Abstract
Tea (Camellia sinensis and C. assamica) possess antimicrobial property due to the presence of bioactive compounds. Qualitative analysis of phytochemicals of both tea species such as alkaloids, flavonoids, carbohydrates, saponins, tannins, steroids, terpenoids and cardiac glycosides was carried out. The antimicrobial activity of both plant species extracts were performed by well and disc agar diffusion methods. The most antimicrobial potential of plant extract was further selected to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) against two most susceptible human pathogens. Phytochemicals and antimicrobial activity assays showed different results according to the type of the applied extract solvent. The methanol extract of C. sinensis shows more inhibitory effect than the other extracts of both tea species. The lowest concentration of C. sinensis was found at 0.048 mg/mL against Staphylococcus aureus. The MIC and MBC of S. aureus and Pseudomonas aeruginosa were ranged from 0.097 to 0.197 mg/mL and 0.19 to 0.39 mg/mL, respectively. Time-killing assay was performed against all tested pathogenic bacteria. After 6 h, P. aeruginosa showed no growth followed by Escherichia coli, Candida albicans, Klebsiella pneumoniae, S. aureus, Salmonella typhi, Listeria monocytogenes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A gradual increase in resistance among microorganisms towards the antibiotics is the leading threat in the present world. About 2 million people attained bacterial diseases in the US hospitals, each year and 70% of cases of bacterial strains resistant to one or more drugs (Infectious Diseases Society of America 2004). Among all the plants, tea plant has been found potential against multi-drug resistance pathogens.
Non-fermented tea has a high range of antibacterial activity than fermented and semi-fermented tea (Toda et al. 1989). In recent years, tea acquired scientific attention as an antimicrobial agent against several bacterial species (Singh et al. 2009). Staphylococcus aureus, P. aeruginosa and S. typhi are some of the common human pathogens, generally causing skin infection, urinary tract infection, and enteric fever respectively.
Tea leaves contain a huge number of polyphenols (flavonoids, catechins, tannins etc.) among which flavonoids are most abundant, possessing the ability to inhibit the spore germination in fungal strains. Besides it also bears several beneficial properties such as anti-inflammatory, antimicrobial, antiallergic, antioxidant, antitumor etc. Therefore, flavonoids are becoming the topic of medicinal research. In contrast, catechins include epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epigallocatechin gallate (EGCG) (Cabrera et al. 2003). Among these, the compounds responsible for the antimicrobial property are EGCG and EGC. EGCG has the potential to decrease the growth of Escherichia coli by about 50% (Nazer et al. 2005). Green tea catechins revealed antimicrobial activity against a wide variety of microorganisms including, Gram-positive, Gram-negative bacteria, some viruses, fungi and prions (Reygaert 2018). Many researches proved that tea polyphenols showed positive effects on human health (Yang and Zhang 2019). Tea attributed biological properties to prevent cardiovascular diseases (Sesso et al. 1999) cancer (Mckay and Blumberg 2002), anti-inflammatory, antiarthritic, antibacterial, antiangiogenic, antioxidative, antiviral, neuroprotective and cholesterol-lowering effects (Chacko et al. 2010). Due to the high content of flavonoids, it has been attributed to antifungal activity against Candida species (Cushnie and Lamb 2005).
Nowadays, the frequency of these pathogens to cause disease has increased. Antibiotics have been used to treat the disease-causing microorganism resulting into side effects on human health. However, plant-based products found to be effective to cure human diseases and for body healthy (Padmini et al. 2010).
Material and methods
Collection of Camellia species
The species of Camellia were collected from Tea Estate, Dehradun, Uttarakhand. The young fresh leaves were washed to remove the dust particle followed by shade-drying and grind to form a powder. Both the plants were identified based on their morphological features and authentic databases by the Botanical Survey of India (BSI). The accession number of C. sinensis and C. assamica are 19a and 19b respectively.
Preparation of Camellia species extract
The dried plant material of both species was weighed and 100 g of each was kept separately into Soxhlet apparatus for 6 h extraction. Different solvents such as methanol, ethanol and water were used for extraction. The solvents were evaporated by using rotary vacuum evaporator and plant extracts were stored in a refrigerator for further use (Siddhuraju et al. 2002) and their yield percentage were calculated by using the formula (Mostafa et al. 2018): Extract yield% = weight of extracted plants residues/weight of plant raw material × 100.
