Abstract
The present study was done to isolate and characterize two strains of phosphate solubilizing bacteria from rhizospheres of acacia, sugar beet, and wheat, then determine synergic effects of nanosilica and these strains on the vegetative growth of land cress plant. Isolates identification was performed using physiological, morphological, biochemical tests, and 16S ribosomal ribonucleic acid sequencing. Nanosilica was extracted from Equisetum telmateia and characterized via X-ray diffraction, scanning electron microscopy, dynamic light scattering, Brunauer–Emmett–Teller, and X-ray fluorescence techniques. The size and the purity of extracted silica powder were about 30 nm, 97.5 %, respectively. Two strains, namely, Pseudomonas stutzeri and Mesorhizobium sp. were the most efficient strains to grow and solubilize phosphorus in the presence of 860 mM NaCl and various pH conditions. The highest growth of these two strains was observed at 0.05 and 0.07 ppm of nanosilica. The highest amount of dry weight of shoot and root of land cress plant was recorded with the simultaneous application of these strains in combination with nanosilica. The combination of nanosilica and these strains enhanced the soil nitrogen and phosphorus content and the vegetative growth of land cress plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, in the agricultural sector, the application of plant growth-promoting rhizobacteria (PGPR) as an alternative method for improving plant growth and productivity as well as reducing environmental pollution was increased (Saharan and Nehra 2011). PGPR can increase plant growth by various mechanisms such as phosphate solubilization, phytohormone production, nitrogen fixation, and biocontrol of plant pathogens (Bhattacharyya and Jha 2012; Vessey 2003). Phosphorus (P) is considered as an essential macronutrient for plant development, and it improves some metabolic pathways such as cell growth, respiration, photosynthesis, cell division, and nutrient uptake (Khan et al. 2014; Bhat et al. 2018).
It has been reported that the most common forms of phosphorus found in chemical fertilizers become insoluble due to the formation of strong bonds between phosphorus and calcium in alkaline soils (Rodríguez and Fraga 1999; Kochian 2012). On the other hand, soil microorganisms play an essential role in converting insoluble forms of phosphorus to available forms by alkaline phosphatases and organic acids (Park et al. 2009; Gulati et al. 2010; Sharma et al. 2013). The phosphate solubilizing activity has been already shown in some bacteria including Pseudomonas, Bacillus, Enterobacter, Rhizobium, Mesorhizobium, Burkholderia, Azotobacter, Azospirillum, and Erwinia genera (Rodríguez and Fraga 1999; Vazquez et al. 2000; Vessey 2003; Hu et al. 2006; Perrig et al. 2007; Saharan and Nehra 2011; Bhattacharyya and Jha 2012; Goes et al. 2012). It should be noted that this activity depends on the physical and chemical properties of soil, which are influenced by factors such as the salinity, temperature, and available elements.
Micronutrients (such as Zn, Mn, Fe, and B) and macronutrients (such as N, Ca, P, S, and Mg) are essential constituents for a plant to support its growth, life cycle, and biological functions; however increasing evidences in the literature have demonstrated the effects of non- or quasi-essential elements on plant growth and development. For example, over 150 years passed before, plant scientists have reported the advantages of silicon (Si) in improving plant resistance/tolerance against different biotic and abiotic stresses (Haynes 2014; Liang et al. 2015; Tubana et al. 2016; Deshmukh et al. 2017; Farouk and EL-Metwally 2019; Farouk and Al-Sanoussi 2019). Despite the progress in discovery and characterization of genes responsible for the molecular mechanisms of Si uptake and transport in plants over the past decade (Tubana et al. 2016; Sahebi et al. 2015; Deshmukh et al. 2017), these mechanisms still remain poorly understood and in need of further research.
While, Si as a quasi-essential element, is the second most abundant element in the earth’s crust (the Si content of soils is about 45 wt. %), but the vast majority of this content is not available to plants. Generally, it is believed that Si is taken up from the soil as monosilicic acid [Si(OH)4] by plant roots, transported to leaves and deposited as phytoliths, amorphous silica (SiO2) bodies. Finally, Si transfer takes place between the biomass and the soil solution (Tubana et al. 2016; Deshmukh et al. 2017). It should be noted that the weathering of silicate minerals is an excellent source of dissolved Si (Basile-Doelsch et al. 2005). However, the dissolution rates of silicate minerals are rather slow and governed by several conditions or parameters. Generally, soils are capable to immediately replenishing lost Si in soil solution, but certain types of soil may take some time to replace lost Si even under accelerated mineral weathering. Consequently, fertilization using different sources rich in Si becomes a logical approach. Therefore, research activities are currently focused on improving Si fertilization and Si sources for crop cultivation. Application of Si fertilizers has been shown to enhance plant resistance to diseases and pests significantly, thus contributing to increased food safety, higher production with lower input costs, and reduced negative impacts on environmental health (Haynes 2014; Tubana et al. 2016).
