Abstract
The main aim of this study was to investigate how phosphorous (P) release would change after the addition of combined sinfluence upon soil properties sugarcane residues – derived biochar (B) and P fertilizer in a calcareous clay loam soil after 7 and 120 days of incubation. Biochar was produced from sugarcane residues (S) at the temperature of 400 °C under limited oxygen condition. A full-factorial experiment was performed in a completely randomized design to evaluate the effects of amendment (without amendment, 1% S, and 1% B) and P (0 and 50 mg kg−1) on availability and P release characteristics over incubation period (7 and 120 days). After incubation, the kinetics of P release in soils was determined by successive extraction with Olsen solution in a period of 2 to 768 h at 25 ± 1 °C. Results showed that the difference between cumulative P release in soil treated with S and B, at two incubation times, was not significant (P > 0.05). At both times, the cumulative P release increased by 5 up to 23% as influenced by amendments and P fertilizer. Difference between treatments at 7 and 120 days of incubation was not significant (P > 0.05). The lowest and highest initial amount of P release was found in control soil (14.62 mg kg−1 h−1) and soil treated with P + B (38.26 mg kg−1 h−1), respectively. The maximum and minimum rates of P release were in control soil (0.328 (mg kg−1)−1) and soil treated with P + B (0.203 (mg kg−1)−1), respectively. Also, the lowest and highest initial release rates were observed in control soil (4.79) and soil treated with P + B (7.76), respectively. Biochar addition to soil treated with P increased initial amount of P release and initial release rate of P. Therefore, addition of sugarcane residues –derived biochar to soil treated with P fertilizer can increase P availability in similar calcareous soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus has many roles in plants and living organisms existing in an extremely low concentration in the soil solution and its uptake by plant leads to reduction of phosphorous (P) availability (Hosseinpur et al. 2012; Moghimi et al. 2018). The chemical reactions of P with different soil components cause a small portion of the chemical fertilizer P to remain available (Jalali 2006; Hosseinpur et al. 2012). The synergistic effect of the P fertilizes and organic fertilizers on the available P was demonstrated (Delgado and Torrent 2000; Fathi Gerdelidani and Mirseyed Hosseini 2018; Moghimi et al. 2018). Organic fertilizers introduce both available essential nutrients and organic matter in soils leading to an increase in soil fertility (Audette et al. 2016).
The accessibility of P from fertilizers in soils is influenced by the rate of P release from soils because of equilibration of P between the solid and solution phases (Toor and Bahl 1999). The P ion concentration in the solution phase is determined by reaction to solid phase such as adsorption, release, and dissolution-precipitation (McDowell and Sharpley 2003). Application of chemical fertilizers P in calcareous soils form insoluble Ca2+-P compounds (Iyamuremye and Dick 1996; Alvarez et al. 2004; Schneider and Haderlein 2016) which have little availability and efficiency for plants (Fathi Gerdelidani and Mirseyed Hosseini 2018). Therefore, the rate of P release from calcareous soils can remarkably influence on P fertility (Jalali and Ahmadi Mohammad Zinli 2011). The change in soluble P concentration with time was significantly related to the rate of P release from the P minerals to the soil solution. Therefore, the kinetics investigations of P release from soil is valuable in evaluating the accessibility of soil P (Shariatmadari et al. 2006). Phosphorus release characteristics was investigated by many researchers (Toor and Bahl 1999; McDowell and Sharpley 2003; Shariatmadari et al. 2006; Nafiu 2009; Jalali and Ahmadi Mohammad Zinli 2011) and in many studies, the effect of pyrolysis on feedstock and feedstock-derived biochar was investigated (Wang et al. 2015; Liang et al. 2018) but limited information is still available on the effect of biochar on the kinetics of P release in calcareous soils.
