Abstract
Food consumption and utilization parameters of Plutella xylostella (L.) were studied on the leaves of eight cabbage cultivars under laboratory conditions (27 ± 2 °C, 70 ± 5 % RH, 16 L: 8D photoperiod). The present study evaluated the effect of different biochemical factors of cabbage cultivars on the food consumption and utilization parameters of P. xylostella. All the food consumption and utilization parameters differed significantly among the cabbage cultivars. The highest values of relative consumption rate (RCR) and relative growth rate (RGR) were observed on the cabbage cultivars ‘NS 183’, ‘DEB 806’, ‘Green Express’ and ‘Mohor’. The highest values of the efficiency of conversion of ingested (3.94 %) and digested (4.95 %) food was observed on the cabbage cultivar ‘DEB 806’. In contrast, the 4th instar P. xylostella larvae fed on the cabbage cultivar ‘Lucky Ball’ exhibited the lowest values of RCR (1.31 mg food consumed/mg body weight/ day), RGR (0.33 mg weight gain/mg body weight/ day), ECI (2.47 %) and ECD (2.95 %). The food consumption and utilization parameters of P. xylostella were positively correlated with the protein: carbohydrate (P:C) ratio of cabbage cultivars. The food consumption and utilization parameters of P. xylostella showed a non-significant correlation with the total glucosinolate content and total phenolic content of cabbage cultivars. The present study will help to identify effective resistant or tolerant cabbage cultivars for the development of Integrated Pest Management strategies against P. xylostella.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cabbage (Brassica oleracea var. capitata L.) is one of the important cruciferous vegetable crops grown in India. India is the second highest producer of cabbage in the world with an annual production of 9369 thousand tonnes and productivity of 23.25 metric tonnes ha− 1 from an area of 403 thousand hectares during the year 2019-20 (Anonymous 2020). The diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae), is the most devastating pest of cabbage causing upto 50–80 % annual loss in marketable yield (Devjani and Singh 1999; Ayalew 2006) and annual losses of about US $ 16.0 million in India (Mohan and Gujar 2003). The outbreak of this pest is due to its wide geographical distribution, ability to migrate over long distances, high reproductive potential, tendency to acquire insecticide resistance and the elimination of its natural enemies due to the widespread use of broad-spectrum insecticides (Gryzwacz 2010; Canico 2013; Furlong 2013). P. xylostella has gained multiple resistance to major insecticide groups including the new generation insecticides and Bacillus thuringiensis Berliner (Bt) formulations under field conditions (Sarfraz and Keddie 2005; Oliveira et al. 2011; Neto et al. 2016; Zhang et al. 2016). This situation has prompted the necessity to formulate a sustainable and eco-friendly management strategy for P. xylostella, capable of minimizing pre-harvest losses in cabbage.
Host Plant Resistance is an integral component of Integrated Pest Management (IPM) and can effectively reduce insect pest infestation (Stout 2014). The host plant biochemicals influence food consumption and utilization by the insect herbivore; thereby, affecting its growth and development (Behmer 2009; Santolamazza-Carbone et al. 2016; Wetzel et al. 2016; Nouri-Ganbalani et al. 2018). The generalist insects usually depend on nutrients such as proteins, carbohydrates and other primary metabolites for their feeding activity on plants (Ratzka et al. 2002). In addition to these nutritional factors, the feeding activity of the crucifer specialist insects is stimulated by specific chemical cues known as glucosinolates (Renwick and Lopez 1999). Glucosinolates are naturally occurring secondary metabolites found mostly in the plants of the family Brassicaceae (Santolamazza-Carbone et al. 2016). Other secondary plant metabolites like phenols, abundant in brassicaceous plants (Cartea et al. 2011), can negatively affect the nutritional physiology of insects and reduce its performance (War et al. 2018).
