Abstract
Present work describes enzyme-based optic fiber biosensor for the estimation of inorganic phosphate. A biosensor is based on the inhibitory effect of inorganic phosphate on acid phosphatase activity. Acid phosphatase catalyzes the conversion of p-nitrophenyl phosphate to a colored product p-nitrophenol, which is detected optically at 405 nm. This reaction is inhibited by inorganic phosphate present in the sample and the extent of inhibition is correlated to its concentration. Acid phosphatase immobilized in agarose–jellose composite is used as biocomponent for the fabrication of optic fiber biosensor. A calibration curve for inorganic phosphate was obtained with KH2PO4 and used for the determination of inorganic phosphate in the sample. This optic fiber biosensor was applied for the estimation of inorganic phosphate in complex urine samples. This biosensor is easy to fabricate moreover it has shown a shelf life of more than 80 days with a linearity range from 20 to 100 µM. The response time of the sensor was found to be 20 min.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The levels of inorganic phosphate in the urine samples are significant in the clinical diagnosis of hyperparathyroidism [1], hypoparathyroidism, and vitamin D deficiency [2]. Normal excretion of inorganic phosphorus in 24 hours urine varies from 0.4 to 2.0 g., the average value is about 1 g [3], which depends upon diet. Normally urine inorganic phosphate is measured colorimetrically by using Fiske and Subbarow method [4]. The principle behind it is an acid-molybdate reagent which reacts with inorganic phosphate to form phosphomolybdic acid. The hexavalent molybdenum of the phosphomolybdic acid is reduced by means of 1, 2, 4-aminonaphtholsulphonic acid to give a blue compound which is estimated colorimetrically.
Apart from this various chemical methods have been [5,6,7,8,9,10] reported for estimation of inorganic phosphate. There are certain disadvantages with the classical colorimetric methods such as strong acidic conditions and stability of color. Biosensors combine the specificity of biological molecules with the sensitivity of the transducer. They offer many advantages and therefore being explored for inorganic phosphate determination. There are few biosensors reported for the same.
Enzyme based optical biosensor is a biosensor that employs an optical fiber or optical fiber bundle, as a platform for the biological recognition element, and works as a transducer for resultant signal (e.g. absorbance, chemiluminescence, fluorescence etc.). The abundance of enzyme-based optical fiber biosensors [11,12,13,14,15,16,17,18,19,20,21,22] have been reported in the literature is likely due to the inherent selectivity of that enzyme for a specific analyte, and the ease with which they are employed in sensing. Our group has developed an optical fiber biosensor based on reflectance measurements for 3, 4-dihydroxyphenylalanine (L-dopa) based on its reaction with the enzyme tyrosinase [23].
There are only a few reports for inorganic phosphate biosensor with optical transducer [24,25,26,27,28]. In all these optical biosensors chemiluminescence signal has been monitored for the determination of phosphate.
Moreover, there are various references where amperometric biosensors have been employed for phosphate detection and estimation. [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Amongst all the electrochemical biosensors only one sensor has been applied for the estimation of inorganic phosphate in urine samples. A sensor comprises a plant tissue electrode in combination with glucose oxidase [32]. Measurements are based on the inhibition of the acid phosphatase activity contained in a thin slice of Solanum tuberosum. However, the main disadvantage of this dual enzyme biosensor is the difficulty of use in biological fluids which already contain glucose.
Recently Chen-Zhong Li et al. developed an apparatus based on reflection-mode fluorescence-sensing, leveraging an environmentally sensitive fluorophore (MDCC) bound to a bacterial phosphate-binding protein to generate a fluorescent optical signal proportional to the concentration of (Pi) present with a response time of only 4 s [50].
All these biosensors are depending upon two or more than two enzymatic reactions, which make the optimization process of biosensor difficult. As per our knowledge, there are no reports on biosensors for estimation of inorganic phosphate comprising of a single enzyme using optical fiber as a transducer (absorbance mode).
2 Materials and methods
2.1 Material
Acid phosphatase extracted and partially purified in our laboratory from Phaseolus vulgaris. The chemicals KH2PO4 and p-nitrophenyl phosphate (pNPP) were purchased from Sisco Research Laboratory (India). All chemicals were of analytical grade. All the solutions were prepared with water from the Millipore Milli-Q system.
The absorbance studies were carried out on a commercially available Ocean Optics PC 1000 spectrophotometer capable of measuring absorption in the wavelength range 330–780 nm. The light source used was a tungsten-halogen lamp. The enzyme membrane was attached to fiber and inserted in a cell from the top. The sensing was in a thin syringe with a bundle of 16 fibers incorporated together.
