Abstract
Purpose
Early diagnosis of ICU-acquired weakness can support the ICU team in applying appropriate interventions which may lead to better results at clinical and functional outcomes. Thus, interest has increased in non-invasive and more feasible methods to diagnose neuromuscular dysfunction, such as the stimulus electrodiagnosis test (SET) and strength-duration test (SDT). The aim of the present study was to assess the agreement of the SET and SDT carried out automatically using an innovative method.
Methods
We performed a prospective observational study to determine the agreement of SET and SDT, performed automatically using a biofeedback circuit to detect muscle contractions, comprised of an accelerometer module connected to an electrical stimulator. These tests were applied in healthy and critically ill participants.
Results
Twenty-one participants were analyzed in the study, and 168 assessments of SET and SDT were performed. The Bland-Altman analysis of automatic SET and SDT in the control group showed a low bias of −25 (95% CI, −94.3 to 44.3 μs) and 0.6 (95% CI, −1.9 to 3.1 μC) respectively. In the critically ill group, automatic SET and SDT presented a low bias of −104.5 (95% CI, −1716 to 1507μs) and −12.6 (95% CI, −119.4 to 94.1 μC) respectively.
Conclusions
We demonstrated that an innovative method to carry out SET and SDT automatically presents low agreement bias and good to excellent reliability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensive care unit-acquired weakness (ICUAW) is a prevalent neuromuscular dysfunction that often arises during a patient’s stay in the intensive care unit (ICU). Its origin appears to be closely tied to the critical illness itself and the treatments administered, with no other readily identifiable causes (Vanhorebeek et al. 2020). Its prevalence is around 43% and ranges from 25 to 75% (Fan et al. 2014), which can be explained by the tool used to assess, the timing of the evaluation and the cause of ICU admission (Hermans and Van den Berghe 2015). The presence of ICU-acquired weakness is related to worse clinical outcomes and impairs short and long-term functional results (Hakiki et al. 2020; Latronico and Bolton 2011). Early diagnosis can support the ICU team in applying appropriate interventions, such as neuromuscular electrical stimulation, which may lead to better clinical and functional outcomes such as decrease ICU length of stay and increase of muscle strength (Moss et al. 2014; Silva et al. 2019).
Nerve and muscle biopsy, as well as electroneuromyography, are validated tools to detect neuromuscular dysfunction caused by ICU-acquired weakness (Latronico and Bolton 2011; Moss et al. 2014). However, carrying out these exams is not always feasible in the ICU. A biopsy is a surgical procedure, and the ICU is an electrically unfriendly setting for electroneuromyography (Lacomis 2013). Moreover, electroneuromyography requires appropriate devices and a specialized team that are commonly unavailable in the ICU.
In this context, there has been increased interest in non-invasive and more feasible surrogate methods to diagnose neuromuscular dysfunctions, such as the stimulus electrodiagnosis test (SET) and the strength-duration test (SDT) (Fagoni et al. 2021; Silva et al. 2018). The SET provides variables such as rheobase and chronaxie which are the minimum intensity and minimum pulse duration needed to excite a given tissue, respectively (Silva et al. 2018). The sensitivity of the SET to detect muscle denervation can reach 100% in the acute phase, compared to electroneuromyography in outpatients (Paternostro-Sulga et al. 2002).
The use of the SDT has been described for more than a century to guide neurophysiologists regarding neuromuscular excitability (Lapicque 1909). It plots the lowest intensities required for stimulation versus pulse durations through predefined steps. The SDT is useful because it describes how the current required changes when the pulse duration is modified, providing information about neuromuscular excitability (Geddes and Bourland 1985); the less excitable the tissue is, the higher the intensity and pulse duration necessary to excite the tissue. The lack of appropriate devices in the ICU and the absence of SDT validation in critically ill patients has hindered the widespread use of SDT in clinical practice.
Recently, the feasibility of the SET and SDT to assess critically ill patients was demonstrated; however, many points still require further clarification (Fagoni et al. 2021; Silva et al. 2018).