Phytochemical analysis of tea extracts
Tannins
About 5 mL of an extract of the plant was boiled with 20 mL of distilled water and filtered, the boiled filtrate was then treated with 0.1% of ferric chloride. Changing in blue-black colours detects tannins.
Flavonoids
5 mL of ammonia solution was added to a 3 mL of plant extract followed by careful addition of sulphuric acid. The presence of flavonoids will be observed if the extracted colour is turned into yellow, but on standing, the yellow colour will be disappeared, subsequently, few drops of aluminium solution (1%) should be added in each plant extract.
Terpenoids
About 5 mL of each plant extract was mixed with 2 mL of chloroform, then 3 mL concentrated sulphuric acid was added carefully. The presence of terpenoids is proved by the formation of a reddish-brown coloured interface.
Saponins
2 mL of each plant extract was boiled with 20 mL distilled water in a water bath and filtered then. 10 mL of the filtrate was mixed with 5 mL of distilled water and shaken vigorously. If a froth is observed, olive oil will be added to froth followed by vigorously shaking; formation of a foam appearance confirms the presence of saponins.
Steroids
Acetic anhydride (2 mL) was added to each plant extract with 2 mL of sulphuric acid. The presence of steroids will be detected as the violet colour is changed into blue or green.
Cardiac glycoside
Each plant extract was separately treated with 2 mL of glacial acetic anhydride with one drop of ferric chloride solution followed by mixing of 1 mL of concentrated sulphuric acid. The formation of interface with brown colour indicates deoxysugar characteristic of cardenolides. Violet colour ring may appear below the brown ring while the acetic acid is added, a greenish colour ring may form moderately throughout the thin layer.
Alkaloids
2 mL of each plant extracts was treated with 3 mL of hexane followed by proper shaking and filtration. 5 mL of hydrochloride acid (2%) was poured in a test tube containing plant-extract-hexane mixture, shaken then filtered. A few drops of picric acid were added in the mixture. The formation of yellow precipitate indicates the presence of alkaloids.
Carbohydrates
2 mL of Benedict’s reagent was mixed with each plant extract and boiled; formation of reddish-brown precipitate documents the presence of carbohydrates.
Bacteria
Six pathogenic bacterial strains viz., P. aeruginosa (MTCC 424), S. aureus (MTCC 7443), E. coli (MTCC 118), Listeria monocytogenes (MTCC 657), Salmonella typhi (MTCC 733), Klebsiella pneumoniae (MTCC 432), Candida albicans (MTCC 227) were collected from Microbial Type Culture Collection (MTCC), Chandigarh, India. These cultures were maintained by sub-culturing on nutrient and Sabouraud dextrose agar slants for bacteria at 37 °C and fungi species at 28 °C, respectively.
Antimicrobial activity of plant extract
Agar well diffusion method
Tea leaves extracts of both species were investigated for their antimicrobial activities. 100 µL of microbial cultures were swabbed on Mueller Hinton agar plate (MHA) for bacterial strains and Sabouraud dextrose agar (SDA) for fungal strain. Wells (6 mm in diameter) were punched using a sterile cork borer. 80 µL of each plant extract was dispensed in each well. All plates of bacteria were incubated at 37 ± 1 °C in bio-oxygen demand incubator (BOD) for 24 h and fungal plates were incubated at 25 ± 1 °C for 24–48 h to measure the zone of inhibition (Mehrotra et al. 2010).
Disc diffusion method
Antimicrobial activity of the plant extracts was also evaluated by disc diffusion method. 100 µL of each bacterial culture (1 × 108 CFU/mL) were dispensed in Mueller Hinton Agar medium and Sabouraud dextrose agar medium plates to prepare a lawn. Whatmann filter paper (disc 5 mm) was impregnated with 10 µL of the plant extract then placed on the bacterial and fungal lawn.
Formed inhibition zones were measured in mm and the most potent antimicrobial plant extract was selected for further studies.
Determination of minimum inhibitory concentration, minimum bactericidal concentration
MIC of the selected plant extract was determined by a double fold serial dilution method against all the pathogenic of both the Camellia species. A stock solution of crude plant extract was prepared (25 mg/mL of the corresponding solvent); then it was diluted in test tubes containing sterile Luria Bertani broth using twofold serial dilution method to obtain concentrations ranging from 25 to 0.048 mg/mL. 100 µL of bacterial inoculum (1 × 108 CFU/mL)/100 µL extract dilution was used for each test tube. The mixtures were incubated at 37 °C for bacterial growth. After the incubation, the lowest concentration that inhibits the growth of bacteria was considered as the MIC. To determine the plant extract MBC, tested bacterial strains were sub-cultured together with the plant extract on agar plates using Nutrient Agar Medium (NAM) and Sabouraud dextrose agar medium (SDA) for the dilution process. MBC was considered as the concentration that ceased the bacterial growth (Mostafa et al. 2018).