The use of nanotechnology in agriculture has become important because it introduces new sustainable strategies under normal or stress conditions (Iavicoli et al. 2017; Rizwan et al. 2017; Ali et al. 2019). Several papers in the literature have studied the effects of nanosilica on plant growth (Siddiqui and Al-Whaibi 2014; Tripathi et al. 2016; Rastogi et al. 2019; Farouk and EL-Metwally 2019). Nanosilica was found to improve germination as well as fresh and dry weight in a known Si-excluder tomato (Siddiqui and Al-Whaibi 2014). Also, silica nanoparticles were shown to protect wheat seedlings against ultraviolet stress by stimulating the antioxidant defense system (Tripathi et al. 2016). Moreover, mesoporous silica nanoparticles were shown to boost the growth, total protein content, and photosynthesis of lupin and wheat seedlings and to induce no changes in the activity of antioxidant enzymes (Sun et al. 2016).
Nanosilica used in agriculture can be synthesized using different methods. Biological methods are valuable compared to other synthetic methods because produced powders by these methods are more stable and more varied in shape and size (Mittal et al. 2013). Also, biological methods are more compatible with the environment and don’t use toxic chemicals (Karunakaran et al. 2013). The production cost of these powders could be lower than the commercial ones available in view of used raw materials (e.g., plant materials) and low energy requirement of the process. Also, nanosilica can be easily produced from different biological sources. Among the creatures, plants are the best choice because they are readily available and are more suitable for large-scale production. So, the biosynthesize of nanoparticles is of interest with the use of plants, e.g., inactivated plant tissues, plant extracts, and live plants (Mittal et al. 2013).
Besides the evidence of nanosilica can be useful for biotic and abiotic stress tolerance of plants, there is a great passion in recent studies for the use of phosphate solubilizing bacteria (PSB) along with nanosilica to improve plant growth and quality of soil (Rangaraj et al. 2014). Some studies have shown that the nanosilica has a better effect than conventional Si sources on the bacterial population and the amount of nutrient in the soil (Karunakaran et al. 2013). These researches have established that both PBS and nanosilica play a key role in plant growth and development; however, the interactions between bacteria and nanosilica in soil need more studies. Based on this hypothesis, the combined effects of applying nanosilica and PBS on the growth of land cress plant were investigated. Therefore, the objectives of the present study were to (i) extract nanosilica from Equisetum telmateia; (ii) isolate PSB from rhizospheres of acacia, sugar beet, and wheat; and (iii) determine the amount of nitrogen and phosphorus content in the soil and the growth of land cress plant.
2 Materials and Methods
2.1 Isolation of PSB
Thirty-seven bacterial strains were isolated from the rhizospheres of Acacia victoriae, sugar beet, and wheat in different zones of Isfahan province, Iran. The isolation was done according to the method described by Behbahani (2011). To isolate rhizosphere bacteria, adhering soils on roots were shaken to collect the rhizosphere soils. Serially diluted soil samples were plated on a Pikovskaya’s (PVK) agar medium (pH 6.8–7.0) containing tricalcium phosphate (TCP) as a sole phosphorus source for selectively screening bacteria, which can release inorganic phosphate from TCP (Nautiyal et al. 2000). After seven days of incubation at 28 °C ± 2 °C, PSB developed clear zones around colonies. Seven strains with potent activity were selected for further analyses.
2.2 Determination of Phosphate Solubilization Potential
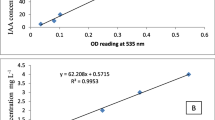
The quantitative and qualitative activities of these seven strains of phosphate solubilization isolates were determined. The quantitative determination was performed based on the solubilization index (SI) in a PVK agar medium. To calculate the value of SI, each isolate was spotted on the PVK agar medium and incubated at 28 °C ± 2 °C for 7 days (Pande et al. 2017). This index was calculated from the ratio of the total diameter (colony + halo zone) to the colony diameter according to the study of Pande et al. (2017). The qualitative analysis of the phosphate solubilization potential of these strains was conducted by determining the available soluble phosphate in a PVK broth medium supplemented with 0.5 vol.% of TCP. Briefly, flasks containing 100 ml of sterilized PVK broth medium were inoculated in triplicate with 1 ml of each bacterial solution at a concentration of 107CFU ml−1. The flasks were incubated at 37 °C on a rotary shaker at 120 rpm. An un-inoculated medium served as the control. After 2 days, the bacterial cell suspensions were centrifuged at about 11,200 xg for 10 min. The phosphorus content of the supernatants was determined using the phosphomolybdate method (Watanabe and Olsen 1965). Finally, based on the value of SI on the PVK agar medium, and the amount of the soluble phosphate in the PVK broth, two isolates were selected as potent phosphate solubilizers to identify and apply in a greenhouse.