Food consumption and utilization parameters generate valuable information about an insect’s relative rate of food intake and its physiological capacity to assimilate the ingested and digested food for growth and development (Waldbauer 1968). These parameters also link the nutritional performance of an insect herbivore with various host plant attributes (Scriber and Slansky 1981; Slansky 1992; Wetzel et al. 2016). Numerous studies have assessed the effects of plant biochemical factors on nutritional physiology of insects (Senthil-Nathan 2013; Robin et al. 2017; Nouri-Ganbalani et al. 2018). Despite the economic importance of P. xylostella, no information is available on how the food consumption and utilization parameters of this pest are influenced by the different biochemical factors of cabbage. Therefore, the present study aimed to investigate the effect of biochemical factors of cabbage on the food consumption and utilization parameters of P. xylostella. This study would provide further insight into the nutritional physiology of the insect-host plant interactions and could help to design a comprehensive Integrated Pest Management strategy against P. xylostella on cabbage.
Materials and methods
Raising of cabbage cultivars
Seedlings of eight cabbage commercial cultivars (Table 1) were raised under the insect-proof nets at Central Research Farm, Bidhan Chandra Krishi Viswavidyalaya, Gayeshpur. Thirty days old cabbage seedlings were transplanted in separate plots measuring 3.0 × 3.0 m with a spacing of 60 cm between rows and 40 cm between plants. Fertilizers at 120:60:40 kg/ha (N: P2O5: K2O) were incorporated into the soil before transplanting. Plants were maintained free of any pests and no chemical pesticides were used.
Insect culture
The culture of field-collected P. xylostella larvae were maintained on fresh leaves of each cabbage cultivar for multiple generations in glass jars (15 cm diameter and 20 cm depth) at a temperature of 27 ± 2 °C, 70 ± 5 % relative humidity and 16 L: 8D photoperiod. The leaves (food) were changed daily until the feeding ceased in the pre-pupal stage. Newly emerged moths were released in separate glass jars of the same size as above with excised leaves of each cabbage cultivar for mating and oviposition. A cotton ball soaked in 10 % honey diet (1 part of honey: 9 parts of water) was kept in each glass jar as food for the adult moths. The leaves of cabbage cultivars with newly laid eggs were carefully put inside the separate glass jars for hatching. The newly moulted 4th instar P. xylostella larvae were utilized for the experiments.
Food consumption and utilization parameters
The food consumption and utilization parameters of 4th instar P. xylostella larvae were studied on the leaves of eight-week-old plants of the selected cabbage cultivars under laboratory conditions at a temperature of 27 ± 2 °C, 70 ± 5 % relative humidity and 16 L: 8D photoperiod. The fresh leaves of each cabbage cultivar were pre-weighed and placed in individual plastic vials (40 mm diameter x 60 mm height) with perforated lids. Newly moulted 4th instar larvae were obtained from the insect culture and were initially pre-starved for 6 h. The larvae were weighed and released individually in each plastic vial with fresh cabbage leaves. Each cabbage cultivar was replicated five times with ten vials per replication. After two days of feeding, the weight of the 4th instar P. xylostella larvae, unconsumed leaves of each cabbage cultivar (food) and faeces were weighed. Simultaneously, a control with the same amount of fresh leaves without larvae was maintained for each cabbage cultivar under similar conditions to determine the natural weight loss.
The food consumption and utilization parameters of P. xylostella were worked out for each cabbage cultivar using the standard formulae as described by Waldbauer (1968):
Relative consumption rate (RCR) (mg food consumed/mg body weight/day) = Q/ (S x T).
Relative growth rate (RGR) (mg weight gain/mg body weight/day) = R/ (S x T).
Efficiency of conversion of ingested food (ECI) (%) = R/ Q x 100.
Efficiency of conversion of digested food (ECD) (%) = R/ (Q − U) x 100.
where, Q is corrected fresh weight of leaves consumed (mg), R is the weight gained by larva (mg), S is mean weight of larva (mg) during the feeding period, U is weight of faeces produced (mg) in each treatment, T is feeding time (in days). The corrected fresh weight of leaves consumed by larva during the feeding period due to natural loss of moisture was calculated using the formula: Q= [1-a/2] [W-(L + bL)], where Q is corrected fresh weight (mg), W is weight of food given (mg), L is weight of uneaten food (mg), a is the ratio of moisture loss to the initial weight and b is the ratio of moisture loss to the final weight.
