, including crop residues such as wheat straw and sewage sludge. Both types of residue could be an excellent raw material to apply in some biostimulation strategies (Almonacid et al. 2015; Kumari et al. 2018). Furthermore, the addition of an organic amendment as a nutrient source for endemic microorganisms improves the physical, chemical, and biological properties of soil, mainly through the increase in overall microbiological activity promoted by the availability of organic and inorganic nutrients (Almonacid et al. 2015).
In this study, we hypothesized that sewage sludge combined with wheat crop residues, used as an organic amendment, can be used as a bioaugmentation-biostimulation strategy to promote simazine dissipation in an Andisol soil. Additionally, Trametes versicolor in combination with the organic amendment can contribute to increasing simazine dissipation rates and improving soil health. For this purpose, a simazine-contaminated Andisol soil was supplemented with T. versicolor inoculated in an organic amendment comprising sewage sludge and wheat straw residues. Soil enzyme activities were assessed to evaluate biochemical changes, and GC-ECD analyses were used to quantify the simazine dissipation rate.
2 Materials and Methods
2.1 Simazine
High-purity simazine (99%; Sigma-Aldrich, San Luis, MO) was used in the experiments. Soils were spiked with 10 ml of simazine diluted in acetone to perform contamination at doses of 2 and 20 mg kg−1 (estimated field dose and 10× field dose, respectively).
2.2 Soil Characteristics
The collected soil was an Andisol from southern Chile belonging to the Freire family (38°50′S and 72°35′W; medial, mesic, Typic Placudands) with a silty loam texture (CIREN 2002). Soil samples were collected from the surface layer (0–20 cm), air-dried, sieved through a 2-mm mesh, and characterized according to the methods described in Sadzawka et al. (2004). Briefly, the organic matter content was measured using the Walkley-Black method. The pH was measured in soil suspensions with deionized water (1:2.5 w:v). Cation exchange capacity (CEC) was calculated from the total exchangeable bases (Mg, Ca, K, and Na extracted by 1 M ammonium acetate at pH 7.0) analyzed by flame atomic absorption spectrophotometry.
2.3 Organic Residues and Saprophytic Fungi
Wheat straw corresponded to crop residues from the Araucania Region and the stabilized sewage sludge was collected from municipal wastewater plant ESSAL S.A., Osorno, Chile. Chemical characterization of the residues were: (i) 43% cellulose, 30% hemicellulose, 9% lignin, C:N ratio 87, pH 5.5, 46% carbon, total N 0.5%, total P content 1.5 mg kg−1, Cu content 2.5 mg kg−1, and Zn content 5.8 mg kg−1 for wheat straw; and (ii) 1.96% cellulose, 11.0%, hemicellulose, 1.3% lignin, C:N ratio 8.5, pH 12.81% organic matter, 46% carbon, total N 0.6%, total P content 19,500 mg kg−1, Cu content 113 mg kg−1, and Zn content 399 mg kg−1 for sewage sludge.
Saprophytic fungi T. versicolor (A-1369) was obtained from the culture collection of the Centro de Investigaciones Biológicas in Madrid. The fungal inoculum was prepared as follows: active mycelia plugs of T. versicolor from 7-day-old culture on potato dextrose agar were placed in 400 mL of potato dextrose broth and incubated at 30 °C and 200 rpm for 15 days. Then, 30 mL aliquots of inoculum were used to start growth in 250-mL Erlenmeyer flasks containing 40 g of sterilized organic residues (10 g of wheat straw and 30 g of sewage sludge), which was the best combination for enhancing enzyme production (Almonacid et al. 2015). The inoculated substrate was incubated for 2 weeks in darkness and then incorporated into the soil microcosms (40 g of inoculated substrate mixed with 460 g of soil). The moisture was monitored gravimetrically and periodically adjusted by adding distilled water.
2.4 Amendment Microcosms
Experiments to evaluate the simazine dissipation rate, enzymatic activities, and effects on soil microbial activity were performed in eight different microcosms. Each microcosm consisted of a glass pot with 500 g of soil, at approximately 60% of water holding capacity (WHC), supplemented with the organic amendment and/or simazine (Table 1). Soil and the organic amendment were mixed and then preincubated for 2 weeks at 25 °C in darkness prior to applying the contamination dose. After that, the simazine solution was slowly spiked over the microcosms. The simazine concentrations were controlled at 2 and 20 mg kg−1. Samples were taken at days 0, 1, 5, 15, and 30 after simazine application for enzymatic determinations and simazine content.