2.3 Characterization and Identification of Bacterial Isolates
Two selected PSB isolates with the highest phosphate solubility were identified based on different morphological, biochemical, and genetical tests such as Gram staining, motility, oxidase, catalase, levan, indole, urease, gelatinase, starch hydrolysis, different carbohydrate fermentation, and 16S ribosomal ribonucleic acid (rRNA) (Richardson 2001). For determination of the 16S rRNA sequences of the PSB isolates, the genomic deoxyribonucleic acid (DNA) was extracted according to the study of Moore et al. (1999) using the phenol/chloroform/isoamyl alcohol method, and quantified by electrophoresis with a 1 % wt./vol. Agarose gel (Cinna-Gene, Iran).
Universal primers 27F-YM (5'-AGAGTTGATYMTGGCTCA-3') and 1429R (5'-CGGTTACCTTGTTGTFACGACTT-3') were used for the amplification of 16S rRNA gene by the polymerase chain reaction (PCR) method (Mao et al. 2012). Then, the amplified PCR products of two isolates 16S rRNA gene were sent to Macrogen™ Company (Seoul, South Korea), for sequencing using forward and reverse 27F and 1429R universal primers. The sequences were analyzed, cut, and aligned to establish the consensus sequences with the Vector NTI AdvanceTM 10 software (Lu and Moriyama 2004). In that line, these consensus sequences were compared with the available standard sequences of bacterial lineage in the National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST 2019).
2.4 Effects of Salt and pH on Phosphate Solubilization Capacity and Bacterial Growth
The effects of salt (NaCl) and pH on phosphate solubilization and bacterial growth were tested for each strain on nutrient broth (NB, Merck) media containing various concentrations of NaCl (0, 180, 350, 530, 600, 700, 780, 860, 950, and 1030 mM) and pH tolerance (3–12). The flasks were incubated for 3 days at 30 °C on an orbital shaker at 180 rpm. Sampling was done at 3, 6, 9, 12, 18, 24, 48, and 72 h for the estimation of bacterial growth. This was carried out by taking a 100 ml sample from each Erlenmeyer flask and transferring to an NB medium to perform colony counting after serial dilution. For the quantitative estimation of phosphate solubilizing ability, the strains were harvested by centrifugation at about 20,000 xg for 10 min.
2.5 Extraction of Silica From Equisetum telmateia
Stems of Equisetum telmateia as the source of silica used in this study were obtained from Tonekabon city, Iran, and cut to small pieces. The pieces were washed with deionized (DI) water to remove adhering soil and dust. An acid-leaching step with a hot hydrochloric acid (0.1 M HCl, Merck) solution was used to remove metallic impurities from the dried stems in an oven at 110 °C for 24 h. Then, the stems were cooled, washed, and dried at 100 °C for 24 h, and converted to powder by calcination at 500 °C for 48 h. The obtained powder was executed using an alkali leaching method. For this reason, 20 g of this powder was mixed with 160 ml of sodium hydroxide (2.5 M NaOH, Merck) solution to convert silica into a sodium silicate solution. To remove metal and carbon residues, the solution was filtered. Sulfuric acid (2.5 M H2SO4, Merck) was added to the filtered solution with constant stirring until getting a pH of 2. Then, ammonium hydroxide (NH4OH, Merck) was used to reach pH 8.5 and left for 3.5 h at room temperature. Silica powder with transparent and colorless appearance was refluxed with HCl (6 M) for further purification after 4 h. The powder was washed repeatedly using DI water to make it acid-free. It was then dissolved in NaOH (2.5 M) by continuous stirring and then concentrated H2SO4 was added to adjust pH in the range of 7.5–8.5. The precipitated silica was washed repeatedly with warm DI water until the filtrate becomes completely alkali-free. After the washing process, the silica powder was dried at 50 °C for 48 h in an oven (Assefi et al. 2015).
2.6 Characterization of Extracted Silica
Morphological characteristics of extracted silica were determined by a scanning electron microscope (SEM, ZEISS SIGMA 500 VP). The phase structure of the powder was determined by X-ray diffraction (XRD) method using a Bruker D8 Advance diffractometer. The X-ray fluorescence (XRF, Bruker S4 Pioneer) semiquantitative analysis was used to estimate the presence of different elements in the sample. The Brunauer–Emmett–Teller (BET, BELSORP-mini ӀӀ) method was used to calculate the specific surface area of the powder. Also, the particle size distribution of silica powder was measured by a Horiba SZ-100 series analyzer based on the dynamic light scattering (DLS) method.