Time-kill efficacy assay/Survival of microbial species
The survival of bacteria, time kill efficacy assay, in the methanolic extract of C. sinensis was carried out with slight modification. Different time intervals were applied in the assay at 0, 2, 4 and 6 h. 50 mg of methanolic extract C. sinensis was dissolved in 1 mL of methanol to achieve 50 mg/mL concentration. Test bacteria were separately inoculated into sterile Luria Bertani broth and incubated at 37 °C for 24 h. The standard tube dilution method was opted to evaluate the time-kill efficacy of bacteria. Bacterial inoculum (1 mL) was diluted by adding 9 mL sterile saline solution and serially diluted upto 10–3. On testing the bacterial dilution (10–3), bacterial colonies were observed in a discrete form which was easily countable. One mL of 10–3 bacterial dilution was incubated with an equal amount of 50 mg/mL plant extract at 37 °C for bacteria and 25 °C for fungi for a different time interval (0, 2, 4 and 6 h). 100 µL incubated suspension was transferred on the agar plates and spread through the spreader. Nutrient agar medium was used for bacteria and for fungi Sabouraud dextrose agar was used. Results were expressed in terms of log10 CFU/mL (Hassan et al. 2014).
Statistical analysis
The experimental data were performed in triplicate and expressed as mean ± standard error. The data were analyzed following Analysis of Variance (ANOVA) using Microsoft Excel 2016. The significance value of data was determined at p ≤ 0.05.
Results
Plants extraction yield
The highest yield was found to be of methanol (4.58%) followed by ethanol (3.27%) in the case of C. sinensis and ethanol (2.44%) followed by water (2.40%), in the case of C. assamica (Table 1).
Phytochemical analysis
The different extract of C. sinensis and C. assamica showed the presence of alkaloids, flavonoids, carbohydrates, terpenoids, saponins, tannins, steroids (Tables 2 and 3).
Antimicrobial activity assay of plant extract
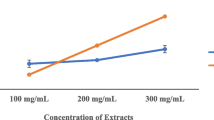
Results of the antimicrobial activity evaluation of Camellia two species by agar-well and disc-diffusion methods were demonstrated in Figs. 1 and 2, respectively. It showed that the C. sinensis extract is more effective against S. aureus followed by P. aeruginosa; whereas, C. assamica extract is more effective against P. aeruginosa followed by S. aureus. The methanolic extract of C. sinensis was found more potential as compared to methanolic extract of C. assamica against the pathogenic bacteria. Hence, it was proved that plant extract is very effective against both bacterial species.
Time-killing assay/Survival of microbial species
Qualitative analysis of bacteria survival/time-killing assay, at different time intervals, was demonstrated in Table 4. The methanolic extract of C. sinensis showed more efficiency against P. aeruginosa, E. coli, C. albicans followed by K. pneumoniae, S. aureus, S. typhi and L. monocytogenes (Table 4).
Discussion
There are many factors that may influence the antibacterial activity of green tea leaves such as the origin of tea plants, breeding, extract preparation, and nature of tea clones and seedlings (Grange and Davey 1990) and geographic differences (Boyanova et al. 2005).
The phytochemical screening and quantitative estimation of the percentage crude yields of chemical constituents of the plants showed that the leaves and stems were rich in alkaloids, flavonoids, tannins, and saponins. They were known to show medicinal activity as well as exhibiting physiological activity (Edeoga et al. 2005). Therefore, both the Camellia species depicts the presence of phytochemical that might be responsible for medicinal and therapeutic activity (Tables 2, 3).