The inter-rater reproducibility of the SET is fair in some conditions, and SDT reproducibility has not yet been evaluated. The critical point related to the reproducibility of these exams is that both depend on the visual and subjective detection of muscle contraction. Thus, in the present study, we propose an innovative method for automatically performing the SET and SDT to detect neuromuscular excitability. This may improve reproducibility and solve inter-rater incongruent diagnoses.
The main aim of the present study was to assess the agreement and reliability of the SET and SDT carried out automatically using an innovative method. Secondarily, we aimed to determine the safety and feasibility of the SET and SDT in critically ill patients.
Methods
Study design
We performed a prospective observational study to assess the agreement of the SET and SDT, performed automatically using a new medical device. The present study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies (von Elm et al. 2007).
Setting
The research was carried out at the department of rehabilitation and at the neurotrauma ICU in a tertiary public reference hospital in the Federal District of Brazil. We conducted our study according to the Declaration of Helsinki, and approval for the project was obtained from the local ethics committee (IHBDF, Brasília, Brazil, protocol 3.629.265). Written consent was obtained from healthy participants in the control group and from the closest responsible family member in the critically ill group, since all participants were intubated and sedated at enrollment. Data collection was conducted between November 2020 and March 2022.
Participants
The control group was composed of healthy male and female participants, aged 18 to 60 years, without a clinical history of orthopedic and neuromuscular disease. The critically ill group was composed of traumatic brain injured participants, of both sexes, aged 18 to 60 years, sedated for at least 6 days on mechanical ventilation. Participants with bone fractures, skin injuries at the assessment location, and pregnant women were excluded. In both groups, participants were recruited consecutively.
Variables
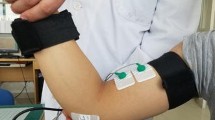
All participants were assessed by SET and SDT. Two manual and two automatic assessments were performed for every test (i.e., SET and SDT). The manual assessments were performed by a single expert examiner (PES). We used a new innovative device, Recare® (Visuri, Belo Horizonte, Brazil), to achieve both manual and automatic assessments.
The automatic assessments were performed using a biofeedback circuit to detect muscle contractions, comprised of an accelerometer module (STMicroelectronics STEVAL-MKI211V1K) connected to the Recare® by an I2C serial protocol. The weight of the printed circuit board was 5.5 g, and the plastic encapsulation weighed 10 g, resulting in a final weight of 15.5 g. The Recare® Scientific Mode (RSM) was configured to start the stimulation and progressively increment the pacing parameter values until a contraction was detected. At this moment, the device automatically interrupts the stimulation and saves the final parameter values on the hardware memory. To determine the contraction, the accelerometer Z-axis was positioned perpendicularly to the surface of the skin, over the muscle belly. The RSM was configured to acquire accelerometer data at a 100 Hz sample rate. The RSM algorithm calculated the standard deviation (SD) for 500 ms of Z-axis data obtained before and after each stimulation. A contraction event was determined every time the Z-axis SD value after the stimulus exceeded 1.2 times the previously calculated value. The value of 1.2 times was defined based on a previous study (Bispo et al. 2019).
Primary outcomes
The primary outcomes of the present study were the agreement and reliability of chronaxie and area under the curve, measured automatically during two repetitive tests from the SET and SDT respectively.
Stimulus electrodiagnosis test (SET)
The SET was performed using two electrodes positioned proximal over the right tibialis anterior muscle, 1/4 of the distance between the inferior border of the patella and the lateral malleolus. All participants were in a supine position with 30° bed elevation.
Neuromuscular excitability was assessed by rheobase and chronaxie. Rheobase is the minimal current intensity necessary to reach the neuromuscular excitability threshold applied with a rectangular pulse with an infinite duration. Chronaxie is the shortest pulse duration required to reach the neuromuscular excitability threshold by a current with twice the intensity of rheobase (Silva et al. 2018).