2.7 Effect of Different Concentrations of Nanosilica on the Bacterial Growth
The effect of different concentrations of nanosilica (0, 0.01, 0.03, 0.05, 0.07, and 0.1 ppm) on the bacterial growth was studied. Firstly, 50 ml of NB medium was mixed with different concentrations of nanosilica powder. Fifty microliters of each isolate with a concentration of 107CFU ml−1 were added to NB media, separately. Then, the tubes were incubated in a rotary shaker at 200 rpm and 37 °C. Every three hours, 100 μl of eightfold serial dilution culture was grown onto the surface of poured agar plates with a sterile glass rod, and the colonies which developed upon incubation of the plates at 37 °C were counted using the surface plate count method.
2.8 Measurement of Nitrogen and Phosphorus (NP) Content in Soils Treated with Strains and Nanosilica
To investigate the effect of two strains and nanosilica on the soil NP level, some pots containing the silty loam were provided without sterilization to prepare a competitive condition for inoculated strains. The results of the soil analysis used in the experiment are presented in Table 1. Firstly, nine pots containing 500 g soil were uniformly mixed with different concentrations of nanosilica (0, 20, 40, 60, 80, 100, and 120 ppm), and two strains in triplicate, separately. In the next step, each strain separately and in combination were added to nineteen pots containing nanosilica in triplicate (fifty-four pots) to study synergic effects of them on the NP content.
2.9 Effect of Nanosilica and Bacterial Isolates on the Growth of Land Cress Plant
Seeds of land cress plant were sterilized with a sodium hypochlorite solution (0.5 vol. %) and washed with DI water. The sterilized seeds were germinated at 25 °Ϲ under 16 h light and 8 h dark conditions. Eight uniform germinated seeds were planted into each pot. The pot soils were mixed thoroughly with nanosilica and bacterial suspensions, as mentioned in Section 2.8. During the study, no organic fertilizer was added to any of the treatment pots, and the pots were regularly irrigated with tap water. The experiment was done with 28 treatments in 3 replicates. After 1 month, aerial and root parts were washed, separated and dried in an oven at 70 °Ϲ for 48 h, and powdered. Dry weights were measured as g pot−1 (four plants) using a sensitive balance.
2.10 Statistical Analysis
The experimental data were subjected to analysis of variance (ANOVA) using SAS ver. 9.1.3 package. The least significant difference (LSD) means comparison was performed to further explore the differences with significant (P ≤ 0.05) ANOVA results.
3 Results
3.1 Molecular Identification of Bacteria
Among seven potent isolates, the highest values of SI were recorded from isolates 1 and 3 (SI = 2.9 and 3.0). The identification of these two strains was confirmed by 16S rRNA sequence analysis with the comparison of reference strains in the NCBI gene database. The sequence analysis data of the 16S rRNA gene showed 99 % sequence similarity of strains 1 and 3 with Pseudomonas stutzeri DSM 10701 and Mesorhizobium sp. strain S1B, respectively. The 16S rRNA gene sequences obtained in this study were deposited in GenBank under accession numbers MK045742 and MK071654.
3.2 Phosphate Solubilization Activity and Bacterial Growth Estimation
The quantitative approximation of phosphate solubilization was considered based on bacterial growth under different stress conditions. The two strains could grow well in media containing 0 to 780 mM NaCl (Fig. 1). However, both the bacterial growth and the solubilized phosphorus (P) were decreased when cultured in media with increased salt concentration. It became more apparent in media containing 860 mM NaCl. Mesorhizobium sp. was a salt-tolerant strain and grew well in a medium containing 950 mM of NaCl, while the isolate Pseudomonas stutzeri was salt-sensitive. Also, these two strains were able to grow under different simulated pH conditions (pH in the range of 6 to 11), but the optimal range of pH for the bacterial growth was between 7 and 9. Figure 2 shows that Pseudomonas stutzeri has a high ability for solubilizing P at alkaline pH, but Mesorhizobium sp. is sensitive to alkaline pH and has no significant influence on the solubilized P.
3.3 Extracted Nanosilica
The results of the XRF analysis showing the chemical composition of ash obtained from the Equisetum telmateia plant are given in Table 2. From this table, it can be seen that the main compound of the Equisetum telmateia ash after the acid treatment and several washing steps is silica (97.5 wt. %). However, other elements remained, and their total content is about 2.5 wt.%. Another study on Equisetum arvenses calcined at 500 °C showed that the obtained ash before acid treatment contained 59.6 wt.% of silica, and this value increased to 93.5 wt.% after acid treatment (Carneiro 2012).
Figure 3 shows the XRD pattern of the silica powder extracted from the Equisetum telmateia ash. As seen from this figure, there is only a very broad peak in the range of 15 to 30 degrees which indicates the amorphous silica structure. In other words, the absence of high-intensity narrow peaks indicates the absence of silica crystalline structures such as quartz or cristobalite. This result is consistent with the results of the silica powder obtained from the rice husk and the coconut endocrine (Freitas et al. 2000). Other researchers also observed the same XRD pattern for the nanosilica obtained from the rice husk after the acid treatment and washing process (Liou and Wu 2010).