Steroids were found in all the plants. Furthermore, some of these investigated plants contained steroidal compounds. It should be noted that steroidal compounds are of much importance and interest in pharmacy due to their relationship with compounds as sex hormones (Okwu 1999). These phytochemicals also called secondary metabolites that are present in plant extracts. They showed potentials antibacterial activity that can be exploited as the alternatives of antibiotics in the treatment of bacterial infections (Vaghasiya et al. 2011). Antimicrobial and antioxidant potential of some medicinal plants has great attention in both food and pharmaceutical industries because it contains natural compounds that replace the synthetic antioxidant and antimicrobial substances (Deba et al. 2008). The high number of polyphenols present in plants has a great significance as a natural destroyer against pathogenic microbes (Baravalia et al. 2009). A large number of flavonoids are capable of showing antioxidant potential which is effective against broad range of microbes (Kasolo et al. 2010). It has been reported that the flavonoids possess anticarcinogenic, anti-mutagenic, anti-inflammatory, and antioxidant potential effects (Hausteen 2005; Li-weber 2009). It helps to reduce high blood pressure as well (Ayinde et al. 2007). Tea plants could be useful to reduce disease severity.
Alkaloids are the naturally occurring nitrogen compound, commonly found to have potent antimicrobial activity because of their capability to disturb the functioning of the DNA of microorganisms (Kasolo et al. 2010). It has been reported that the phytochemicals present in plant-based food also help to improve blood pressure, glucose level, cholesterol rate etc. (Broadhurst et al. 2000; Kelble 2006). Cardiac glycoside has been pharmacologically active. It might be used to treat heart failure and give strength to the heart muscles (Sospeter et al. 2013). The role of tannins helps to prevent the degenerative diseases and exhibit antimicrobial potential against pathogenic microorganisms (Ngoci et al. 2011). Many researches reported that catechins are present in Camellia species are also responsible to damage bacterial membrane (Cho et al. 2008). It has been reported that extracts of tea leaves contain polyphenolic compounds with activity against a broad spectrum of microbes. Studies conducted over the last 20 years have depicted that the green tea polyphenolic catechins, including (−)-epigallocatechingallate (EGCg) and (−)-epicatechingallate (ECg), can impede the growth of wide range of Gram-positive, Gram-negative bacteria and fungal species with significant potency (Inamdar et al. 2014).
Survival of bacteria/time-killing assay outcomes showed that it depends upon the concentration of extract and time-dependent. The time kill assay show the bactericidal activity and the duration of a bacteriostatic effect of a fixed concentration of the antimicrobial agent, thereby contributes a clear analysis of the relationship between the mortality microbial population and the antimicrobial agent concentration (Igbinosa and Idemudia 2016). At a fixed dilution (10–3) methanol extract of C. sinensis kills P. aeruginosa at (50 µg/mL) concentration. The survival of bacteria assay could be useful to evaluate the particular dose and time interval to inhibit the pathogen. The compound Epigallocatechin gallate in tea leaves has also been delineated to interact with the outer membrane of bacteria and may prevent the adhesion to epithelial cells (HEp-2), in mammals and probably without amendment in epithelial cells (Sharma et al. 2012). Another mechanism is C. sinensis extract may affect the activity of dihydrofolate reductase, as well as increase the thickness of the epidermis (Chung et al. 2003).
Conclusion
It has been well known the rate of resistance of pathogens towards the antibiotics increasing day-by-day. Therefore, the extracts of medicinal plants and their compounds creates a new interest as an antimicrobial agent. The compounds present in the tea extracts might be pharmacologically acceptable because the natural compounds that are extracted from tea do not show any side effects on human health. Screening of phytochemical compounds in tea extract that have potent antimicrobial and antioxidant properties. The background of the manuscript indicate that C. sinensis and C. assamica phyto-complex extracts have an efficacious antimicrobial potential, as evidenced by the inhibitory effect on bacterial growth of different human pathogens.
The antimicrobial properties of C. sinensis and C. assamica extracts are of great interest in the light of the ongoing threat of microbial strains developing resistance to conventional antibiotics. These outcomes are also interesting in the perspective of biologically active molecules present in Camellia species from agro-industrial by-products.