Extraction and assay of biochemical constituents
Standard protocols were used to assess the biochemical constituents in the leaves of each cabbage cultivar. Before the start of the experiment, fresh leaf samples from eight-week-old cabbage plants were oven-dried at 60 °C for 36 h. Total phenol content in leaves (mg gallic acid equivalent/g dry weight) was determined using the Folin & Ciocalteu’s reagent (Sigma-Aldrich, India) method (Vinson et al. 1998). The total protein and carbohydrate content of each cabbage cultivar was estimated using the method of Sadasivam and Manickam (2007). The total protein and carbohydrate content of each cabbage cultivar was expressed as a ratio of protein: carbohydrate (P:C).
The total glucosinolate content in cabbage leaves (µmoles/g dry weight) was calculated from the sinigrin hydrate (Sigma-Aldrich, India) analytical standard (McGhee et al. 1965). The enzyme myrosinase catalyzes the conversion of glucosinolates into unstable isothiocyanates in brassicaceous plants (Ratzka et al. 2002; Wallace and Eigenbrode 2002). To deactivate this myrosinase enzyme, 0.3 g of oven-dried cabbage leaf sample was extracted by boiling at 80 °C for 15 min with 0.3 ml of 60 % methanol (Merck, India). Four ml of 0.2 mM sodium (II) tetrapalladate (Sigma-Aldrich, India) was added to the supernatant (40 µl) obtained after centrifugation of the extracted sample.
Data analysis
Data on food consumption and utilization parameters were used to compute the analysis of variance (ANOVA) for testing the significance of differences among the cabbage cultivars using completely randomized design (CRD). A Tukey’s Honestly Significant Difference test at a 5 per cent probability was used to compare the significance of differences among the cabbage cultivars. Before statistical analyses, the percentage data on ECI and ECD were subjected to arcsine transformation (Gomez and Gomez 1984). The correlation of different biochemical constituents in leaves of cabbage cultivars with the food consumption and utilization parameters was also worked out. The data analysis was carried out with the software IBM SPSS version 23.0 for Windows (IBM Corporation, Armonk, New York, USA).
Results and discussion
Food consumption and utilization parameters
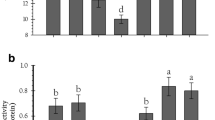
The results on the food consumption and utilization parameters of 4th instar P. xylostella larvae on different cabbage cultivars are presented and discussed herein. Food consumption and utilization parameters viz.. RCR, RGR, ECI and ECD of P. xylostella differed significantly among all the eight cabbage cultivars tested in the present study. Relative consumption rate (RCR) indicating the relative rate of food intake by 4th instar P. xylostella larvae was significantly higher on the cabbage cultivars ‘NS 183’, ‘Green Express’ and ‘Mohor’ (Fig. 1; F = 4.33; df = 7, 32; p = 0.002). The lowest values of relative consumption rate were recorded on the cabbage cultivar ‘Lucky Ball’. Relative growth rate (RGR) was highest for P. xylostella larvae fed on the leaves of the cabbage cultivars ‘DEB 806’, ‘NS 183’, and ‘Green Express’ (Fig. 2; F = 10.42; df = 7, 32; p < 0.0001). Significantly lower values of RGR was recorded on the cabbage cultivars ‘Lucky Ball’. The highest and lowest values for Efficiency of conversion of ingested food (ECI) (Fig. 3; F = 20.39; df = 7, 32; p < 0.0001) and Efficiency of conversion of digested food (ECD) (Fig. 4; F = 54.64; df = 7, 32; p < 0.0001) were recorded on the cultivars ‘DEB 806’ and ‘Lucky Ball’, respectively.
Food consumption and utilization parameters are appropriate indices to assess the nutritional physiology of insect herbivores on host plants (Hemati et al. 2012). The results of the present investigation showed a significant decline in the RCR values of 4th instar P. xylostella larvae when fed on the leaves of the cabbage cultivar ‘Lucky Ball’. Lower consumption rates of P. xylostella might be due to the differences in the nutritional quality of food or presence of secondary plant metabolites (Scriber and Slansky 1981; Hariprasad and Emden 2010). Lower RCR was associated with a decrease in faeces production which might be due to prolonged retention of the ingested food in the larval digestive tract (Scriber and Slansky 1981).