2.2 Isolation of jellose from tamarind seed
Tamarind seeds (Tamarindus indica) are available in large quantities in India and South East Asia. The isolation of polysaccharide from the seeds was carried out by roasting the seeds at 80 °C for nearly 4 h in an oven. Afterward, seeds were soaked in warm water (40 °C) for 48 h. The brown coat of the seeds was removed completely. The inner white kernels were washed thoroughly and blended with distilled water to form a paste. The paste was then soaked in enough amount of n-butanol and stirred thoroughly. This was kept for 24 h; about 350 ml of n-butanol was used to defatten 250 g of the kernel paste. This defatted paste was dried and the creamy white granular powder was obtained. This powder was then pulverized in a mortar and pestle to get a fine powder called Tamarind kernel powder (TKP) and this, in an airtight bottle. 1 g of the fine Tamarind kernel powder (TKP) was weighed and dissolved in 1000 ml of boiling water with constant stirring and kept overnight in a hot water bath. The next day, the solution was filtered through a cheesecloth to remove insoluble proteins and other impurities. The filtrate was concentrated by a flash evaporator and precipitation was carried out with 3–4 volumes of ethanol, with constant stirring using a mechanical stirrer. The solution as vacuum filtered to obtain the residue which contained polysaccharides. The residue was dried at room temperature, by keeping in a desiccator. This was used as one of the components of the composite biopolymer matrix.
2.3 Extraction and partial purification of acid phosphatase enzyme
The enzyme acid phosphatase was extracted from the seeds of Phaseolus vulgaris by using acetate buffer saline (pH 5.5, 0.1 M). It was further purified by 70% ammonium sulphate salt saturation concentration and DEAE-ion-exchange chromatography.
2.4 Entrapment of acid phosphatase in agarose–jellose membrane
1 ml of 3% agarose was mixed with an equal volume of 1% jellose and the solution was allowed to cool to ~ 40 °C. Then partially purified ACP (25 µg) added to this mixture and thoroughly mixed and cast on a plastic sheet (2.4 × 3.7 cm2) supported by a glass slide. It was allowed to dry at 37 °C. The membrane was slowly and carefully detached from the plastic support and stored under refrigeration in order to prevent any bacterial growth on the membrane. A small piece of the immobilized membrane (5 mm2) containing 1.3 units of the enzyme was cut and fixed at the tip of the optic fiber and then the fiber was inserted in the cell from the top.
2.5 Construction of optic fiber biosensor
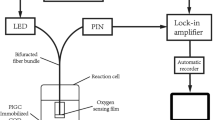
The acid phosphatase inhibition based biosensor was developed for the estimation of inorganic phosphate in the urine sample as shown in Fig. 1. Fabrication of the biosensor was carried out by employing ACP immobilized membrane as biocomponent of the sensor, para-nitrophenyl phosphate (pNPP) was used as a substrate. Acid phosphatase catalyzes the hydrolysis of pNPP to para-nitrophenol, which was measured in terms of absorbance at 405 nm. The liberation of para-nitrophenol is inversely proportional to inorganic phosphate. All the measurements were carried out on a commercially available ocean optics PC 1000 spectrophotometer.
2.6 Measurement procedure
All the measurements were carried out in a cell at room temperature by using the following assay system.
The acetate buffer (pH 5.5, 0.1 M) and varying concentrations of inorganic phosphate (KH2PO4) were taken (in the range 40–140 µM) in the cell. The optrode was kept in the cell for 5 min. Later on substrate p-nitrophenyl phosphate (60 µM) was added and the reaction was allowed to take place for 10 min at room temperature. The reaction was arrested by 1 ml Tris–HCl (pH 9, 1 M). The colored product pNP formed was measured at 405 nm. The percentage inhibition of acid phosphatase for inorganic phosphate was calculated using the expression 3.1.
where \(E_{o}\) is the absorbance in the absence of inhibitor and \(E_{m}\) is the absorbance at 405 nm with different concentrations of inorganic phosphate.
The lower limit of detection was calculated by following the same procedure as described above with a concentration of inorganic phosphate as 20 µM and 10 µM.
Before measuring the colored product formed due to the action of acid phosphatase, a blank polymer was attached to the tip of the fiber and was inserted in the cell and response was recorded as reference spectra (baseline). Then a fresh piece of acid phosphatase immobilized in the composite matrix (A–J) was placed on the optic fiber tip and inserted in the cell, the photometric response was recorded and compared in terms of absorbance of the solution.