2.5 Soil Biochemical Determinations
Soil biochemical analyses were performed on the different eight microcosms at days 0, 1, 5, 15, and 30 after simazine application as follows: (i) Soil acid phosphatase activity was determined using p-nitrophenyl phosphate as an orthophosphate monoester analog according to Sannino and Gianfreda (2001); (ii) β-glucosidase activity was measured according to Tabatabai and Bremner (1969); (iii) Total microbial activity was measured by monitoring FDA hydrolysis (Adam and Duncan 2001); and (iv) Manganese peroxidase (MnP) activity was measured by mixing 1 g of soil, 2.0 mL of sodium tartrate (0.1 M pH 5.0), 2.0 mL of H2O2 (0.1 mM), and 2.0 mL of MnSO4 (0.1 mM). Samples were incubated at 25 °C for 30 min and measured at an absorbance of 420 nm. All activities were assayed in triplicate and reported on a dry soil basis.
2.6 Determination of Residual Simazine
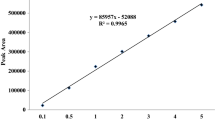
Ten grams of soil (60% of its WHC) were submitted to simazine extraction by shaking the soil and 20 mL of acetone for 60 min at 150 rpm, followed by sonication for 30 min. The resulting suspension was transferred to 50-mL Falcon tubes and centrifuged at 5000 rpm for 10 min. One milliliter of supernatant was passed through activated florisil (2 g) and sodium sulfate (1 g) columns with 5 mL of acetone. Samples were lyophilized and concentrated to 1 mL. Samples were filtered and analyzed for simazine quantifications. The concentrations of simazine in extracts were analyzed with a Shimadzu (Shimadzu Corp., Kyoto, Japan) gas chromatograph coupled with an electron capture detector (GC-ECD) using an RTX-5 column (Restek Corp., Bellefonte, PA). The column was programmed from an initial temperature of 60 °C for 1 min to 140 °C at a rate of 12 °C min−1, held for 1 min, and then ramped at a rate of 8 °C min−1 to 240 °C with a final hold time of 4 min. The detector and injector were maintained at 320 °C and 250 °C, respectively. The injector was in splitless mode for detection. To determine the detection limits, aliquots of soil were spiked with simazine. Recovery percentages were 83.25 ± 1.70 for S2; 81.19 ± 7.83 for SR2; 80.74 ± 3.21 for SRT2; 96.89 ± 3.88 for S20; 94.68 ± 4.82 for SR20; and 91.98 ± 1.24 for SRT20. The first-order rate of degradation and the DT50 (time required for 50% of the initial dose of pesticide to be degraded) of each compound in each soil were determined with the following equations:
Where Ct is the concentration of pesticide remaining in soil (mg kg−1) after t (days), Co is the initial concentration of pesticide (mg kg−1), and k is the rate of degradation (day−1) (Swarcewicz and Gregorczyk 2013).
2.7 Greenhouse Experiments
Solanum lycopersicum was used as the test plant. Seeds were surface-sterilized with NaClO for 15 min, thoroughly rinsed with sterilized distilled water. Four weeks after germination, ten uniform seedlings were transplanted to individual 1 L pots containing the remaining soil to evaluate the presence of simazine and/or to evaluate the toxicity of the degradation products. The plants were grown in a greenhouse for 45 days and the dry shoot and root biomass were measured.
2.8 Statistical Analyses
All results were analyzed by one-way ANOVA. Means and standard errors of ten replicates were calculated for enzymatic activity, simazine dissipation, and shoot and root biomass. Statistical significance was set at p < 0.05. All statistical tests were conducted using the R software (R Core Team 2018; https://www.R-project.org).
3 Results
3.1 Soil Enzyme Activities
Results for acid phosphatase, β-glucosidase, Mn peroxidase, and FDA hydrolysis are shown in Table 2. FDA hydrolysis was similar for all treatments at the beginning of the experiments. The higher values were obtained at day five (p < 0.05) for both doses. The simazine application caused a decrease in the enzymatic activity at day 1, mainly due to the change induced by the presence of the herbicide. Higher activity values were obtained in treatments with the organic amendment inoculated with T. versicolor (SRT, SRT2, and SRT20) (Table 2).