Biochar is a carbon-rich substance that is produced from pyrolysis of feedstock in limited or no oxygen condition. Because of biochar’s properties (increasing soil cation exchange capacity (CEC), organic carbon, enzyme activities, and available nutrients), there is an interest to apply biochar as soil amendment for supplying essential nutrients such as P, in recent years (Amin and Eissa 2017; Fathi Gerdelidani and Mirseyed Hosseini 2018; Liang et al. 2018; Hong and Lu 2018). Meanwhile, P may be released from organic fertilizers more rapidly than it can be used by crops (Liang et al. 2013; 2018). Liang et al. (2014) reported that the cumulative amount of released P from manure - derived biochar reduced 76% in comparison with manure. In some studies, the release kinetics of P from different biochars was investigated (Wang et al. 2015 and Liang et al. 2018). Liang et al. (2014) concluded that the slow but steady release of P from soils treated with biochar caused to this amendment as a possible slow released P amendment.
Detailed information of the biochar effect on the P cycle in soils is still scanty (Hong and Lu 2018). Interaction mechanism between biochar and P fertilizer should be better understood to show biochar as a soil amendment, especially in calcareous soils (Lentz and Ippolito 2012; Jin et al. 2016). This study aimed to investigate changes in kinetics characteristics of P release after adding combined sugarcane residues –derived biochar and P fertilizer. The hypotheses tested were as follows: (1) biochar addition to the soil will modify rate and cumulative amount of P release in 768 h and (2) the biochar effect will differ during incubation period (7 and 120 days).
2 Materials and Methods
2.1 Soil Sampling and Analysis
A soil (0–30 cm) was selected from Typic Calcixerepts of the Shahrekord University (50° 49′ E, 32° 21′ N) that is located in the Chaharmahal-Va-Bakhtiari Province, Central Iran. The soil sample was air-dried and ground to pass through a 2-mm sieve for future studies. Electrical conductivity (EC), pH, organic carbon (OC), cation exchange capacity (CEC), calcium carbonate equivalent (CCE), total N, available P (P Olsen), and total P were determined adopting standard methods (Sparks et al. 1996). Particle size distribution of soil was determined by the hydrometer method (Gee and Bauder 1986).
2.2 Biochar Production
In this study, sugarcane residues were used to produce biochar collected from a sugarcane farm in Khuzestan Province, Iran. The sugarcane residues were air-dried at room condition, and then them was cut into small pieces and pyrolized at the temperature of 400 °C for 2 h under limited oxygen condition. The sugarcane residues and their biochar were sieved (< 2 mm). Electrical conductivity and pH of sugarcane residues and their biochar were measured in a 1 to 5 amendment to deionized water. Total C, N, and CEC of amendments were determined (Sparks et al. 1996). The yield of biochar was calculated by dried matter of biochar to the dried matter of sugarcane residues.
2.3 Experimental Design and Incubation Study
A completely randomized design with 3 × 2 full-factorial treatment combination was performed in 3 replicates with the following factors: (1) amendment (without amendment, sugarcane residues (S), and B at 1% (w/w), equivalent to 25 Mg ha−1, based on the depth (0.2 m) and soil bulk density (1250 kg m−3)) and (2) P fertilizer (0 and 50 mg kg−1 P as Ca(H2PO4)2.H2O, equivalent to 125 kg ha−1, based on the above depth and soil bulk density) over the incubation period (7 and 120 days). All amounts of amendments and soil were calculated based on dry weight. Three thousand grams of air-dried soil (< 2 mm) was filled in each jar, mixed with the amendments and P fertilizer. Deionized water was used to adjust soil moisture content at 80% field capacity. The soils were incubated for 120 days at 25 ± 1 °C. After 7 and 120 days of incubation, soil samples were air-dried and used for other analysis.
Available P (P Olsen) was measured by the 0.5 M NaHCO3 (pH = 8.2) (Sparks et al. 1996) at 7 and 120 days of incubation.