Figure 4 shows the DLS curve of silica powder extracted from the Equisetum telmateia ash after the acid treatment and washing steps. From this figure, the silica powder shows a single population of particles with an average hydrodynamic diameter of about 150 nm.
The size and the morphology of the extracted silica powder were also investigated by the SEM analysis, as shown in Fig. 5. This micrograph illustrates that the silica particles have a spherical shape and a narrow size distribution with an average diameter of about 30 nm. Interestingly, the average particle size obtained from the SEM analysis as a direct method is much smaller than the DLS method, which measures the hydrodynamic size of particles. However, the silica nanoparticles are in the agglomerated form. Another study on the silica nanoparticles obtained from the Equisetum arvenses ash showed that the average size of the agglomerated particles was about 300 nm (Carneiro et al. 2015).
Also, the results of the BET analysis of the extracted silica powder show that the silica powder has a specific surface area of about 410 m2/g with an average pore diameter of about 12 nm. In comparison, the value of the specific surface area for the extracted silica powder in this study is relatively high. For example, this value for Equisetum arvenses calcined at 500 °C before and after the acid treatment was reported as 54, and 330 m2/g, respectively (Carneiro 2012). The high value of the specific surface area for the extracted silica powder in this work could be related to the utilized acid treatment and the several washing steps which led to the significantly leaching of impurities from the silica structure, and forming of the porosity.
3.4 Effect of Nanosilica Concentration on the Bacterial Growth
Figure 6a shows the effect of different concentrations of nanosilica on the total Pseudomonas stutzeri and Mesorhizobium sp. count. The increasing trend of Pseudomonas stutzeri count with an increase in the dosage of nanosilica was observed. The growth observed was time and concentration dependent. Among the different concentrations of nanosilica, the highest Pseudomonas stutzeri population was obtained at 0.07 ppm treatment and followed by 0.05 ppm. However, the 0.1 ppm concentration resulted in a decline in Pseudomonas stutzeri population. A similar trend in media containing Mesorhizobium sp. populations with the different concentrations of nanosilica was also observed with respect to the control sample (Fig. 6b). However, the effect of nanosilica on the Pseudomonas stutzeri growth was significantly more than Mesorhizobium sp.
3.5 Effect of Nanosilica and Phosphate Solubilizing Isolates on Soil NP Level
The results demonstrated that both nanosilica and strains could significantly (P < 0.05) increase the amount of solubilized nitrogen and phosphorus in the soil (Fig. 7a to d). The comparison study using the independent effect of the bacterial isolates showed that the bacteria application resulted in an increase in the soil NP content compared to the control (Fig. 7a and b). Also, the NP content of soils inoculates with Pseudomonas stutzeri alone or in conjunction with the inoculation of Mesorhizobium sp. was significantly higher than soils containing Mesorhizobium sp. alone (Fig. 7a and b). The comparison study of the different concentrations of nanosilica demonstrated that the application of nanosilica could increase NP levels in the soil. The highest NP content was obtained at 100 ppm nanosilica (Fig. 7c and d). The results of the interaction of nanosilica and strains indicated that the soil inoculates with Pseudomonas stutzeri alone or in conjunction with the inoculation of Mesorhizobium sp. had the highest NP content in all treatments at 100 ppm nanosilica concentration (Fig. 7e and f).
The effects of bacterial isolates (a, b), nanosilica concentrations (c, d), and the interaction of nanosilica and bacteria (e, f) on the amount of solubilized nitrogen and phosphorus in the soil. Mean values for bars with the same letter above them are not significantly different (the LSD test, P ≤ 0.05). Error bars are standard errors (n = 3)
3.6 Effect of Nanosilica and Phosphate Solubilizing Isolates on the Growth of Land Cress Plant
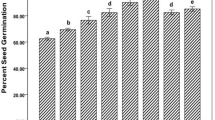
The effects of the bacterial strains and different concentrations of nanosilica on the dry weight of the shoot and root of land cress plant are shown in Fig. 8a to d. The results demonstrated that the inoculation of soil with these strains could improve the plant growth compared to non-inoculated control (Fig. 8a and b). Analysis of variance showed that the effect of treatments on the dry weight of the shoot and root at 5 % level was significant. The highest dry weights of shoot and root were recorded in soils inoculated with Pseudomonas stutzeri in combination with Mesorhizobium sp. In the case of a single strain, the highest dry weights of shoot and root were achieved in a soil inoculated with Pseudomonas stutzeri.