The response of the optrode was measured as a function of wavelength. The absorbance of the colored complex formed was recorded from wavelength 330–500 nm to find out the λmax, where the maximum absorbance was observed. This wavelength was selected for further absorbance measurements.
2.7 Optimization of substrate concentration
The response of the reaction catalyzed by acid phosphatase was checked for various substrate concentrations in the range 10–80 µM and a spectrum, as absorbance against para nitrophenyl phosphate.
2.8 Effect of pH on the response of optrode
The stability of the ACP sensor was studied with respect to pH. Buffers of different pH (3.6–6.0) with the same molarity (0.1 M) were prepared and the response of optrode was measured with constant substrate concentration (60 µM). Absorbance was measured and a graph of absorbance versus pH was plotted to find out the optimum pH of the sensor.
2.9 Real sample analysis
The urine samples were obtained from Ruby Hall clinic; those samples were collected over a period of 24 h by the clinic.
The fabricated sensor measures the change in absorbance by optic fiber. The change in absorbance is due to the inhibition of the ACP enzyme. % inhibition of enzyme is directly proportional to inorganic phosphate concentration. The urine sample diluted 50 times with acetate buffer (pH 5.5, 0.1 M) and then the response was checked under identical conditions as for standard KH2PO4 solution. The signal obtained was related to a calibration curve to calculate inorganic phosphate concentration in the urine sample.
2.10 Effect of interferents on the response of the biosensor
The interference of uric acid, urea, glucose, and chloride in the estimation of inorganic phosphate was checked by measuring the optrode response in presence of a fixed concentration of KH2PO4 (40 µM) and substrate pNPP (60 µM) as mentioned earlier. Thereafter the optrode response was measured for each interferent (urea 65 g/L, uric acid 25 g/L, oxalic acid 50 mg/L, glucose 200 mg/L, and chloride as NaCl 10 g/L) in presence of the same concentration of KH2PO4 and pNPP. The % inhibition of acid phosphatase for each interferent was calculated and compared with the % inhibition of acid phosphatase obtained with only inorganic phosphate in the absence of interferents.
2.11 Shelf life
The Storage stability of the biosensor was checked by employing the agarose–jellose membrane at the tip of the fiber. The response of optrode for hydrolysis of pNPP was monitored every 7 days until there is a considerable decrease in enzymatic activity. Agarose–jellose acid phosphatase membranes were stored at 4 °C under dry conditions when not in use.
3 Results and discussion
3.1 Partial purification and immobilization of acid phosphatase
The acid phosphatase enzyme fraction obtained after partial purification showed 38 fold purification with a 5% yield. Immobilization is an important step in the fabrication of biosensor; therefore acid phosphatase has been immobilized in the blend of biopolymers agarose and jellose. Various compositions and concentrations of biopolymers were tested for the proper confinement of acid phosphatase. For the first time, acid phosphatase has been successfully immobilized in a composite of agarose–jellose. The % retention of enzyme activity was calculated as follows
In the present studies, 66% retention of enzyme activity was observed. The maximum retention of enzyme activity with negligible leaching was observed due to the fine-tuning of porosity achieved by mixing two polysaccharides in the desired proportion. Comparable Km and Vmax values (Table 1) of free and immobilized enzyme shows that there is no change in the conformation of the enzyme as well as no constraint on the diffusion of substrate and subsequent release of the product.
3.2 Optrode response
The response of the constructed optrode was measured in terms of the absorbance of the solution. The agarose–jellose membrane was employed for the hydrolysis of pNPP, the colored product pNP formed after the enzymatic reaction was easily dispersed in the solution completely. Inhibition of the immobilized acid phosphatase was measured using KH2PO4 as a standard for inorganic phosphate. The overall reaction taking place in the cell is given as follows.

This reaction, catalyzed by acid phosphatase is inhibited in the presence of inorganic phosphate present in the sample which results in a reduction of color production that is inversely proportional to the inorganic phosphate concentration.
3.3 Optimization of substrate concentration for estimation of inorganic phosphate
The substrate (pNPP) concentration to be used for the calibration curve for the estimation of inorganic phosphate was determined from varying substrate (pNPP) assay carried out in the range of 10–80 µM (Fig. 2). The determination of the colored complex in the absorbance mode gave a stable and concentration-dependent increase in the absorbance signal at 405 nm.