2.4 Kinetics of P Release
Successive extraction was performed to kinetics study of P release in the studied soils. One gram of incubated soils (36 tubes) suspended in 20 ml Olsen solution (Toor and Bahl 1999) was equilibrated at 25 ± 1 °C for 2, 4, 8, 24, 48, 72, 96, 240, 336, 456, 600, and 768 h by shaking for 15 min before incubation and before suspensions was centrifuged (3000 rpm). The P in the extracts was measured by the colorimetric method (Murphy and Riley 1962). Different kinetics models (Table 1) were fitted on cumulative concentration of P release. Coefficient of determination (R2) was calculated as follows:
Moreover, standard error of the estimate (SEE) (estimate of cumulative concentration of P release by the kinetics models) was calculated as follows:
where P and P* represent the determined and calculated concentration of P in soil at selected time t, respectively, and n is the number of data points evaluated.
2.5 Statistical Analysis
Two-way repeated measures ANOVA was performed to analyze the effect of amendments and P fertilizer on available P and cumulative amount of P release over the incubation period after treatments. Treatment means were separated at the 5% significance level using Duncan’s multiple range tests. Pearson correlation was performed between available P and parameters of kinetics models. Statistical analysis of data was performed by Statistica 8.
3 Results
3.1 Soil and Biochar Properties
Table 2 shows some properties of soil, biochar, and sugarcane residues. The soil has low OC (0.45%) and calcareous soil (CCE = 34%). Total and available P in the biochar increased 20 and 19%, respectively, compared with sugarcane residues. The P concentration in biochar was higher than sugarcane residues because the volume of residues was reduced 60 up to 90% during pyrolysis process (Brown 2009). The pH of sugarcane residues was equal to 6.5, while their biochar was equal to 8.8. Biochar had an alkaline pH due to alkalis materials formed during pyrolysis process (Yuan et al. 2011). The EC values of biochar and sugarcane residues were equal to 12.6 and 5.0 dS m−1, respectively. Therefore, EC of biochar was 2.5 times higher than sugarcane residues.
3.2 P Availability
The results of ANOVA showed that the effect of amendment, P fertilizer, and time on available P was significant (P < 0.01) (data not shown). The results of Fig. 1 showed that available P in soil treated with P increased significantly (P < 0.05) by the biochar application. In contrast, available P in soil treated with P did not change significantly (P > 0.05) after the application of sugarcane residues. The difference between available P in soils treated with B was not significant (P > 0.05) at 7 and 120 days of incubation.
Effect of biochar addition on available P (mg kg−1) in the soils. Values are mean ± standard errors. Different letters show significant difference (P < 0.05) between means of treatments by Duncan’s multiple range test. B biochar, S sugarcane, P phosphorous. Initial (t = 0) is concentration of Olsen-P in soil + intrinsic Olsen-P in amendments or 50 mg kg−1 P (Table 2)
Total P addition from 1% sugarcane residues and biochar addition to the soils was equal to 5.75 ± 0.01 and 6.89 ± 0.02 mg kg−1, respectively. However, the results of Fig. 1 demonstrate that compared with the control soil, available P increased in soil treated with biochar (11.1 ± 0.07 and 12.3 ± 0.04 mg kg−1 at 7 and 120 days of incubation, respectively). Therefore, available P was augmented in biochar -amended soil more than introduced P as biochar to soil.
Available P in soil treated with S increased 9.3 ± 0.06 and 3.1 ± 0.04 mg kg−1 at 7 and 120 days of incubation. Therefore, available P had a more moderate increase in sugarcane-amended soils than added P as sugarcane to the soil after 120 days of incubation. Available P was remarkably lower (P < 0.05) in untreated soils with P fertilizer than in treated soils with P fertilizer at all times (Fig. 1). Phosphorus extracted with Olsen method reduced (P < 0.05) during another incubation time, compared with the 7-day period except soil treated with B.