Acid phosphatase activity was similar in the control treatments (S, SR, and SRT). The results also showed little variations induced by the addition of simazine in the control treatments S, S2, and S20. Higher values of enzymatic activity were noted in the treatments with the organic amendment (SR2, SR20, SRT2, and SRT20) (Table 2).
β-Glucosidase was higher in treatments with the organic amendment (SR, SR2, SR20, SRT2, SRT2, and SRT20) at days 0, 1, and 5. However, treatments S2 and S20 showed results similar to treatments with the residues after day 5. At day 15, results were similar for all treatments. In general, values of enzymatic activity decreased over time, as in the control (S, SR, and SRT). In treatments with the organic amendment, β-glucosidase activity was higher on the first day (p < 0.05). In soils with simazine, the enzymatic activity increased significantly, which was higher in S20, SR20, and SRT20 at day 5.
MnP activity increased at day 5 for all treatments, with the highest values for treatments SR2 and SRT2. After the peak, the values decreased. At day 30, values were similar to the control (S, SR, and SRT) for both doses.
3.2 Simazine Removal from the Soil Microcosm
Results of simazine degradation are shown in Fig. 1A for the estimated field dose (2 mg kg−1) and in Fig. 1B for the 10× field dose (20 mg kg−1). The recovery percentages of simazine by GC ECD were approximately 83.25% for the treatment S2, 81.19% for SR2 and SRT2, 90.59% for S20, and 94.08% for SR20 and SRT20. For treatments with the estimated field dose 2 mg kg−1 (Fig. 1A), results showed that simazine degradation started at day 1 with no significant differences. Degradation of simazine at day 5 was higher in treatments with the residue (inoculated or not) than the control soils (S2) (p < 0.05). After that, the degradation processes were slow but continued. At day 30, total removed simazine was approximately 78.79% for the control treatment S2; approximately 82.22% for SR2 and 88.62% for SRT2. For treatments with simazine at 20 mg kg−1 (Fig. 1B), the degradation kinetic was similar to the field dose. The results show that the dissipation process also began within 24 h, even though the differences were not significant until day 5, where there were clear differences (p < 0.05) between treatments with the residue (SR20 and SRT20) and the control treatment S20, which had a higher simazine content. At day 30, the end of the analysis, the removed simazine was about 43.75% for the control treatment (S20), approximately 62.28% for treatment SR20, and approximately 73.90% for SRT20.
For simazine persistence, a first-order kinetic was used, and the estimation of half-life times is reported in Table 3. The half-life times were higher in treatments without organic amendment (S2 and S20). In particular, for 2 mg kg−1, the half-life values were reduced from 13.6 to 12.0 days for the biostimulation strategy (SR2), and from 13.6 to 9.8 days with the organic amendment inoculated with T. versicolor (SRT2). Similarly, in treatments with 20 mg kg−1, the DT50 values were reduced from 35.9 to 21.3 days with the organic amendment (SR20) and from 35.9 to 15.8 days with the organic amendment inoculated with T. versicolor (SRT20).
3.3 Greenhouse Experiments
After 45 days of culture of S. lycopersicum in the spiked soils, there were statistical differences between controls and treatments without the organic amendment (S2 and S20) (Fig. 2). In general, we showed a tendency to increase plant biomass in treatments with the organic amendments, in particular, aerial dry weight (p < 0.05). Furthermore, the application of simazine to the soils induced a decrease in the production of biomass, which was stronger in treatments with the higher simazine dose (Fig. 2). These negative effects were lower when the organic residue was biotransformed by T. versicolor, showing biomass production similarly to that obtained in the control treatments (S; Fig. 2).
Shoot and root dry weight of Solanum lycopersicum plants established in the treated soil. The plants were harvested 45 days after sowing. (S) soil control; (S2) soil plus simazine at 2 mg kg−1; (S20) soil plus simazine at 20 mg kg−1; (SR) soil plus organic residues; (SR2) soil plus organic residues and simazine at 2 mg kg−1; (SR20) soil plus organic residues and simazine at 20 mg kg−1; (SRT) soil plus organic residues + T. versicolor; (SRT2) soil plus organic residues + T. versicolor and simazine at 2 mg kg−1; (SRT20) soil plus organic residues + T. versicolor and simazine at 20 mg kg−1. The same letter is not significantly different according to Tukey’s multiple range test (p < 0.05)
4 Discussion
Our study showed that the application of simazine induces several changes in soil enzymatic activities, especially in the FDA hydrolysis. FDA hydrolysis occurs by the action of lipase, esterase, protease, and hydrolase enzymes, hydrolyzing nonspecifically fluorescein diacetate. Thus, FDA hydrolysis measures the direct action of soil microorganisms (Casucci et al. 2003). In general, the higher values of FDA hydrolysis in treatments SRT, SRT2, and SRT20 are caused by T. versicolor, which has been characterized as a fungus with a strong extracellular enzymatic apparatus, and is a suitable candidate for use in bioremediation strategies (Bastos and Magan 2009).