Rheobase and chronaxie were measured with a single-biphasic pulse. For rheobase assessment, the intensity was increased from 1 to 240 mA, with individual 1 mA increments until eliciting a slight and visible muscle contraction. The evaluation was performed with a pulse duration of 500 ms and intervals of 2 s between pulses. For the assessment of chronaxie, the current amplitude was set to twice the rheobase value. Subsequently, the pulse duration was increased from 50 μs to 500 ms in increments of 50 μs, until eliciting a slight but visible muscle contraction (Silva et al. 2018).
The examiner performed the SET manually and automatically, twice each, separated by 5-min intervals.
Strength-duration test (SDT)
The SDT was carried out 5 min after the SET. Patients and electrodes were maintained in the same position as the SET. A rectangular, biphasic, and symmetric pulse was used with different pulse durations: 100 μs, 200 μs, 400 μs, 700 μs, 1000 μs, 1500 μs, 2000 μs, 2500 μs, 5000 μs, 10 ms, 15 ms, 20 ms, 50 ms, 100 ms, 200 ms, up to 500 ms. For every pulse duration, the examiner (manual) or the device (automatic) was required to find a minimal muscle contraction using an intensity ranging from 1 to 240 mApp. The assessment was stopped when a plateau was reached in the intensity axis in three incremental steps of pulse duration.
First, the examiner manually performed the SDT twice, with a 5-min interval. In sequence, the device automatically performed the SDT twice with 5-min intervals.
The total charge applied in the SDT was measured by the area under the curve (AUC). Before calculating the AUC, the curve was determined by joining the “n” straight lines between the “n+1” subsequent points on the graph obtained by the pulse duration (x-axis, μs) and intensities (y-axis, mA) from each SDT. The AUC (Fig. 1A) was obtained by calculating the sum of the areas under the “n” functions fi(x), which represent the “n” equations of straight lines between points xi and xi+1, with i ranging from “1” to “n.” Each of the “n” section areas was calculated by the respective definite integrals of fi(x) from xi to xi+1. According to the International System of Units, the unit of area used was μs × mA, equivalent to the electrical charge in microcoulombs (μC). The AUC calculations were carried out in Matlab R2022b.
Calculation of the area under the curve from the strength-duration test. A presents the function used to calculate AUC. B, C, and D show examples of the strength-duration test plotted automatically by the device in two representative participants of both groups. The AUC was calculated according to the equations shown in A. B presents two curves plotted in the same graph from a healthy and a critically ill participant. C and D present the AUC values from healthy and critically ill participants respectively. AUC, area under the curve; μC, microcoulomb; SDT, strength-duration test
Secondary outcomes
Safety and feasibility
Safety was assessed by the incidence of a skin burn and feasibility by the time spent performing the assessments.
Study size
Sample size was calculated a priori using the formula proposed by Walter et al. (Walter et al. 1998) for reliability studies. Initially, 30 subjects were proposed in the critically ill group; however, due to the SARS-CoV-2 pandemic, the present study needed to be stopped with 12 participants. Thus, the post hoc power (1-β) was calculated, demonstrating a mean value of 83%. This calculation was carried out taking into account 11 subjects, a number of observations of 2, significance level of 0.05, acceptable reliability of 0.8, and a mean of measured reliability of 0.96 in the AUC analysis.
Statistical methods
The normality of the data was tested with the Shapiro-Wilk test, and parametric and non-parametric tests were used, given the characteristics of the variables. The variables were described as mean and standard deviation or median and interquartile range. Agreement was assessed by the Bland-Altman method and reliability by the intraclass correlation coefficient. The correlation coefficients were classified as suggested by Mukaka (2012): > 0.9, excellent; 0.7 to 0.9, good; 0.5 to 0.7, moderate; 0.3 to 0.5, fair; 0 to 0.3, slight.