3.3 Characteristics of P Release
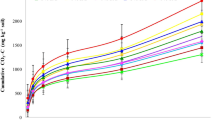
Figure 2 shows the plot of successive extraction of P from the amended soils. The amounts of P release differed among the soils treated with amendments. Figure 2 demonstrates that release of P from all soils was rapid at initial few hours and then continued slowly until the apparent equilibrium was attained. Result showed that cumulative amount of P release ranged from 122 ± 3 to 150 ± 3 mg kg−1. The highest concentration of P release was found in soil treated with P + B (Fig. 3). At both times, the cumulative concentration of P release increased by 5 to 23% as modified by the application of amendments and P fertilizer. The difference between cumulative P release in soils treated with S and B was not significant (P > 0.05) at two incubation times. In comparison with 7 days of incubation, concentration of P release from soil treated with P decreased 7% at 120 days of incubation. The difference between P release in treated soil with B and S was not significant (P > 0.05). Results showed that the cumulative amount of P released in soil treated with P and amendments was higher than other soils (soils treated with S and B).
Different kinetics mathematical models (Table 1) were tested for modeling release of P from the soils into Olsen solution. Among them, power function (averaged R2 = 0.989–0.992, SEE = 4.06–4.63 mg kg−1) and parabolic diffusion equation (R2 = 0.969–0.990, SEE = 3.52–6.14 mg kg−1) can describe P release characteristics at both incubation times (Table 3).
Table 4 shows the coefficients of parabolic diffusion and power function equations. The parabolic diffusion model may be applied to describing diffusion-controlled phenomena (Sparks 1989). The “a” and “R” coefficients in parabolic diffusion are initial concentration of P and diffusion rate constant, respectively (Toor and Bahl 1999). The minimum and maximum amounts of “a” coefficient in parabolic diffusion equation were observed in control soil (20.33 mg kg−1) and soil treated with P + B (51.73 mg kg−1), respectively. This coefficient in soils treated with B and S was lower than those in other treatments. The “R” coefficients in control soil (4.13 mg kg−1 h−1/2) and soil treated with P + B (3.82 mg kg−1 h−1/2) were higher than those in other soils. The “R” in soil treated with P + B (3.82 mg kg−1 h−1/2) was higher than that in soil treated with P + S (3.78 mg kg−1 h−1/2) and soil treated with P (3.73 mg kg−1 h−1/2).
The power function equation can be applied to describing diffusion process (Havlin et al. 1985). The two constants in the power function can be considered initial indicators of the amount of P release (a) and the rate of P release (b) (McDowell and Sharpley 2003). In power function equation, the lowest and highest initial concentration of P release (“a” coefficient) were observed in control soil (14.62 mg kg−1 h−1) and soil treated with P + B (38.26 mg kg−1 h−1). The a coefficient in soil treated with P + S was equal to 35.07 mg kg−1 h−1. This coefficient in soil treated with B (27.06 mg kg−1 h−1) was close to soil treated with S (27.21 mg kg−1 h−1). The rate of P release (“b” coefficient) ranged from 0.203 (mg kg−1)−1 (soil treated with P + B) up to 0.328 (mg kg−1)−1 (control soil). This coefficient in soil treated with P + S was equal to 0.211 (mg kg−1)−1. The rate of P release in soil treated with S and soil treated with B was equal to 0.235 and 0.236 (mg kg−1)−1, respectively.
The “a × b” coefficient is used as initial release rate estimation (Allen et al. 1995; Motaghian and Hosseinpur 2013). The highest value of “a × b” was observed in soil treated with P + B, P + S, P, B, and S, respectively. The initial release rate in soil treated with P + B was 62% higher than control soil. Also, the initial release rate in soil treated with P + B was 11% higher than soil treated with P. In control soil, the initial release rate was reduced after 120 days of incubation (4.79) compared with 7 days of incubation (4.60). This parameter in soil treated with P was reduced by 10% after 120 days of incubation. In soil treated with P + S, “a × b” was equal to 7.41 and 7.06 at 7 and 120 days of incubation. Also, “a × b” in soil treated with P + B was 7.06 at both incubation times.
The “a” coefficient is as an indicator of initial P release concentration in power function and parabolic diffusion equations. In this study, correlation between the “a” coefficient in power function and parabolic diffusion equations was significant (r = 0.98, p < 0.01). Therefore, by raising its value in soil, concentration of P release rises.