The intensity of the colored complex is inversely proportional to the inorganic phosphate after reaction with immobilized acid phosphatase. Under the present experimental conditions, the acid phosphatase optrode exhibited a concentration-dependent response for inorganic phosphate. Spectra for varying concentrations of inorganic phosphate were recorded (Fig. 3). The outermost spectrum is without inhibitor. The calibration curve was constructed by evaluating the inhibition degree against the concentration of inorganic phosphate. Figure 4 shows the calibration graph obtained, from which it can be noticed that the dynamic range of the biosensor for inorganic phosphate is in the range 40–140 µM. The response of the sensor was measured for 10 µM and 20 µM concentration of inorganic phosphate and it was detectable only for 20 µM which is the lower detection limit of the sensor. The lower detection limit of the fabricated sensor is comparable with that of chemiluminescence based optical biosensor for phosphate ion using pyruvate oxidase enzyme [24]. To check the reproducibility of the measurements, the experiment was carried out three times; the relative standard deviation was calculated and found to be 4.3%.
3.4 Effect of pH
The response of the acid phosphatase optrode to the variation of pH was determined by using different buffers in the pH range from 3.6 to 6.0. The optimum pH of the sensor (Fig. 5) was found to be comparable with the reported immobilized acid phosphatase [51]. Therefore all measurements were carried out at pH 5.5.
3.5 Storage stability
The ACP immobilized A–J membranes were stored at 4 °C under dry conditions. The activity of immobilized acid phosphatase was periodically measured after every 7 days. It has been observed that the immobilized enzyme could retain its activity for more than 84 days. A lifetime of acid phosphatase is very high compared to the acid phosphatase employed in electrochemical biosensor for the estimation of inorganic phosphate in a urine sample [32]. It may be due to the suitable microenvironment provided by the support matrix A–J.
3.6 Application of optic fiber biosensor for the determination of inorganic phosphate in a urine sample
The fabricated sensor was used for the estimation of inorganic phosphate in real urine samples. The inhibitory effect exerted by inorganic phosphate present in the urine sample was correlated with standard inorganic phosphate to determine its concentration. Here sample pretreatment is not required except dilution (1:50). Six successive measurements of each sample were taken and the average optical response was considered to calculate inorganic phosphate level. The values obtained by the present optical biosensor were in good agreement with the auto-analyzer values as shown in Table 2.
Normally inorganic phosphate values are expressed in grams. Therefore the values obtained from the standard graph are calculated as
3.7 Interference studies
Uric acid, urea, glucose, etc. are the major constituents of the multicomponent urine sample; inhibition assays are susceptible to interferents, therefore, the interference of these substances was studied to evaluate the selectivity of the biosensor. No interference was detected from urea ≤ 65 g/L, uric acid ≤ 25 g/L, oxalic acid ≤ 50 mg/L, glucose ≤ 200 mg/L, chloride as NaCl ≤ 10 g/L.
A comparison of the analytical features of the fabricated optical biosensor with that of reported one is given in Table 3.
4 Conclusions
In the present work optic, a fiber-based biosensor has been developed for the estimation of inorganic phosphate in the urine sample. For the construction of biosensor, acid phosphatase entrapped in a blend of polysaccharide agarose and jellose has been successfully employed. This biopolymer composite forms a thin, transparent and flexible membrane that did not show any interference in the absorbance measurement. The colored product formed after the hydrolysis of para-nitrophenyl phosphate was completely dispersed in the solution without any retention on the membrane. From the results obtained, it is concluded that the present optic fiber biosensor is easy to fabricate compared to its electrochemical as well as optical (chemiluminescence) counterpart since amperometric measurements require co-immobilization of two or more enzymes. Further, for designing biosensor based on two or more enzymes need to optimize the compatibility of working conditions for each enzyme reaction especially for pH and co-reactant concentration. In addition to this, the shelf life of the ACP based biosensor is more than 80 days, much higher than the earlier reported sensors. The constructed optic fiber sensor was employed successfully for the quantification of inorganic phosphate in multicomponent urine samples.