We also showed negative effects of simazine on the phosphatase activity. Acid phosphatase plays an important role in soils, specifically in the conversion of organic phosphorous to bioavailable forms for plants and microorganisms (Huang et al. 2003). When the organic amendment was incorporated, the overall acid phosphatase activity was improved, establishing an effective strategy for improving the soil biochemical properties of the simazine-contaminated soil.
Our study tested whether simazine affects the β-glucosidase activity, mainly by stimulating native soil microorganisms, which respond to stress and thus raise the level of enzyme production. Glucosidase activity is important for organic matter decomposition, specifically in the hydrolysis of β-glucosidase bonds of large carbohydrate chains present in lignocellulosic residues (Han and Chen 2008).
In the case of MnP, our results showed that this enzyme can play an important role in early degradation stages of the herbicide, improving the microbiological activity. MnP activity is one of the enzymes involved in the degradation of soil pollutants because the enzyme is directly involved in the oxidation of chemical compounds (Pizzul et al. 2009). Cea et al. (2010) showed a positive correlation between the activity of this enzyme and the degradation of pentachlorophenol as a result of a bioaugmentation strategy using Anthracophyllum discolor in an Andisol soil. These results agree with those reported in our study, where T. versicolor is a strong candidate to promote biodegradation of the herbicide.
Our study showed simazine removal from the soil microcosms. The differences in final simazine concentrations in treatments S2 and S20 (without biostimulation and bioaugmentation) are explained by the presence of native soil microorganisms, which were able to degrade the herbicide under the conditions of this study. These degradation rates increased when the soil incorporated the organic amendment (biostimulation strategies) in treatments SR2 and SR20, because the organic amendment promoted the development and enzymatic activity of certain strains of microorganisms, improving the simazine dissipation rates. On the other hand, when the organic amendment inoculated with T. versicolor was used (treatments SRT2 and SRT20), the dissipation rates increased due to the metabolic activity of T. versicolor. The incorporation of the organic amendment inoculated with T. versicolor makes it possible to obtain free nutrient sources for natural soil microbiota. The main form of herbicide degradation involves soil microbiological communities (Van Eerd et al. 2003). In addition, pesticide adsorption to organic matter and clays also plays a fundamental role in decreasing the amount of herbicide available in the soil solution (Đurović et al. 2009). Bastos and Magan (2009) studied the behavior of T. versicolor in a soil contaminated with high levels of atrazine, finding optimal degradation rates, classifying this fungus as a candidate for use in bioremediation strategies of soils contaminated with s-triazine herbicides. Similar results were reported by Morgante et al. (2012), who found simazine degradation by soil microorganism strains similar to those used in our study, showing microbial degradation rates of simazine, which is the main degradation mechanism of simazine under natural conditions. Likewise, the use of a bioaugmentation technique with a strain of Pseudomonas sp. can reduce the half-life times of the herbicide in soil. In our study, we showed that soil preincubation with an organic amendment (inoculated or not) can improve microbial responses to simazine contamination and improve the soil quality (Leskovar and Othman 2018). This preincubation caused an increase in the enzymatic activity and improved the soil conditions in order to obtain better simazine degradation rates and a lower half-life time.