3.4 Relation Between the Characteristics of P Release with Available P
Table 5 shows the correlations between the parameters of kinetics models and available P. It shows that significant correlation was observed between “a” coefficient in power function (0.932, P < 0.01) and parabolic diffusion (0.922, P < 0.01) with available P. The b coefficient in power function equation significantly correlated with Olsen-P (− 0.856, P < 0.01), while correlation between the “R” coefficient in parabolic diffusion equation and Olsen-P was not significant (− 0.396, P > 0.05). Significant correlation was found between concentration of P release and P extracted adopting Olsen method (0.921, P < 0.01).
4 Discussion
Results showed that biochar derived from sugarcane residues was more effective than sugarcane residues in enhancing available P in soils treated with P fertilizer. In agreement with obtained results, Zolfi-Bavariani et al. (2016) reported that available P in calcareous soil treated with poultry manure (33.4 mg kg−1) was lower than the soil treated with its alkaline biochar (87.2 mg kg−1) at 1 to 150 days of incubation. More than 80% of the P added to calcareous soils becomes unavailable because of adsorption and precipitation and converted to the organic P (Holford 1997). Wang et al. (2015) reported that Ca2+ and Mg2+ released from soil treated with poultry litter was higher than the soil treated with its biochar (produced at 400 °C) in 30 days of sequential extraction. Also, Khadem and Raiesi (2019) reported that microbial population in calcareous soils treated with maize residue was higher than the soils treated with its biochar. Therefore, biochar increases available P compared with sugarcane residues mainly by (a) reducing Ca2+ and Mg2+ release through immobilization of these metals during pyrolysis (Wang et al. 2015); (b) higher concentration of P compared with sugarcane residues (Table 2); and (c) increasing microbial population in soil treated with feedstock than soil treated with biochar (Khadem and Raiesi 2019), hence immobilization of P in soil treated with feedstock.
Phosphorous extracted by Olsen in soils treated with S and B increased after 7 days of incubation (Fig. 1). Singh Mavi et al. (2018) observed available P increased with increasing rate of rice residue – derived biochar application. This may be attributed to higher CO2 release from the decay of B and S (Smith et al. 2010; Ippolito et al. 2016; Yan et al. 2018), phosphatase activity (Jin et al. 2016), release of dissolved organic matter (Schneider and Haderlein 2016), and change in mineral P fractions in soils (Fathi Gerdelidani and Mirseyed Hosseini 2018; Yan et al. 2018). Olsen-P in soil treated with B did not change after 120 days of incubation compared with 7 days of incubation. However, Olsen-P in soil treated with S declined after 120 days of incubation compared with 7 days of incubation (Fig. 1). This may be pertinent to higher amounts of Ca2+ and Mg2+ release in S than B treatments (Wang et al. 2015) and increasing microbial population (Luo et al. 2017; Nie et al. 2018; Khadem and Raiesi 2019; Karimi et al. 2019).
Organic materials and their decomposition products could reduce P fixation in the soils (Velásquez et al. 2016; Yan et al. 2018). Moreover, many P compounds maintained after the pyrolysis of feedstock. They were not volatilized during the pyrolysis process (Uchimiya and Hiradate 2014). Fathi Gerdelidani and Mirseyed Hosseini (2018) reported that in the application of bagasse biochar in a loamy calcareous soil, P concentration extracted by Olsen method augmented significantly (P < 0.01) after 120 days of incubation. They stated that the available P could rise when more CO2 is released via organic matter decay and cation complexion under the influence of P fixation in calcareous soils. Many studies have shown that higher P availability can be attributed to the application and decomposition of organic fertilizers forming complexes and changing P fractions (Jin et al. 2016; Schneider and Haderlein 2016; Fathi Gerdelidani and Mirseyed Hosseini 2018; Yan et al. 2018). Conversely, Liang et al. (2014) observed that concentration of P released from an acidic soil (pH = 6.85) decreased when the manure was converted to biochar due to crystalline P formation [(Ca,Mg)3(PO4)2]. They reported that the cumulative amount of P released from manure-derived biochar was reduced 76% compared with manure. In this research, P water-soluble concentration in manure (762 mg kg−1) decreased in biochar (39 mg kg−1). Despite that, total P in biochar increased by 193% (8.6 g kg−1 and 25.2 g kg−1 in manure and biochar, respectively).