The effects of bacterial isolates (a, b), nanosilica concentrations (c, d), and the interaction of nanosilica and bacteria (e, f) on the dry weight of shoot and root of land cress. Mean values for bars with the same letter above them are not significantly different (the LSD test, P ≤ 0.05). Error bars are standard errors (n = 3)
The effect of nanosilica on the plant growth (Fig. 8c and c) showed that the nanosilica at different concentrations led to an increase in plant growth in a dose-dependent manner. The highest plant dry weight was achieved at a concentration of 100 ppm and followed by 80 and 120 ppm. The synergistic effect of the strains in combination with nanosilica has been shown (Fig. 8e and f). The highest amount of shoot and root dry weight was recorded with simultaneous application of Pseudomonas stutzeri and Mesorhizobium sp. Since the root growth increases the plant nutrition and growth of shoot, these results were nearly correlated with each other.
4 Discussion
Although phosphorus is a key limiting factor in agriculture production, it is almost insoluble in soil. The microbial activation seems to be an effective way to solve the solidified phosphorus in soil. The inoculation of phosphate solubilizers in P-deficient soil has been reported to increase the available P content in soil and P uptake in plants (Kumar and Narula 1999). The PSB can be regarded as one kind of PGPR, which are usually considered as alternatives to conventional fertilizers (Adesemoye et al. 2009; Yu et al. 2011; Majeed et al. 2015).
In this study, Pseudomonas stutzeri and Mesorhizobium sp. were isolated using the PVK medium. The PSB solubilize inorganic phosphates by several mechanisms, including the production of organic acids, polysaccharides, and phosphatase enzymes (Rodríguez et al. 2000). The acidification of culture indicated the production of organic acid that seemed to be the primary mechanism for phosphate solubilization (Vassilev et al. 2006; Ma et al. 2009). In this work, the decline in pH was also occurred during phosphate solubilization by the PSB that confirmed the production of organic acid.
In general, the investigated Pseudomonas stutzeri and Mesorhizobium sp. had good growth performance at various concentrations of NaCl. These bacteria showed a similar pattern of pH tolerance. Also, these strains tolerated high salt concentrations. The high concentration of NaCl solution can give high competitive value in the rhizosphere to survive in harsh environmental conditions. This result was somewhat according to the studies of Zhu et al. (2011) and Haile et al. (2016). The sensitive isolate for salt tolerance was Pseudomonas stutzeri, which was able to grow up to 780 mM salt concentration. Mesorhizobium sp. were able to grow well up to 1030 mM NaCl, which was similar to the work of Amardip and Ghosh (2011), who found that isolates could tolerate the high salt concentration.
The effect of the application of different concentrations of nanosilica showed an increase in the bacterial count with an increase in the concentration of the nanosilica up to applied in comparison to the control treatment. The increase in the population of microorganisms due to the effect of nanosilica (0.07 ppm) was in accordance with an earlier study (Rangaraj et al. 2014). These observations reveal that the nanosilica may either act as a substrate for microorganisms or a stimulant that resulted in an increased microbial population. It has been reported that the application of nanosilica doubled the colony forming unit, favoring the beneficial effect on the bacterial population (Karunakaran et al. 2013). The increase in the NP content was observed with the application of nanosilica with respect to control, which may be either due to an enhanced population of nitrogen fixers and other P-solubilizing microbes in the soil. This increase was more observed at a concentration of 100 ppm nanosilica. The plants treated with nanosilica and bacterial isolates showed a significant increase in the dry weight of shoot and root, as compared to the control. The highest plant dry weight was observed in nanosilica at 100 ppm concentration, which indicates a lower dosage of nanosilica could promote the beneficial growth of land cress plant. It should be noted that at in a high concentration of nanosilica in a culture medium, the possibility of direct contact of silica particles with the bacterial cell wall increases, and hence, the bacterial growth is reduced; however, the soil conditions are quite different, and all of the silica particles are not in direct contact with the cell wall. Therefore, they can be used in the soli at much higher concentrations than in the culture medium. It also appears that nanosilica at a concentration of 120 ppm has no toxic effects on plant growth and only reduces it.
Some researchers reported that nanosilica improved leaf fresh and dry weight and chlorophyll content under salinity stress (Siddiqui and Al-Whaibi 2014; Kalteh et al. 2018). Similar results demonstrated that the treatment of coriander and wheat plants with Si fertilizer could improve plant growth and photosynthetic pigments (Al-Garni et al. 2019). Another study showed that nanosilica could enhance seed germination of cucumber and the development of seedlings under elevated concentrations of Na+ (Na+-derived salinity) (Alsaeedi et al. 2018). Silicon supply was also used to increase inorganic P in the leaves and tubers under low soil P conditions (Soratto et al. 2018). Our results also demonstrated that the highest growth of land cress plant was obtained in pots containing nanosilica and PSB in combination.