Differences in SDT, SET, and time spent during the test were analyzed using the unpaired Student T-test. Statistically significant differences were assumed when p < 0.05. Data were analyzed using the software Graphpad Prism version 8.3 (Graphpad Software, Inc., San Diego, California, USA).
Results
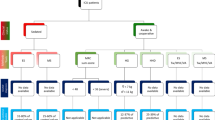
From the fifty-four participants assessed for eligibility, twenty-two were included in the study. The recruitment process is described in the flow diagram (Fig. 2). Participants’ characteristics are presented in Table 1.
Stimulus electrodiagnosis test (SET)
The SET agreement was calculated using chronaxie, as this parameter is used to define neuromuscular excitability dysfunction (Paternostro-Sulga et al. 2002; Silva et al. 2018). The results of these assessments in the control and critically ill groups performed manually and automatically are presented in Fig. 3A to D. There were no statistical differences in the chronaxie assessed manually by the examiner and automatically by the device in both groups (Fig. 3E and F). The overall mean chronaxie in the control group and in the critically ill group were respectively 236 ±106 μs vs. 851 ±1159 μs (p < 0.0028).
Intra-rater agreement of chronaxie measured from stimulus electrodiagnosis test, manually and automatically. The Bland-Altman plot of the intra-rater comparison between two repeated measures of chronaxie during the SET taken by the examiner manually and the device automatically. Differences and averages were calculated from the repeated measures recorded on the tibialis anterior muscle. Upper and lower discontinuous traces show respectively the bounds of a 95% range of the LOA; the continuous line presents the bias mean. The ICC between repeated measures is indicated for each graph. Squares in the graphs represent manual assessments, and circles represent automatic assessments. ICC, intraclass correlation coefficient; LOA, limit of agreement; SET, stimulus electrodiagnosis test
Strength-duration test (SDT)
The agreements of the SDT carried out manually and automatically are presented in Fig. 4A–D. There was no statically difference in the AUC plotted by manual and automatic assessments in both groups (Fig. 4E–F). The overall mean (manual and automatic) of the AUC in the control group and the critically ill group were respectively 10.5 ±3.5 μC vs. 71.6 ±100.9 μC (p < 0.0002).
Intra-rater agreement of the area under the curve measured in the strength-duration test, manually and automatically. The Bland-Altman plot of the intra-rater comparison between two repeated measures of area under the curve from SDT taken by the examiner manually and the device automatically. Differences and averages were calculated from the repeated measures recorded on the tibialis anterior muscle. Upper and lower discontinuous traces show respectively the bounds of a 95% range of the LOA; the continuous line presents the bias mean. The ICC between repeated measures is indicated for each graph. Squares in the graphs represent manual assessments, and circles represent automatic assessments. ICC, intraclass correlation coefficient; LOA, limit of agreement; SDT, strength-duration test
Safety and feasibility
In total, 168 SET and SDT were performed manually by the examiner and automatically by the device. No cases of skin burns or any side effects were detected. There were no statistical differences between the time spent in the manual or automatic assessments to perform the SET: automatic, mean (±SD) of 1.76 ±0.42 min vs. manual, 1.6 ±0.3 min respectively, p = 0.13. The automatic assessment spent more time than the manual to assess SDT: mean (±SD) of 4.98 ±2.52 min vs. 3.35 ±0.87 min respectively, p = 0.0076.
Discussion
The present study demonstrated low agreement bias in the automatic assessment of SET and SDT. The agreements of the SET and SDT were similar when performed manually and automatically. Both presented a good to excellent reliability, and there was no significant difference between the absolute values of these two forms of assessment of SET and SDT. Moreover, it was shown that both the manual and automatic assessments are feasible and safe.
Electrodiagnostic tests in the ICU, such as nerve conduction studies and needle electromyography, can be a difficult task due to the electrically unfriendly environment (Lacomis 2013). Thus, the SET can be viewed as a surrogate screening test that can be used to detect neuromuscular abnormalities (Paternostro-Sulga et al. 2002; Silva et al. 2019).