The efficiency with which the ingested and digested food is assimilated for growth and development determines the degree of food utilization by the insect herbivore (Namin et al. 2014). ECI and ECD indicate the physiological efficiency of an insect to assimilate the ingested and digested food for its growth and development (Hemati et al. 2012). The reduced RGR of P. xylostella larvae on the cabbage cultivars ‘Lucky Ball’ and ‘Blue Jays’ was apparently due to a decrease in ECI and ECD values. The P. xylostella larvae fed on these cabbage cultivars were least efficient at assimilating the ingested and digested food for growth and development as indicated by the low ECI and ECD values. A significant decline in ECI and ECD values of P. xylostella larvae might be due to the metabolic costs associated with the biosynthesis and maintenance of detoxification enzymes for plant secondary metabolites in the larval gut (Ratzka et al. 2002). The present results on food consumption and utilization parameters of P. xylostella are in agreement with the findings of Kaur (2001) and Nouri-Ganbalani et al. (2018) on Brassica oleracea var. botrytis L. and Brassica napus L., respectively.
Glucosinolates
The present results revealed a significant variation in the total glucosinolate content of leaves among the tested cabbage cultivars (Fig. 5; F = 16.52; df = 7, 32; p < 0.0001). The total glucosinolate content was significantly higher in the leaves of cabbage cultivar ‘Green Express’ and ‘Blue Jays’. The cultivars ‘Rare Ball’, ‘Mohor’ and ‘NS 183’ exhibited significantly lower values for total glucosinolate content. The present results are in close agreement with Kusznierewicz et al. (2008) and Poelman et al. (2008), who found that the total glucosinolate content in cabbage cultivars ranged from 3.3 to 7.7 µmoles of total glucosinolate/g dry weight and 0.67 to 8.91 µmoles of total glucosinolate/g dry weight, respectively.
Total phenols
The cabbage cultivars differed statistically for the total phenol content in leaves (Fig. 6; F = 3.58; df = 7, 32; p = 0.006). The highest and lowest values for total phenol content were recorded from the leaves of the cultivars ‘Rare Ball’ and ‘Mohor’, respectively. Previous studies by Kamath et al. (2015), Karoui et al. (2018) and Kusznierewicz et al. (2008) also showed that the total phenol content of cabbage cultivars varied from 0.81 to 4.9 mg gallic acid equivalent/g dry weight of leaf tissue, which supports the present findings.
Protein: Carbohydrate (P:C) ratio
The P:C ratio in cabbage leaves differed statistically among the cabbage cultivars (Fig. 7; F = 3.04; df = 7, 32; p = 0.014). The highest and lowest values for P:C ratio were recorded from the leaves of the cultivars ‘DEB 806’ and ‘Lucky Ball’, respectively. The present results are in close agreement with the findings of Adelanwa and Medugu (2015).
Correlation studies
Correlation of different biochemical constituents in leaves of cabbage cultivars with food consumption and utilization parameters of P. xylostella was worked out and presented in Table 2. The results showed a positive and non-significant correlation of the total glucosinolate content with RCR (r = 0.248; p = 0.554), RGR (r = 0.315; p = 0.447), ECI (r = 0.265; p = 0.527) and ECD (r = 0.190; p = 0.652) of P. xylostella (Table 2). Total phenol content registered a negative, non- significant correlation with RCR (r= -0.658; p = 0.076), RGR (r= -0.631; p = 0.093), ECI (r= -0.469; p = 0.241) and ECD (r= -0.353; p = 0.392) of P. xylostella (Table 2). Highly significant and positive correlation was recorded between the protein: carbohydrate (P:C) ratio in cabbage leaves and RCR (r = 0.799; p = 0.017), RGR (r = 0.966; p < 0.0001), ECI (r = 0.946; p < 0.0001) and ECD (r = 0.910; p = 0.002) of P. xylostella (Table 2).