The ability of different kinetic models to explain P release has been reported in many studies (Agbenin and Tiessen 1995; McDowell and Sharpley 2003; Shariatmadari et al. 2006; Nafiu 2009; Jalali and Ahmadi Mohammad Zinli, 2011; Liang et al. 2014). Jalali and Ahmadi Mohammad Zinli (2011) reported that parabolic diffusion (R2 = 0.972–0.992) and power function (R2 = 0.958–0.981) equations can explain mechanism of P release from different calcareous soils in Iran.
The amount of “a” coefficient rapidly released P pools augmented with concentration of P in soil; this is shown by lower “b” coefficient (the rate of P release) and higher “a” coefficient (the initial pool P release) (Lookman et al. 1995). The results of Table 4 shows that “a” and “b” coefficients in soil treated with P + B were the lowest and the highest. In this study, concentration of P in B was higher than S (Table 2). Therefore, the amount and rate of P release in soil treated with B were higher than soil treated with S. In addition, biochar enhances P release and availability mainly by (a) changing Ca2+, Fe3+, and Al3+ activities through increasing CEC of soils (Joseph and Lehmann 2009); (b) using biochar as a source of soluble P that can increase P in soil solutions (Parvage et al., 2013); (c) improving phosphatase activities, which plays a crucial role in transforming organic P into inorganic P in soils (Jin et al. 2016; Khadem and Raiesi 2019); (d) releasing dissolved organic matter, which competes with P for sorption sites (Schneider and Haderlein 2016); and (e) changing mineral P fractions in soils (Fathi Gerdelidani and Mirseyed Hosseini 2018; Hong and Lu 2018).
The best supporting evidence for any kinetic models to describe the rate of nutrient release is presented to investigate the relation between parameters of kinetics models and available nutrient (Motaghian and Hosseinpur 2013). Therefore, “a” coefficient in power function and parabolic diffusion equations, a × b coefficient in power function, and concentration of P release after 768 h may be applied as indictors of the P supplying power in amended calcareous soils with biochar.
Biochar improves phosphatase activities (Jin et al. 2016; Khadem and Raiesi 2019) and microbial population (Luo et al. 2017; Nie et al. 2018; Khadem and Raiesi 2019). Therefore, microorganisms immobilize soluble P and reduce availability of P during incubation. Moreover, dissolved organic carbon (amino acids, proteins, organic acids, and so on) can reduce precipitation rate of Ca-phosphate in calcareous soils (Alvarez et al. 2004; Ahmad et al. 2018). Also, amendments can change mineral fractions of P and increase soluble P in calcareous soils (Yan et al. 2018). Meanwhile, increases in microbial population and immobilization of P, but activity of soil microbes, have positive roles in releasing dissolved organic carbon and changing mineral fractions of P in calcareous soils (Ahmad et al. 2018).
5 Conclusion
Maximum amount of P was released in the soils treated with P + B. At both incubation times, the concentration of P release from soil treated with P fertilizer increased as affected by amendments application, but biochar increased the availability of P more than its sugarcane residues. Also, biochar increased the availability of P more than sugarcane residues. The difference between cumulative amount of P release in soil treated with S and B was not significant at two incubation times. The results showed that adding sugarcane-derived biochar to similar calcareous soils treated with P can increase availability of P over time.