5 Conclusions
The results demonstrated that nanosilica significantly enhanced the nitrogen and phosphorus content of the soil. By inoculation, nanosilica of 100 ppm showed the best growth enhancement of land cress plant in terms of the increase of dry weight. Thus, nanosilica extracted from Equisetum telmateia is promising to apply as a growth promoter and elicitor for plants as well as an environmentally friendly agrochemical for sustainable development of agriculture. The combined application of nanosilica and phosphate solubilizing bacteria isolates at the same concentration showed a higher effect as compared to the control and can be used as efficient inoculants for integrated nutrient management for plant production under sustainable agriculture.
References
Adesemoye A, Torbert H, Kloepper J (2009) Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microbial ecology 58:921–929
Al-Garni SM, Khan MMA, Bahieldin A (2019) Plant growth-promoting bacteria and silicon fertilizer enhance plant growth and salinity tolerance in Coriandrum sativum. Journal of Plant Interactions 14:386–396
Ali S, Rizwan M, Hussain A, ur Rehman MZ, Ali B, Yousaf B, Wijaya L, Alyemeni MN, Ahmad P (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiology and Biochemistry 140:1–8
Alsaeedi A, El-Ramady H, Alshaal T, El-Garawani M, Elhawat N, Al-Otaibi A (2018) Exogenous nanosilica improves germination and growth of cucumber by maintaining K+/Na+ ratio under elevated Na+ stress. Plant physiology and biochemistry 125:164–171
Amardip S, Ghosh A (2011) Characterization, identification & cataloguing of agriculturally important microorganisms isolated from selected wetland and rain-fed ecosystem of Bihar. Asian Journal of Experimental Biological Sciences 2:575–582
Assefi M, Davar F, Hadadzadeh H (2015) Green synthesis of nanosilica by thermal decomposition of pine cones and pine needles. Advanced Powder Technology 26:1583–1589
Basile-Doelsch I, Meunier JD, Parron C (2005) Another continental pool in the terrestrial silicon cycle. Nature 433:399–402
Behbahani M (2011) Investigation of biological behavior and colonization ability of Iranian indigenous phosphate solubilizing bacteria. Scientia horticulturae 124:393–399
Bhat SA, Singh J, Vig AP (2018) Earthworms as organic waste managers and biofertilizer producers. Waste and Biomass Valorization 9:1073–1086
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World Journal of Microbiology and Biotechnology 28:1327–1350
BLAST. http://www.ncbi.nlm.nih.gov. 2019
Carneiro, M. (2012) Obtaining nano silica from Equisetum arvenses L and their use in the modification of veneers of schizolobium parahyba var. amazonicum (Huber ex Ducke) wood, Ph. D. thesis, Federal University of Parana, Curitiba, Brazil (in Portuguese).
Carneiro ME, Magalhães WL, BOLZON DE MUÑIZ, G., Nisgoski, S., and Satyanarayana, K. G. (2015) Preparation and characterization of nano silica from Equisetum arvenses. Embrapa Florestas-Artigo em periódico indexado (ALICE).
Deshmukh RK, Ma JF, Bélanger RR (2017) Role of silicon in plants. Frontiers Media, Lausanne
Farouk S, Al-Sanoussi AJ (2019) The role of biostimulants in increasing barley plant growth and yield under newly cultivated sandy soil. Cercetări Agronomice în Moldova (Agronomical Research in Moldavia) LII(178):114-125. https://doi.org/10.2478/cerce-2019-0012.
Farouk S, EL-Metwally IM (2019) Synergistic responses of drip-irrigated wheat crop to chitosan and/or silicon under different irrigation regimes. Agricultural Water Management 226:105807. https://doi.org/10.1016/j.agwat.2019.105807
Freitas JC, Emmerich FG, Bonagamba TJ (2000) High-resolution solid-state NMR study of the occurrence and thermal transformations of silicon-containing species in biomass materials. Chemistry of materials 12:711–718
Goes K, de Castro Fisher ML, Cattelan AJ, Nogueira MA, Portela de Carvalho CG, Martinez de Oliveira AL (2012) Biochemical and molecular characterization of high population density bacteria isolated from sunflower. J. Microbiol. Biotechnol 22:437–447
Gulati A, Sharma N, Vyas P, Sood S, Rahi P, Pathania V, Prasad R (2010) Organic acid production and plant growth promotion as a function of phosphate solubilization by Acinetobacter rhizosphaerae strain BIHB 723 isolated from the cold deserts of the trans-Himalayas. Archives of microbiology 192:975–983
Haile, D., Mekbib, F., and Assefa, F. (2016) Isolation of phosphate solubilizing bacteria from white lupin (Lupinus albus L.) rhizosphere soils collected from Gojam, Ethiopia, J Fertil Pestic 7, 2.