Correlation studies showed a positive but non-significant correlation of the total glucosinolate content with relative consumption rates (RCR) of P. xylostella on the tested cabbage cultivars. Previous authors also found no direct association between total glucosinolate content of cabbage cultivars and larval feeding rates of P. xylostella (Bodnaryk 1992; Poelman et al. 2008; Robin et al. 2017). The present results conform to the findings of Arany et al. (2008) and Bodnaryk (1997), who also reported a non-significant association of total glucosinolate content with larval feeding rates of P. xylostella on Arabidopsis thaliana L. and Brassica juncea L. The total glucosinolate content of cabbage cultivars did not affect the relative growth rates and food utilization parameters of P. xylostella larvae in the present investigation. Our results are in agreement with previous authors who also found no correlation between total glucosinolate content and larval performance of crucifer specialist feeders like cabbage stem flea beetle (Doring and Ulber 2020), stem weevil (Eickermann et al. 2011) and root fly (Birch et al. 1992). Blau (1978) and Li et al. (2000) reported that artificial diets with a higher concentration of allyl glucosinolates did not affect the performance of Pieris rapae L. and P. xylostella larvae, which supports the present findings.
Glucosinolates are secondary metabolites containing non-volatile nitrogen and sulphur-linked glycosides and are known to occur in plants belonging to the family Brassicaceae (Charron and Sams 2004). The enzyme myrosinase catalyzes the conversion of glucosinolates into toxic isothiocyanates in brassicaceous plants (Ratzka et al. 2002; Wallace and Eigenbrode 2002). These toxic isothiocyanates usually act as feeding deterrents and inhibit growth rates in generalist insects (Hopkins et al. 2009; Santolamazza-Carbone et al. 2016). However, specialist crucifer feeders have evolved different detoxification strategies to counteract myrosinase-isothiocyanate toxicity (Jeschke et al. 2016). P. xylostella can avoid this myrosinase hydrolysis by employing a desulfation strategy and minimize the post-ingestion toxicity of glucosinolates (Heidel-Fischer et al. 2019). The ingested glucosinolates are rapidly desulfated into non-toxic desulfo-glucosinolates by glucosinolate-sulfatase (GSS) enzyme in the gut lumen. These non-toxic desulfoglucosinolates no longer act as substrates for myrosinase and are excreted out with faecal matter (Ratzka et al. 2002).
The present investigation showed a negative association of total phenolic content with all the food consumption and utilization parameters of P. xylostella. Numerous studies have highlighted the deleterious effects of plant phenolics in insects as feeding deterrents and anti-digestion factors (Appel 1993; Bennett and Wallsgrove 1994; Resse 1978). Plant phenols impair insect gut metabolism and prevent nutrient uptake by covalently binding to dietary proteins, lipids and digestive enzymes (Felton et al. 1992). These compounds also inhibit insect growth through oxidative stress by the generation of oxygen and phenoxy radicals (War et al. 2012).
In addition to these secondary metabolites, nutrients such as proteins, carbohydrates play an essential role in determining the total food intake and its utilization by the insect herbivore; thereby influencing its growth and development (Scriber and Slansky 1981; Slansky 1993). In the present study, the food consumption and utilization parameters of P. xylostella showed a highly significant, positive correlation with the protein: carbohydrate (P:C) ratio in leaves of cabbage cultivars. Dietary protein is an important limiting factor than carbohydrate for insect growth (Scriber and Slansky 1981; Wilson et al. 2019), as plant tissue generally have a low concentration of usable nitrogen and essential amino acids (Felton 1996). Insects fed on imbalanced food (low protein: carbohydrate ratio) compensate for the low nutritional value of food by an increase in its feeding rates (Lee et al. 2004; Slansky 1993). A compensatory increase in relative consumption rates was also exhibited by P. xylostella for low P:C ratio on all the cabbage cultivars in the present investigation. Such compensation in feeding rates might have increased the ingestion of plant secondary metabolites by P. xylostella on cabbage cultivars (Bernays 1990). The metabolic costs incurred for the detoxification of these secondary metabolites possibly resulted in low RGR, ECI and ECD values on cabbage cultivars.