Our study showed that the presence of simazine in the soil affects the growth of Solanum lycopersicum, especially in the soil treatments with the highest residual simazine. Although the biomass production was higher in the amendment treatments, we showed that the non-amendment soil also can allow plant development, in spite of the lower biomass obtained, which agrees with the natural simazine dissipation rate obtained in treatments without the organic amendment. The fact that some microorganisms can use simazine as a nitrogen source for growth is a relevant factor that contributes to simazine dissipation and could also help reduce ground water contamination through leaching (Chris Wilson et al. 2011; Dinamarca et al. 2007). These natural processes are possible due to natural microorganisms, which can promote the mineralization of xenobiotic compounds to less contaminating forms. However, these processes are slower in non-amendment soils and require more time to complete the dissipation to less contaminating forms such as NH3 and CO2 (Morgante et al. 2012), producing smaller plants than in amendment soils. With respect to amended microcosms, the presence of organic matter improves the interchangeable sites in the soil and therefore induces lower pesticide availability in the soil soluble fractions (Flores et al. 2009). Furthermore, adding fungal strains to the soil as a bioaugmentation strategy is an effective technique for improving biological and chemical soil properties. The use of bioaugmentation to degrade simazine using bacterial strains has been reported (Flores et al. 2009), but little is known about the ability of fungal strains to dissipate simazine residues themselves. The effect on plant growth promotion of S. lycopersicum may be an indirect process in which the fungus produces different extracellular enzymes (Nakatani et al. 2010), degrading both the pollutant and the organic residues; a process that indirectly stimulates the proliferation of certain bacterial and/or fungal strains involved in the degradation of simazine, improving the soil conditions to promote plant growth (Morgante et al. 2012). Organic amendment, either natural or inoculated, can be an effective strategy to apply in bioremediation processes because it contains available organic carbon, mineral elements essential for the growth of microorganisms and plants (Almonacid et al. 2015). Previous research has described these techniques as addressing the degradation of xenobiotic compounds (Ghazali et al. 2004; Suja et al. 2014). In this study, we tested two bioremediation techniques: biostimulation and bioaugmentation, and both can be effectively used to contribute to improving simazine dissipation rates in the Andisol soil.
5 Conclusions
This study showed that T. versicolor contributes to simazine dissipation. In addition, the use of a combined biostimulation and bioaugmentation strategy with T. versicolor can improve simazine dissipation rates, demonstrating the potential of this technique to design bioremediation strategies to recover simazine-contaminated soils. Also, we saw negative effects of simazine on the growth of Solanum lycopersicum plants and the enzymatic activities of simazine-treated soils; however, these effects can be reduced by the use of the biostimulation and bioaugmentation strategy performed.
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Almonacid L, Fuentes A, Ortiz J, Salas C, Garcia-Romera I, Ocampo J, Arriagada C (2015) Effect of mixing soil saprophytic fungi with organic residues on the response of Solanum lycopersicum to arbuscular mycorrhizal fungi. Soil Use Manag 31:155–164
Arias-Estévez M, López-Periago E, Martínez-Carballo E, Simal-Gándara J, Mejuto J-C, García-Río L (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123:247–260
Arriagada C, Pereira G, García-Romera I, Ocampo JA (2010) Improved zinc tolerance in Eucalyptus globulus inoculated with Glomus deserticola and Trametes versicolor or Coriolopsis rigida. Soil Biol Biochem 42:118–124
Bastos AC, Magan N (2009) Trametes versicolor: potential for atrazine bioremediation in calcareous clay soil, under low water availability conditions. Int Biodeterior Biodegrad 63:389–394
Camacho-Morales RL, Gerardo-Gerardo J, Guillén KN, Sánchez JE (2017) Ligninolytic enzyme production by white rot fungi during paraquat (herbicide) degradation. Rev Argent Microbiol 49:189–196
Casucci C, Okeke BC, Frankenberger WT (2003) Effects of mercury on microbial biomass and enzyme activities in soil. Biol Trace Elem Res 94:179–191
Cea M, Jorquera M, Rubilar O, Langer H, Tortella G, Diez MC (2010) Bioremediation of soil contaminated with pentachlorophenol by Anthracophyllum discolor and its effect on soil microbial community. J Hazard Mater 181:315–323
Cheng H, Jones DL, Hill P, Bastami MS (2017) Biochar concomitantly increases simazine sorption in sandy loam soil and lowers its dissipation. Arch Agron Soil Sci 63:1082–1092