The assessment of chronaxie by the SET seems to be fundamental not only for diagnosis, but also for the prescription of neuromuscular electrical stimulation (NMES) (Silva et al. 2019, 2018). Chronaxie defines the minimal level of neuromuscular excitability, and its value should be applied to pulse duration to evoke effective contractions, in order to avoid muscle atrophy (Silva et al. 2017). The precise definition of chronaxie could be the starting point for an adequate diagnosis of neuromuscular excitability and NMES treatment.
A previous study found that SET has a sensitivity of 100% in the acute phase and 86% in the subacute/chronic phase of muscle denervation in outpatients, compared to needle electromyography (Paternostro-Sulga et al. 2002). However, these authors did not assess all clinimetric properties of this test. A more recent study showed that the SET can present fair agreement in the inter-rater evaluation in an ICU setting when the tibialis anterior is assessed (de Araujo et al. 2019).
The level of reproducibility (reliability and/or agreement) of a test can be related to the previous training of the professionals (Brooks and Thomas 1995). Furthermore, both the SET and SDT have another critical point: the subjectivity of visual detection of the muscle contractions (de Araujo et al. 2019). Thus, the heterogeneity in the expertise of the ICU team and the subjectivity can preclude adequate levels of inter-examiner reproducibility in these tests. Accordingly, the use of new automated technology that does not need a human decision can improve the clinimetric properties of SET and SDT. Consistent with the previous assertion, the present study showed that automatic SET assessment achieved high reliability in both the control and critically ill groups.
It has been advocated that SDT may be more robust to detect neuromuscular excitability dysfunctions than SET (Paternostro-Sulga et al. 2002). Chronaxie, in fact, is a point in the SDT curve. However, since the first studies that described SDT (Lapicque 1909; Ritchie 1944), few advances have been made to improve its assessment and decrease the subjectivity. The analysis of the SDT curve has been performed visually, meaning that clinicians can provide subjective and imprecise descriptions.
The current study presented a novel way to objectively measure the movement of the curves using the calculation of the AUC. Thus, it is possible to numerically assess signals of worsening or improvement in neuromuscular excitability; the higher the AUC, the worse the neuromuscular excitability. Moreover, it was demonstrated that the automatic SDT assessment has a low agreement bias, which allows different examiners to carry out this test.
Recently, Fagoni et al. (2021) carried out the SDT in critically ill patients and concluded that this is a feasible tool to assess neuromuscular excitability dysfunctions. Moreover, SDT can provide additional information to that obtained by electroneuromyography. The authors performed a manual assessment and used a low charge protocol, with a pulse duration ranging from 50 up to 450 μs. This protocol can limit the assessment of patients with neuromuscular excitability dysfunctions as demonstrated by the authors. It has been shown that patients with neuromuscular excitability dysfunctions need high values of charge in SDT to be assessed (Ritchie 1944; Silva et al. 2018). Classically, therefore, this test has been carried out using pulse duration ranges up to infinite values (i.e., >200 ms) as in the present study (Geddes and Bourland 1985).
A possible application of the SDT, beyond diagnosis, is to guide the treatment of neuromuscular dysfunctions. It has been demonstrated that many patients in the ICU are “non-responders” to NMES, i.e., their muscles are unexcitable with traditional protocols: pulse width up to 500 μs and intensity up to 120 mA (Grunow et al. 2019; Segers et al. 2014). The present results reinforce that neuromuscular excitability depends on the charge applied and that the SDT, like the SET, can guide the ICU team on the more precise dose to be used during NMES.
Our results demonstrate that manual and automatic assessments of both tests are safe and feasible, overall requiring less than 5 min to perform. The major complication of this type of test is the incidence of skin burns, caused in this context by the high current density (i.e., values > 50 μA/mm2) (Scheiner et al. 1990). No cases of skin burns were documented in the present study, which could be due to the safety algorithm applied in this new technology. The device is programmed to avoid a current density > 35 μA/mm2; the examiner is required to inform the electrode size, and the intensity is limited based on that cut-off. Due to this safety tool, one participant in the critically ill group could not be completely assessed. The participant presented generalized edema, and to perform SDT, it would have been necessary to overcome the safety limits of current density.