Conclusions
The food consumption and utilization parameters of 4th instar P. xylostella larvae on the cabbage cultivar ‘Lucky Ball’ was characterized by significantly lower rates of food intake and reduced efficiency to assimilate the ingested and digested food for growth and development. The present findings suggest that the cabbage cultivar ‘Lucky Ball’ was not suitable as host for food consumption and its utilization by the 4th instar P. xylostella larvae. The total glucosinolate content and total phenol content of cabbage cultivars did not affect the food consumption and utilization parameters of P. xylostella. The food consumption and utilization parameters of P. xylostella were positively correlated to the protein: carbohydrate (P:C) ratio of cabbage cultivars. The present study helps to identify effective resistant or tolerant cabbage cultivars for the development of Integrated Pest Management strategies against P. xylostella. Further studies are required to ascertain the role of specific plant metabolites on the food consumption and utilization parameters of P. xylostella.
References
Adelanwa EB, Medugu JM (2015) Variation in the nutrient composition of red and green cabbage (Brassica oleracea) with respect to age at harvest. J Appl Agric Res 7:183–189
Anonymous (2020) Agricultural situation in India. Directorate of Economics and Statistics. Department of Agriculture, Cooperation & Farmers Welfare, New Delhi, p 7
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 18:1521–1552
Arany AM, de Jong TJ, Kim HK, van Dam NM, Choi YH, Verpoorte R, van der Meijden E (2008) Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology 18:65–71
Ayalew G (2006) Comparison of yield loss on cabbage from diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) using two insecticides. Crop Prot 25:915–919
Behmer ST (2009) Insect herbivore nutrient regulation. Annu Rev Entomol 54:165–187
Bennett RN, Wallsgrove RM (1994) Secondary Metabolites in Plant Defense Mechanisms. New Phytol 127:617–633
Bernays EA (1990) Insect-Plant Interactions-Volume II, first edn. CRC Press, Boca Raton
Birch ANE, Griffiths DW, Hopkins RJ, Smith WHM, McKinlay RG (1992) Glucosinolate responses of swede, kale, forage and oilseed rape to root damage by turnip root fly (Delia floralis) larvae. J Sci Food Agric 60:1–9
Blau PA, Feeny P, Contardo L, Robson DS (1978) Allylglucosinolate and herbivorous caterpillars: a contrast in toxicity and tolerance. Science 200:1296–1298
Bodnaryk RP (1992) Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 31:2671–2677
Bodnaryk RP (1997) Will low-glucosinolate cultivars of the mustards Brassica juncea and Sinapis alba be vulnerable to insect pests? Can J Plant Sci 77:283–287
Canico A, Santos L, Massing R (2013) Development and adult longevity of diamondback moth and its parasitoids Cotesia plutellae and Diadegma semiclausum in uncontrolled conditions. Afr Crop Sci Conf Proc. 11:257–262
Cartea ME, Francisco M, Soengas P, Velasco P (2011) Phenolic compounds in Brassica vegetables. Molecules 16:251–280
Charron CS, Sams CE (2004) Glucosinolate content and myrosinase activity in rapid-cycling Brassica oleracea grown in controlled environment. J Am Hort Sci 129:321–330
Devjani P, Singh TK (1999) Field density and biology of diamondback moth P. xylostella L. (Lepidoptera: Yponomeutidae) on cauliflower in Manipur. J Adv Zool 20:53–55
Doring A, Ulber B (2020) Performance of cabbage stem flea beetle larvae (Psylliodes chrysocephala) in brassicaceous plants and the effect of glucosinolate profiles. Entomol Exp Appl 168:200–208
Eickermann M, Ulber B, Vidal S (2011) Resynthesized lines and cultivars of Brassica napus L. provide sources of resistance to the cabbage stem weevil (Ceutorhynchus pallidactylus (Marsh.)). Bull Entomol Res 110:1–8
Felton GW (1996) Nutritive quality of plant protein: sources of variation and insect herbivore responses. Arch Insect Biochem Physiol 32:107–130