References
Bronsky D, Kushner DS, Dubin A, Snapper I (1958) Idiopathic hyperparathyroidism and pseudohypoparathyroidism: case reports and review of the literature. Medicine (Baltimore) 37:317–352
Singer M, Bone J (1954) Fanconi’s syndrome: treatment by massive dosage of calciferol and two-stage osteotomy. Joint Surg Br 36-B:633
Nath RL (1990) Practical biochemistry in clinical medicine, 2nd edn. Academic Publishers, Calcutta
Fiske CH, Subbarow YJ (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Kuttner T, Lichtenstein L (1930) Microcolorimetric studies II. Estimation of phosphorus: molybdic acid-stannous chloride reagent. J Biol Chem 86:671–676
Gomorri GJ (1942) A modification of the colorimetric phosphorous determination for use with the photoelectric colorimeter. Lab Clin Med 27:955–960
Bartlett RG (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–468
Upreti GC (1984) Colorimetric estimation of inorganic phosphate in colored and/or turbid biological samples: assay of phosphohydrolases. Anal Biochem 137:85–492
Katewa SD, Katyare SS (2003) A simplified method for inorganic phosphate determination and its application for phosphate analysis in enzyme assays. Anal Biochem 323:180–187
Patel SP, Patel MA, Modi HR, Katyare SS (2007) Improved method for estimation of inorganic phosphate: implications for its application in enzyme assays. Indian J Biochem Biophys 44:88–93
Kuswandi B (2003) Simple optical fiber biosensor based on immobilised enzyme for monitoring of trace heavy metal ions. Anal Bioanal Chem 376:1104–1110
Schubert F (1993) A fiber-optic enzyme sensor for the determination of adenosine diphosphate using internal analyte recycling. Sens Actuators B Chem 11:531–535
Anders KD, Wehnert G, Thordsen O, Schepere T, Rehr B, Sahm H (1993) Biotechnological applications of fiber-optic sensing: multiple uses of a fiber-optic fluorimeter. Sens Actuators B Chem 11:395–403
Lee SJ, Saleemuddin M, Scheper T, Loos H, Sahm H (1994) A fluorometric fiberoptic biosensor for dual analysis of glucose and fructose using glucose-fructose-oxidoreductase isolated from zymomonas-mobilis. J Biotechnol 36:39–44
Narayanaswamy R, Sevilla F (1988) Optical fibre sensors for chemical species. Anal Lett 21:1165–1175
Schaffar BPH, Dremel BAA, Schmid RD (1989) Ascorbic acid determination in fruit juices based on a fibre optic ascorbic acid biosensor and flow injection analysis. GBF Monogr 13:229–232
Yerian TD, Christian GD, Ruzicka J (1986) Enzymatic determination of urea in water by opto-sensing flow injection analysis. Analyst 111:865–873
Trettnak W, Wolfbeis OS (1990) Fiber optic cholesterol biosensor with an oxygen optrode as the transducer. Anal Biochem 184:124–127
Dremel BAA, Schmid RD, Wolfbeis OS (1991) Comparison of two fiber-optic L-glutamate biosensors based on the detection of oxygen or carbon dioxide and their application in combination with flow injection analysis to the determination of glutamate. Anal Chim Acta 248:351–359
Trettnak W, Wolfbeis OS (1989) A fiber optic biosensor with an oxygen optrode as the transducer. Anal Lett 22:2191–2197
Opitz N, Lübbers DW (1988) Electrochromic dyes, enzyme reactions and hormone—protein interactions in fluorescence optic sensor (optode) technology. Talanta 35:123–127
Wolfbeis OS, Posch HE (1988) A fiber optic ethanol biosensor. Fresenius J Anal Chem 332:255–260
Paranjpe P, Dutta S, Karve M, Padhye S, Narayanaswamy R (2001) A disposable optrode using immobilized tyrosinase films. Anal Biochem 294:102–107
Nakamura H, Ikebukuro K, McNiven S, Karube I, Yamamoto H, Hayashi K, Suzuki M, Kubo I (1997) A chemiluminescent FIA biosensor for phosphate ion monitoring using pyruvate oxidase. Biosens Bioelectron 12:959–966
Kawasaki H, Sato K, Ogawa J, Hasegawa Y, Yuki H (1989) Determination of inorganic phosphate by flow injection method with immobilized enzymes and chemiluminescence detection. Anal Biochem 182:366–370
Noguchi A, Aoki T, Oshima T (1995) Determination of phosphate using immobilized reactor chemiluminescence detector by FIA. J Flow Inject Anal 12:209–214
Ikebukuro K, Nishida R, Yamamoto H, Arikawa Y, Nakamura H, Suzuki M, Kubo I, Takeuchi T, Karube I (1996) A novel biosensor system for the determination of phosphate. J Biotechnol 48:67–72