References
Agbenin JO, Tiessen H (1995) Phosphorus farms in particle-size fractions of a toposequence from Northeast Brazil. Soil Sci Soc Am J 59:1687–1693
Ahmad M, Ahmad M, El-Naggar AH, Usman ARA, Abduljabbar A, Vithanage M, Al-Wabel MI (2018) Aging effect of organic and inorganic fertilizers on phosphorus fractionation in a calcareous sandy loam soil. Pedosphere 28:873–883
Allen ER, Ming DW, Hossner LR, Henninger DL (1995) Modeling transport kinetics in clinoptilotite-phosphate rock systems. Soil Sci Soc Am J 59:248–255
Alvarez R, Evans LA, Milham PJ, Wilson MA (2004) Effects of humic material on the precipitation of calcium phosphate. Geoderma 118:245–260
Amin AEEAZ, Eissa MA (2017) Biochar effects on nitrogen and phosphorus use efficiencies of zucchini plants grown in a calcareous sandy soil. J Soil Sci Plant Nutr 17(4):912–921
Audette Y, O'Halloran IP, Evans LJ, Martin RC, Voroney RP (2016) Kinetics of phosphorus forms applied as inorganic and organic amendments to a calcareous soil II: effects of plant growth on plant available and uptake phosphorus. Geoderma 279:70–76.
Brown R (2009) Biochar production technology. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earth scan, London, pp 127–146
Delgado A, Torrent J (2000) Phosphorus forms and desorption patterns in heavily fertilized calcareous and limed soils. Soil Sci Soc Am J 64:2031–2037
Fathi Gerdelidani A, Mirseyed Hosseini H (2018) Effects of sugar cane bagasse biochar and spent mushroom compost on phosphorus fractionation in calcareous soils. Soil Res 56:136–144
Gee GW, Bauder JW (1986) Particle size analysis. In: Klute A (ed) Methods of soil analysis, part 1, 2nd edn. Agron. Monogr. 9. ASA and SSSA, Madison, pp 383–411
Havlin JL, Westfall DG, Olsen SR (1985) Mathematical models for potassium release kinetics in calcareous soils. Soil Sci Soc Am J 49:371–376
Holford I (1997) Soil phosphorus: its measurement, and its uptake by plants. Soil Res 35(2):227–240
Hong C, Lu S (2018) Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ Sci Pollut Res 25:8725–8734
Hosseinpur AR, Kiani S, Halvaei M (2012) Impact of municipal compost on soil phosphorus availability and mineral phosphorus fractions in some calcareous soils. Environ Earth Sci 67:91–96
Ippolito JA, Stromberger ME, Lentz RD, Dungan RS (2016) Hardwood biochar and manure co-application to a calcareous soil. Chemosphere 142:84–91
Iyamuremye F, Dick RP (1996) Organic amendments and phosphorus sorption by soils. Adv Agron 56:139–451
Jalali M (2006) Soil phosphorus buffer coefficient as influenced by time and rate of P addition. Arch Agron Soil Sci 52:269–279
Jalali M, Ahmadi Mohammad Zinli N (2011) Kinetics of phosphorus release from calcareous soils under different land use in Iran. J Plant Nutr Soil Sci 174(1):38–46
Jin Y, Liang X, Miaomiao H, Liu Y, Tian G, Shi J (2016) Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: a microcosm incubation study. Chemosphere 142:128–135
Joseph S, Lehmann J (2009) Biochar for environmental management: science and technology. Earthscan, London
Karimi A, Moezzi A, Chorom M, Enayatizamir N (2019) Chemical fractions and availability of Zn in a calcareous soil in response to biochar amendments. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-019-00084-1
Khadem A, Raiesi F (2019) Response of soil alkaline phosphatase to biochar amendments: changes in kinetic and thermodynamic characteristics. Geoderma 337:44–54
Lentz R, Ippolito J (2012) Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J Environ Qual 41:1033–1043
Liang XQ, Li L, Chen XY, Li H, Liu J, He MM, Ye YS, Tian GM, Lundy M (2013) Dissolved phosphorus losses by lateral seepage from swine manure amendments for organic rice production. Soil Sci Soc Am J 77(3):765–773
Liang Y, Cao X, Zhao L, Xu X, Harris W (2014) Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: effects of phosphorus nature and soil property. J Environ Qual 43:1504–1509