Felton GW, Donato KK, Broadway RM, Duffey SS (1992) Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38:277–285
Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: Problems, progress and prospects. Ann Rev Entomol 58:517–554
Gomez KA, Gomez AA (1984) Statistical Procedure for Agricultural Research. Wiley Inter-Science Publications, New York
Gryzwacz D, Rosbach A, Rauf A, Russel DA, Srivansan R, Shelton AM (2010) Current control methods for diamondback moth and other brassica insect pests and the prospects for improved management with lepidopteran resistant Bt vegetable brassicas in Asia and Africa. J Crop Prot 29:68–79
Hariprasad KV, van Emden HF (2010) Mechanisms of partial plant resistance to diamondback moth (Plutella xylostella) in brassicas. Int J Pest Manag 56:15–22
Heidel-Fischer HM, Kirsch R, Reichelt M, Ahn SJ, Wielsch N, Baxter SW, Heckel DG, Vogel H, Kroymann J (2019) An insect counteradaptation against host plant defenses evolved through concerted neofunctionalization. Mol Biol Evol 36:930–941
Hemati SA, Naseri B, Nouri-Ganbalani G, Rafiee-Dastjerdi H, Golizadeh A (2012) Digestive proteolytic and amylolytic activities and feeding responses of Helicoverpa armigera on different host plants. J Econ Entomol 105:1439–1446
Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83
Jeschke V, Gershenzon J, Vassão DG (2016) Insect detoxification of glucosinolates and their hydrolysis products. Adv Bot Res 80:199–245
Kamath SD, Arunkumar D, Avinash NG, Samshuddin S (2015) Determination of total phenolic content and total antioxidant activity in locally consumed food stuffs in Moodbidri, Karnataka, India. Adv Appl Sci Res 6:99–102
Karoui IJ, Jalloul AB, Jihene A, Abderrabba M (2018) Characterization of bioactive compounds, antioxidant properties and antimicrobial activity of red and white cabbage leaves extracts. J Chem Edu Res Prac 2:1–8
Kaur A (2001) Behavioural physiology of susceptible and insecticide-resistant populations of diamondback moth, Plutella xylostella (Linnaeus). Dissertation, Punjab Agricultural University
Kusznierewicz B, Bartoszek A, Wolska L, Drzewiecki J, Gorinstein S, Namiesnik J (2008) Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT 41:1–9
Lee KP, Raubenheimer D, Simpson SJ (2004) The effects of nutritional imbalance on compensatory feeding for cellulose-mediated dietary dilution in a generalist caterpillar. Physiol Entomol 29:108–117
Li Q, Eigenbrode SD, Stringham GR, Thiagarajah MR (2000) Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J Chem Ecol 26:2401–2419
McGhee JF, Kirk LD, Mustake GC (1965) Method for determination of thioglucosides in Crambe abyssinica. J Am Oil Chem Soc 42:889–891
Mohan M, Gujar GT (2003) Local variation in susceptibility of diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot 22:495–504
Namin FR, Naseri B, Razmjou J (2014) Nutritional performance and activity of some digestive enzymes of the cotton bollworm, Helicoverpa armigera, in response to seven tested bean cultivars. J Insect Sci 14:93
Neto JEL, Amaral MHP, Siqueira HAA, Barros R, Silva PAF (2016) Resistance monitoring of Plutella xylostella (L.) (Lepidoptera: Plutellidae) to risk-reduced insecticides and cross resistance to spinetoram. Phytoparasitica 44:631–640
Nouri-Ganbalani G, Borzoui E, Shahnavazi M, Nouri A (2018) Induction of resistance against Plutella xylostella (L.) (Lep: Plutellidae) by jasmonic acid and mealy cabbage aphid feeding in Brassica napus L. Front Physiol 9:859
Oliveira AC, Siqueira HAA, Oliveira JV, Silva JE, Filho MM (2011) Resistance of Brazilian diamondback moth populations to insecticides. Sci Agric 68:154–159
Poelman EH, Galiart RJFH, Raajimakers CE, van Loon JJA, van Dam NM (2008) Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol Exp Appl 127:218–228
Ratzka A, Vogel H, Kliebenstein DJ, Mitchell-Olds T, Kroymann J (2002) Disarming the mustard oil bomb. Proc Natl Acad Sci USA 99:11223–11228