Haynes RJ (2014) A contemporary overview of silicon availability in agricultural soils. J. Soil Sci. Plant Nutr 177:831–844
Hu X, Chen J, Guo J (2006) Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain. Zhejiang, China, World journal of Microbiology and Biotechnology 22:983–990
Iavicoli I, Leso V, Beezhold DH, Shvedova AA (2017) Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol 329:96–111
Kalteh M, Alipour ZT, Ashraf S, Marashi Aliabadi M, Falah Nosratabadi A (2018) Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. Journal of Chemical Health Risks 4
Karunakaran G, Suriyaprabha R, Manivasakan P, Yuvakkumar R, Rajendran V, Prabu P, Kannan N (2013) Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET nanobiotechnology 7:70–77
Khan, M. S., Zaidi, A., and Ahmad, E. (2014) Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms, In Phosphate solubilizing microorganisms, pp 31-62, Springer.
Kochian LV (2012) Plant nutrition: rooting for more phosphorus. Nature 488:466
Kumar V, Narula N (1999) Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biology and Fertility of Soils 28:301–305
Liang Y, Nikolic M, Bélanger R, Gong H, Song A (2015) Silicon in agriculture. Springer, Dordrecht
Liou T-H, Wu S-J (2010) Kinetics study and characteristics of silica nanoparticles produced from biomass-based material. Industrial & Engineering Chemistry Research 49:8379–8387
Lu G, Moriyama EN (2004) Vector NTI, a balanced all-in-one sequence analysis suite. Briefings in bioinformatics 5:378–388
Ma Y, Rajkumar M, Freitas H (2009) Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. Journal of Environmental Management 90:831–837
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Frontiers in microbiology 6:198
Mao D-P, Zhou Q, Chen C-Y, Quan Z-X (2012) Coverage evaluation of universal bacterial primers using the metagenomic datasets. BMC microbiology 12:66
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnology advances 31:346–356
Moore E, Arnscheidt A, Krüger A, Strömpl C, Mau M (1999) Simplified protocols for the preparation of genomic DNA from bacterial cultures. Molecular microbial ecology manual 1:1–15
Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R, Verma D (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiology Letters 182:291–296
Pande A, Pandey P, Mehra S, Singh M, Kaushik S (2017) Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. Journal of Genetic Engineering and Biotechnology 15:379–391
Park KH, Lee CY, Son HJ (2009) Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Letters in applied microbiology 49:222–228
Perrig D, Boiero M, Masciarelli O, Penna C, Ruiz O, Cassán F, Luna M (2007) Plant-growth-promoting compounds produced by two agronomically important strains of Azospirillum brasilense, and implications for inoculant formulation. Applied microbiology and biotechnology 75:1143–1150
Rangaraj S, Gopalu K, Muthusamy P, Rathinam Y, Venkatachalam R, Narayanasamy K (2014) Augmented biocontrol action of silica nanoparticles and Pseudomonas fluorescens bioformulant in maize (Zea mays L.). RSC Advances 4:8461–8465
Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živčák M, Ghorbanpour M, El-Sheery NI, Brestic M (2019) Application of silicon nanoparticles in agriculture. 3. Biotech 9:90
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Functional Plant Biology 28:897–906
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Rehman MZ, Farid M, Abbas F (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology advances 17:319–339
Rodríguez H, Rossolini GM, Gonzalez T, Li J, Glick BR (2000) Isolation of a gene from Burkholderia cepacia IS-16 encoding a protein that facilitates phosphatase activity. Current microbiology 40:362–366
Saharan B, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:30
Sahebi M, Hanafi MM, Siti Nor Akmar A, Rafii MY, Azizi P, Tengoua FF, Nurul Mayzaitul Azwa J, Shabanimofrad M (2015) Importance of silicon and mechanisms of biosilica formation in plants. BioMed research international 2015:396010
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi journal of biological sciences 21:13–17
Sun D, Hussain HI, Yi Z, Rookes JE, Kong L, Cahill DM (2016) Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 152:81–91
Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK (2016) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–80
Tubana BS, Babu T, Datnoff LE (2016) A review of silicon in soils and plants and its role in US agriculture: history and future perspectives. Soil Sci 181:393–411
Vassilev N, Medina A, Azcon R, Vassileva M (2006) Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake. Plant and Soil 287:77
Vazquez P, Holguin G, Puente M, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biology and Fertility of Soils 30:460–468
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant and soil 255:571–586
Watanabe F, Olsen S (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil 1. Soil Science Society of America Journal 29:677–678
Yu X, Liu X, Zhu TH, Liu GH, Mao C (2011) Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biology and Fertility of Soils 47:437–446
Zhu, F., Qu, L., Hong, X., and Sun, X. (2011) Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China, Evidence-Based Complementary and Alternative Medicine 2011.
Acknowledgment
The authors appreciate the financial support of this investigation by the research council of the University of Isfahan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boroumand, N., Behbahani, M. & Dini, G. Combined Effects of Phosphate Solubilizing Bacteria and Nanosilica on the Growth of Land Cress Plant. J Soil Sci Plant Nutr 20, 232–243 (2020). https://doi.org/10.1007/s42729-019-00126-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00126-8