Nakamura H, Hasegawa M, Nomura Y, Arikawa Y, Matsukawa R, Ikebukuro K, Karube I (1999) Development of highly sensitive chemiluminescence flow-injection analysis sensor for phosphate-ion detection using maltose phosphorylase. J Biotechnol 75:127–133
Menzel C, Lerch T, Scheper T, Schügerl K (1995) Development of biosensors based on an electrolyte isolator semiconductor (EIS) capacitor structure and their application for process monitoring. Part I. Development of the biosensors and their characterization. Anal Chim Acta 317:259–264
Schubert F, Renneberg R, Scheller F, Kirstein L (1984) Plant tissue hybrid electrode for determination of phosphate and fluoride. Anal Chem 56:1677–1682
Linders C, Vincké B, Patriarche G (1985) Electrode hybridepour la determination de phosphates et de polyphosphates. Application aux problemes lies a l’environnement. Anal Lett. 18:2195–2208 (In French)
Campanella L, Cordatore M, Mazzei F, Tomassetti M (1990) Determination of inorganic phosphate in drug formulations and biological fluids using a plant tissue electrode. J Pharm Biomed Anal 8:711–716
Campanella L, Cordatore M, Mazzei F, Tomassetti M, Volpe G (1992) Phosphate determination in foodstuffs using a plant tissue electrode. Food Chem 44:291–297
Matsunaga T, Suzuki T, Tomoda R (1984) Photomicrobial sensors for selective determination of phosphate. Enzyme Microb Technol 6:355–358
Guilbault G, Nanjo M (1975) A phosphate-selective electrode based on immobilized alkaline phosphatase and glucose oxidase. Anal Chim Acta 78:69–80
Su Y, Mascini M (1995) AP-GOD biosensor based on a modified poly (phenol) film electrode and its application in the determination of low levels of phosphate. Anal Lett 28:1359–1378
Guilbault G, Cserfalvi T (1976) Ion selective electrodes for phosphate using enzyme systems. Anal Lett 9:277–289
Wollenberger U, Scheller F (1993) Enzyme activation for activator and enzyme activity measurement. Biosens Bioelectron 8:291–297
Watanabe E, Endo H, Toyama K (1988) Determination of phosphate ions with an enzyme sensor system. Biosensors 3:297–306
Haemmerli S, Suleiman A, Guilbault G (1990) Amperometric determination of phosphate by use of a nucleoside phosphorylase-xanthine oxidase enzyme sensor based on a clark-type hydrogen peroxide or oxygen electrode. Anal Biochem 191:106–109
d’Urso E, Coulet P (1990) Phosphate-sensitive enzyme electrode: a potential sensor for environmental control. Anal Chim Acta 239:1–5
d’Urso E, Coulet P (1993) Effect of enzyme ratio and enzyme loading on the performance of a bienzymatic electrochemical phosphate biosensor. Anal Chim Acta 281:535–542
Male K, Luong J (1991) An FIA biosensor system for the determination of phosphate. Biosens Bioelectron 6:581–587
Wollenberger U, Schubert F, Scheller F (1992) Biosensor for sensitive phosphate detection. Sens Actuators B Chem 7:412–415
Kulys J, Higgins I, Bannister J (1992) Amperometric determination of phosphate ions by biosensor. Biosens Bioelectron 7:187–191
Kubo I, Inagawa M, Sugawara T, Arikawa Y, Karube I (1991) Immobilized pyruvate oxidase. Anal Lett 24:1711–1727
Conrath N, Gründig B, Hüwel S, Cammann K (1995) A novel enzyme sensor for the determination of inorganic phosphate. Anal Chim Acta 309:47–52
Mousty C, Cosnier S, Shan D, Mu S (2001) Trienzymatic biosensor for the determination of inorganic phosphate. Anal Chim Acta 443:1–8
Fernández J, López J, Correig X, Katakis I (1998) Reagentless carbon paste phosphate biosensors: preliminary studies. Sens Actuators B Chem 47:13–20
Mehenur S, Jared L, Ghinwa M, Chen-Zhong L (2019) Smart-phone, paper-based fluorescent sensor for ultra-low inorganic phosphate detection in environmental samples. Microsyst Nanoeng 5:2–10
Kurita K, Yoshino H, Nishimura SI, Ishii S, Mori T, Nishiyama Y (1997) Mercapto-chitins—a new type of supports for effective immobilization of acid phosphatase. Carbohyd Polym 32:171–175
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kulkarni, S.J., Karve, M.S. Fabrication of enzyme-based optical biosensor for estimation of inorganic phosphate in a urine sample. SN Appl. Sci. 2, 1118 (2020). https://doi.org/10.1007/s42452-020-2748-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2748-6