Renwick JAA, Lopez K (1999) Experience-based food consumption by larvae of Pieris rapae: addiction to glucosinolates? Entomol. Exp Appl 91:51–58
Resse JC (1978) Chronic effects of plant allelochemics on insect nutritional physiology. Entomol Exp Appl 24:425–431
Robin AHK, Hossain MR, Park JI, Kim HR, Nou IS (2017) Glucosinolate profiles in cabbage genotypes influence the preferential feeding of diamondback moth (Plutella xylostella). Front Plant Sci 8:1244
Sadasivam S, Manickam A (2007) Biochemical methods, second ed. New Age International (P) Limited Publishers, New Delhi and TNAU, Coimbatore, India
Santolamazza-Carbone S, Sotelo T, Velasco P, Cartea ME (2016) Antibiotic properties of the glucosinolates of Brassica oleracea var. acephala similarly affect generalist and specialist larvae of two lepidopteran pests. J Pest Sci 89:195–206
Sarfraz M, Keddie BA (2005) Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae). J Appl Entomol 129:149–157
Scriber JM, Slansky F (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26:183–211
Senthil-Nathan S (2013) Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front Physiol 4:359
Slansky F (1992) Allelochemical-nutrient interactions in herbivore nutritional ecology. In: Rosenthal GA, Berenbaum MR (eds) Herbivores: Their Interactions with Secondary Plant Metabolites, vol 2. Academic Press, New York, pp 135–174
Slansky F (1993) Nutritional ecology: the fundamental quest for nutrients. In: Stamp N, Caesy TM (eds) Caterpillars: Ecology and Evolutionary Constraints on Foraging. Chapman & Hall, New York, pp 29–91
Stout M (2014) Host-Plant Resistance in Pest Management. In: (ed.) Integrated Pest Management: Current Concepts and Ecological Perspective. Academic Press, San Diego, pp 1–21 ). Abrol DP
Vinson JA, Hao Y, Su X, Zubik L (1998) Phenol Antioxidant Quantity and Quality in Foods: Vegetables. J Agric Food Chem 46:3630–3634
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5:229–288
Wallace SK, Eigenbrode SD (2002) Changes in the glucosinolate-myrosinase defense system in Brassica juncea cotyledons during seedling development. J Chem Ecol 28:243–256
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320
War AR, Taggar GK, Hussain B, Taggar MS, Nair RM, Sharma HC (2018) Plant defence against herbivory and insect adaptations. AoB Plants 10:ply037
Wetzel W, Kharouba H, Robinson M, Holyoak M, Karban R (2016) Variability in plant nutrients reduces insect herbivore performance. Nature 539:425–427
Wilson JK, Ruiz L, Davidowitz G (2019) Dietary Protein and Carbohydrates Affect Immune Function and Performance in a Specialist Herbivore Insect (Manduca sexta). Physiol Biochem Zool 92:58–70
Zhang S, Zhang X, Shen J, Mao K, You H, Li J (2016) Susceptibility of field populations of the diamondback moth, Plutella xylostella, to a selection of insecticides in Central China. Pestic Biochem Physiol 132:38–46
Acknowledgements
The authors are thankful to the Director of Research, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur for providing necessary facilities to carry out the present research work. We highly acknowledge the help of Dr. Srikumar Pal, Professor, Department of Agricultural Biochemistry, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur for his valuable support and guidance during the biochemical analyses. We would also thank Dr. Arindam Ghosh, Georg-August-University, Göttingen, Germany and Dr. Mehraj din Dar, Punjab Agricultural University, Ludhiana for the proofreading of final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karmakar, P., Pal, S. & Chakraborty, G. Effects of cabbage cultivars on the food consumption and utilization parameters of diamondback moth, Plutella xylostella (L.). Int J Trop Insect Sci 42, 83–92 (2022). https://doi.org/10.1007/s42690-021-00520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00520-9