Liang X, Jin Y, He M, Niyungeko C, Zhang J, Liu C, Tian G, Arai Y (2018) Phosphorus speciation and release kinetics of swine manure biochar under various pyrolysis temperatures. Environ Sci Pollut Res Int 25:25780–25788
Lookman R, Freese D, Marks R, Vlassak K, Van Riemskdijk WH (1995) Long-term kinetics of phosphate release from the soil. Soil Environ Sci Technol 29:1569–1575
Luo S, Wang S, Tian L, Li S, Li X, Shen Y, Tian C (2017) Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl Soil Ecol 117:10–15
McDowell RW, Sharpley AN (2003) Phosphorus solubility and release kinetics as a function of soil test P concentration. Geoderma 112:143–154
Moghimi N, Hosseinpur AR, Motaghian HR (2018) The effect of vermicompost application on transformation rate of available P applied as chemical fertilizer in a calcareous clay soil. Commun Soil Sci Plant Anal 49:2131–2142
Motaghian HR, Hosseinpur AR (2013) Zinc desorption kinetics in wheat (Triticum Aestivum L.) rhizosphere in some sewage sludge amended soils. J Soil Sci Plant Nutr 13(3):664–678
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nafiu A (2009) Effects of soil properties on the kinetics of desorption of phosphate from Alfisols by anion-exchange resins. J Plant Nutr Soil Sci 172:101–107
Nie C, Yang X, Niazi NK, Xu X, Wen Y, Rinklebe J, Ok YS, Xu S, Wang H (2018) Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: a field study. Chemosphere 200:274–282
Parvage MM, Ulén B, Eriksson J, Strock J, Kirchmann H (2013) Phosphorus availability in soils amended with wheat residue char. Biol Fertil Soils 49:245–250
Schneider F, Haderlein SB (2016) Potential effects of biochar on the availability of phosphorus -mechanistic insights. Geoderma 277:83–90
Shariatmadari H, Shirvani M, Jafari A (2006) Phosphorus release kinetics and availability in calcareous soils of selected arid and semiarid toposequences. Geoderma 132:261–272
Singh Mavi M, Singh G, Singh BP, Singh Sekhon B, Choudhary OP, Sagi S, Berry R (2018) Interactive effects of rice-residue biochar and N-fertilizer on soil functions and crop biomass in contrasting soils. J Soil Sci Plant Nutr 18:41–59
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42:2345–2347
Sparks DL (1989) Kinetics of Soil Chemical Processes. Academic Press. San Diego, CA.
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (1996) Methods of soil analysis. Part 3: chemical methods. Soil Science Society of America, Madison
Toor GS, Bahl GS (1999) Kinetics of phosphate desorption from different soils as influenced by application of poultry manure and fertilizer phosphorus and its uptake by soybean. Bioresour Technol 69:117–121
Uchimiya M, Hiradate S (2014) Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J Agric Food Chem 62:1802–1809
Velásquez G, Ngo P, Rumpel C, Calabi-Floody M, Redel Y, Turner BL, Condron LM, de la Luz Mora M (2016) Chemical nature of residual phosphorus in Andisols. Geoderma 271:27–31
Wang Y, Lin Y, Chiu PC, Imhoff PT, Guo M (2015) Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci Total Environ 512-513:454–463
Yan Z, Chen S, Dari B, Sihi D, Chen Q (2018) Phosphorus transformation response to soil properties changes induced by manure application in a calcareous soil. Geoderma 322:163–171
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zolfi-Bavariani M, Ronaghi A, Ghasemi-Fasaei R, Yasrebi J (2016) Influence of poultry manure-derived biochars on nutrients bioavailability and chemical properties of a calcareous soil. Arch Agron Soil Sci 62(11):1578–1591
Funding
This study is supported by funds allocated by the Vice President for research of Shahrekord University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motaghian, H., Hosseinpur, A. & Safian, M. The Effects of Sugarcane-Derived Biochar on Phosphorus Release Characteristics in a Calcareous Soil. J Soil Sci Plant Nutr 20, 66–74 (2020). https://doi.org/10.1007/s42729-019-00101-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00101-3