Some limitations should be addressed in our study. This was a single-center observational study with healthy participants and critical traumatic brain injury patients; thus, the findings may not be generalizable to different settings and patients. As a result of the SARS-CoV-2 pandemic, the study needed to be stopped before reaching the established sample size. Nevertheless, the power post hoc analysis demonstrated a β error < 20%. Finally, the weight of our sensor (15.5 g) exceeded the recommendation by Ahn et al. (2016) of 13 g as ideal for mechanomyography measurements. However, for the proposed application in our work, which uses accelerometry to determine whether a muscle contraction has occurred or not, the high-frequency components present in the mechanomyography signal were not necessary.
Further studies are required to define the cut-off of the SET and SDT to diagnose ICU-acquired weakness. The use of electroneuromyography may be an adequate tool to be used as the gold standard.
Conclusion
The current study demonstrated that the novel technology used to perform the SET and SDT automatically presents low agreement bias and good to excellent reliability. Furthermore, the results have shown its feasibility and safety. Further studies in different settings with larger sample sizes are necessary to confirm the present findings.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
References
Ahn S, Shin I, Kim Y. The effect of accelerometer mass in mechanomyography measurements. J J Vibroengineering. 2016;18:4736–4742. doi: https://doi.org/10.21595/jve.2016.17420.
Bispo J, Eugênio P, Durigan J, Martins H. Development of equipment for automatic diagnosis of the neuromuscular condition. IFMBE Proc. 2019;70:439–44. https://doi.org/10.1007/978-981-13-2119-1_67.
Brooks D, Thomas J. Interrater reliability of auscultation of breath sounds among physical therapists. Phys Ther. 1995;75:1082–8.
de Araujo AET, Silva PE, Carvalho KLD, Fachin-Martins E, Babault N, Durigan JLQ. Intra- and inter-raters reliability and agreement of stimulus electrodiagnostic tests with two different electrodes in sedated critically ill patients. Phys Ther Theory Pract. 2019;36:1447–56. https://doi.org/10.1080/09593985.2019.1567890.
Fagoni N, Ferretti G, Piva S, Barbieri S, Rasulo F, Latronico N, Gobbo M. A reappraisal of the strength-duration test to assess neuromuscular impairment of critically ill patients. J Electromyogr Kinesiol. 2021;59 https://doi.org/10.1016/J.JELEKIN.2021.102555.
Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, Hopkins RO, Hough CL, Kress JP, Latronico N, Moss M, Needham DM, Rich MM, Stevens RD, Wilson KC, Winkelman C, Zochodne DW, Ali NA. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–46. https://doi.org/10.1164/RCCM.201411-2011ST.
Geddes LA, Bourland JD. The strength-duration curve. IEEE Trans Biomed Eng. 1985;32:458–9.
Grunow JJ, Goll M, Carbon NM, Liebl ME, Weber-Carstens S, Wollersheim T. Differential contractile response of critically ill patients to neuromuscular electrical stimulation. Crit Care. 2019;23 https://doi.org/10.1186/s13054-019-2540-4.
Hakiki B, Draghi F, Scarpino M, Portaccio E, Romoli A, Mannini A, Atzori T, Lolli F, Macchi C, Grippo A. Critical illness polyneuromyopathy: functional impact after severe acquired brain injuries. Acta Neurol Scand. 2020;142:574–84. https://doi.org/10.1111/ANE.13324.
Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274.
Lacomis D. Electrophysiology of neuromuscular disorders in critical illness. Muscle Nerve. 2013;47:452–63. https://doi.org/10.1002/mus.23615.
Lapicque L. Définition expérimentale de l’excitabilité. Comptes Rendus Soc Biol. 1909;77:280–3.
Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–41.
Moss M, Yang M, Macht M, Sottile P, Gray L, McNulty M, Quan D. Screening for critical illness polyneuromyopathy with single nerve conduction studies. Intensive Care Med. 2014;40:683–90. https://doi.org/10.1007/s00134-014-3251-6.
Paternostro-Sulga T, Schuhfried O, Vacariu G, Lang T, Fialka-Moser V, Paternostro-Sluga T, Schuhfried O, Vacariu G, Lang T, Fialka-Moser V. Chronaxie and accommodation index in the diagnosis of muscle denervation. Am J Phys Med Rehabil. 2002;81:253–60.
Ritchie AE. The electrical diagnosis of peripheral nerve injury. Brain. 1944;67:314–30. https://doi.org/10.1093/brain/67.4.314.
Scheiner A, Mortimer JT, Roessmann U. Imbalanced biphasic electrical stimulation: muscle tissue damage. Ann Biomed Eng. 1990;18:407–25.
Segers J, Hermans G, Bruyninckx F, Meyfroidt G, Langer D, Gosselink R. Feasibility of neuromuscular electrical stimulation in critically ill patients. J Crit Care. 2014;29:1082–8.
Silva PE, Babault N, Mazullo JB, Oliveira TP, Lemos BL, Carvalho VO, Durigan JLQ. Safety and feasibility of a neuromuscular electrical stimulation chronaxie-based protocol in critical ill patients: a prospective observational study. J Crit Care. 2017;37:141–8. https://doi.org/10.1186/1741-7015-11-137.
Silva PE, de Cássia MR, Livino-de-Carvalho K, de Araujo AET, Castro J, da Silva VM, Vieira L, Souza VC, Dantas LO, Cipriano G Jr, Nóbrega OT, Babault N, Durigan JLQ. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: a randomized controlled trial. J Intensive Care. 2019;7:59. https://doi.org/10.1186/s40560-019-0417-x.
Silva PE, Maldaner V, Vieira L, de Carvalho KL, Gomes H, Melo P, Babault N, Cipriano G, Durigan JLQ. Neuromuscular electrophysiological disorders and muscle atrophy in mechanically-ventilated traumatic brain injury patients: new insights from a prospective observational study. J Crit Care. 2018;44:87–94. https://doi.org/10.1016/J.JCRC.2017.10.026.
Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46:637–53. https://doi.org/10.1007/S00134-020-05944-4.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X.
Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–10.
Acknowledgements
The authors are grateful to Robin Hambly for the English review. The authors are also grateful to Amaro Eduardo de Araujo for all his support during the data collection in the ICU.
Funding
This study was supported by the Instituto de Gestão Estratégica de Saúde do Distrito Federal (IGESDF; Process number: 01/2019-04). This grant was conceded to Paulo Eugênio Silva.
Author information
Authors and Affiliations
Contributions
PES and HRS designed the study and drafted the article. PES was responsible for applying the SET and SDT. JRDM, AVQ, and AU selected participants and conducted primary data extraction. EFM participated in the interpretation of results, and PES performed the statistical analysis. All authors revised the article and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review boards approved the protocol (IHBDF, Brasília, Brazil, protocol number 3.629.265).
Consent to participate
Participants in the control group signed the informed consent form, and the consent form of participants in the critically ill group was signed by their legal guardian.
Consent for publication
This manuscript has not been published elsewhere and is not under consideration by another journal.
Competing interests
The authors PES and HRM have patents in neuromuscular electrical stimulation as inventors. They also have equity in Visuri SA and serve as scientific advisors. The remaining authors have no relevant conflicts to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva, P.E., Martins, H.R., de Deus Macedo, J.R. et al. Towards innovative electrodiagnosis tests to investigate neuromuscular excitability dysfunction in critically ill patients: an agreement study. Res. Biomed. Eng. 39, 949–958 (2023). https://doi.org/10.1007/s42600-023-00318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-023-00318-3