References
Ayinde BA, Onwukaeme DN, Omogbai EKI (2007) Isolation and characterization of two phenolic compounds from the stem bark of Musanga cecropioides R. Brown (Moraceae). Acta Pol Pharm 64:183–185
Baravalia Y, Kaneria M, Vaghasiya Y, Parekh J, Chanda S (2009) Antioxidant and antibacterial activity of Diospyros ebenum Roxb. leaf extracts. Turk J Biol 33(2):159–164
Boyanova L, Gergova G, Nikolov R, Derejian S, Lazarova E, Katsarov N, Mitov I, Krastev Z (2005) Activity of Bulgarian propolis against Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J Med Microbiol 54:481–483
Broadhurst CL, Polansky MM, Anderson RA (2000) Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem 48:894–952
Cabrera C, Gimenez R, Lopez MC (2003) Determination of tea components with antioxidant activity. J Agric Food Chem 51:4427–4435
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med 5(1):13
Cho YS, Schiller NL, Oh KH (2008) Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr Microbiol 57(6):542–546
Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH et al (2003) Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 17(13):1913–1915
Cushnie TT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26(5):343–356
Deba F, Xuan TD, Yasuda M, Tawata S (2008) Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 19(4):346–352
Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4(7):685–688
Grange JM, Davey RW (1990) Antibacterial properties of Propolis (bee glue). J R Soc Med 83:159–160
Hassan HM, Jiang ZH, Asmussen C, McDonald E, Qin W (2014) Antibacterial activity of northern Ontario medicinal plant extracts. Can J Plant Sci 94(2):417–424
Hausteen BH (2005) The biochemistry and medical significance of the flavonoids. Pharmacol Ther J 96:67–202
Igbinosa EO, Idemudia OG (2016) Anti-vibrio potentials of acetone and aqueous leaf extracts of Ocimum gratissimum (Linn). Trop J Pharm Res 15(4):743–750
Inamdar P, Jelamvazir DS, Patel D, Meshram D (2014) Phytochemical screening and in vitro antifungal activity of Camellia sinensis. Int J Pharm Pharm Sci 6(5):148–150
Infectious Diseases Society of America (2004) Bad bugs, no drugs; as antibiotic discovery stagnates, a public health crisis brews. Infectious Diseases Society of America, Alexandria, VA
Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW (2010) Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res 4(9):753
Kelble A (2006) Spices and type 2 diabetes. Nutr Food Sci 35:81–87
Li-Weber M (2009) New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituent Wogonin, Baicalein and Bacalin. Cancer Treat Rev 35:57–68
McKay DL, Blumberg JB (2002) The role of tea in human health: an update. J Am Coll Nutr 21:1–13
Mehrotra S, Srivastava AK, Nandi SP (2010) Comparative antimicrobial activities of Neem, Amla, Aloe, Assam Tea and Clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res 4(23):2473–2478
Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 25(2):361–366
Nazer A, Kobilinsky A, Tholozan JL, Dubois-Brissonnet F (2005) Combinations of food antimicrobials at low levels to inhibit the growth of Salmonellasv.Typhimurium: a synergistic effect? Food Microbiol 22(5):391–398
Ngoci SN, Mwendia CM, Mwaniki CG (2011) Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. J Anim Plant Sci 11(1):1364–1373. https://www.biosciences.elewa.org/JAPS
Okwu DE (1999) Flavouring properties of spices on cassava Fufu. Afr J Roots Tuber Crops 3(2):19–21
Padmini E, Valarmathi A, Rani MU (2010) Comparative analysis of chemical composition and antibacterial activities of Mentha spicata and Camellia sinensis. Asian J Exp Biol Sci 1(4):772–781
Reygaert WC (2018) Green tea catechins: their use in treating and preventing infectious diseases. BioMed Res Int 2018:9105261. https://doi.org/10.1155/2018/9105261
Sesso H, Gaziano J, Buring J, Hennekens C (1999) Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol 149:162–167
Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R (2012) Green tea extract: possible mechanism and antibacterial activity on skin pathogens. Food Chem 135:672–675
Siddhuraju P, Mohan PS, Becker K (2002) Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem 79(1):61–67
Singh Arora D, Jeet Kaur G, Kaur H (2009) Antibacterial activity of tea and coffee: their extracts and preparations. Int J Food Prop 12(2):286–294
Sospeter NN, Josphat M, Charles GM, Charles MM, George KK (2013) A review of some phytochemicals commonly found in medicinal plants. Photon Int J Med Plants 105:135–140
Toda M, Okubo S, Hiyoshi R, Shimamura T (1989) Antibacterial and bactericidal activities of Japanese green tea. Nippon Saikingaku Zasshi 44(4):669–672
Vaghasiya Y, Dave R, Chanda S (2011) Phytochemical analysis of some medicinal plants from western region of India. Res J Med Plant 5(5):567–576
Yang Y, Zhang T (2019) Antimicrobial activities of tea polyphenol on phytopathogens: a review. Molecules 24(4):816
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pradhan, S., Dubey, R.C. Evaluation of phytochemical, antimicrobial and time-killing assay of Camellia species. Vegetos 33, 759–765 (2020). https://doi.org/10.1007/s42535-020-00153-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-020-00153-2