Chris Wilson P, Lu H, Lin Y (2011) Norflurazon and simazine removal from surface water using a constructed wetland. Bull Environ Contam Toxicol 87:426–430
CIREN. 2002. Descripciones de suelos. Materiales y símbolos. Estudio Agrológico IX Región. Publicación CIREN N° 122. 360 p. Centro de Información de Recursos Naturales (CIREN), Santiago
Coelho-Moreira J, Brugnari T, Sá-Nakanishi AB, Castoldi R, de Souza CG, Bracht A, Peralta RM (2018) Evaluation of diuron tolerance and biotransformation by the white-rot fungus Ganoderma lucidum. Fungal Biol 122:471–478
Dinamarca MA, Cereceda-Balic F, Fadic X, Seeger M (2007) Analysis of s-triazine-degrading microbial communities in soil using most-probable-number enumeration and tetrazolium-salt detection. Int Microbiol 10:209
Đurović R, Gajić-Umiljendić J, Đorđević T (2009) Effects of organic matter and clay content in soil on pesticide adsorption processes. Pest Fitomed 24:51–57
Flores C, Morgante V, González M, Navia R, Seeger M (2009) Adsorption studies of the herbicide simazine in agricultural soils of the Aconcagua valley, Central Chile. Chemosphere 74:1544–1549
Gao Y, Truong YB, Cacioli P, Butler P, Kyratzis IL (2014) Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym Microb Technol 54:38–44
Ghazali FM, Rahman RNZA, Salleh AB, Basri M (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeterior Biodegrad 54:61–67
Han Y, Chen H (2008) Characterization of β-glucosidase from corn stover and its application in simultaneous saccharification and fermentation. Bioresour Technol 99:6081–6087
Hashmi MZ, Kumar V, Varma A (2017) Xenobiotics in the soil environment: monitoring, toxicity and management. Springer
Huang Q, Zhao Z, Chen W (2003) Effects of several low-molecular weight organic acids and phosphate on the adsorption of acid phosphatase by soil colloids and minerals. Chemosphere 52:571–579
Jensen LC, Becerra JR, Escudey . (2018) Impact of physical/chemical properties of volcanic ash-derived soils on mechanisms involved during sorption of ionisable and non-ionisable herbicides
Kumari K, Prasad J, Solanki IS, Chaudhary R (2018) Long-term effect of crop residues incorporation on yield and soil physical properties under rice - wheat cropping system in calcareous soil. J Soil Sci Plant Nutr 18:27–40
Leskovar D, Othman YA (2018) Organic and conventional farming differentially influenced soil respiration, physiology, growth and head quality of artichoke cultivars. J Soil Sci Plant Nutr
Martínez-Iñigo J, Gibello A, Lobo C, Nande M, Vargas R, Garbi C, Fajardo C, Martín M (2010) Evaluation of the atzB gene as a functional marker for the simazine-degrading potential of an agricultural soil. Appl Soil Ecol 45:218–224
Morgante V, Flores C, Fadic X, González M, Hernández M, Cereceda-Balic F, Seeger M (2012) Influence of microorganisms and leaching on simazine attenuation in an agricultural soil. J Environ Manag 95:S300–S305
Nakatani M, Hibi M, Minoda M, Ogawa J, Yokozeki K, Shimizu S (2010) Two laccase isoenzymes and a peroxidase of a commercial laccase-producing basidiomycete, Trametes sp. Ha1. New Biotechnol 27:317–323
Pinochet D, Clunes J, Gauna C, Contreras A (2018) Reasoned fertilization of potato in response to nitrogen supply in Andisols. J Soil Sci Plant Nutr 18:790–803
Pizzul L, Castillo MdP, Stenström J (2009) Degradation of glyphosate and other pesticides by ligninolytic enzymes. Biodegradation 20:751–759
R Core Team (2018) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed January 2018
Sadzawka A, Carrasco M, Grez R, Mora M, Flores H, Neaman A (2004) Métodos de análisis recomendados para los suelos chilenos. Comisión de Normalización y Acreditación Sociedad Chilena de la Ciencia del Suelo, Santiago
Salem HM, Abdel-Salam A, Abdel-Salam MA, Seleiman MF (2017) Soil xenobiotics and their phyto-chemical remediation, xenobiotics in the soil environment. Springer, pp 267–280
Sannino F, Gianfreda L (2001) Pesticide influence on soil enzymatic activities. Chemosphere 45:417–425
Suja F, Rahim F, Taha MR, Hambali N, Rizal Razali M, Khalid A, Hamzah A (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeterior Biodegrad 90:115–122
Swarcewicz MK, Gregorczyk A (2013) Atrazine degradation in soil: effects of adjuvants and a comparison of three mathematical models. Pest Manag Sci 69:1346–1350
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Funding
This study was funded by “Fondo Nacional De Desarrollo Científico y Tecnológico” [grant numbers 1170931 to C.A. and 3160699 to A.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herrera, H., Palma, G., Almonacid, L. et al. Improving Soil Simazine Dissipation Through an Organic Amendment Inoculated with Trametes versicolor. J Soil Sci Plant Nutr 19, 262–269 (2019). https://doi.org/10.1007/s42729-019-0019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-0019-7