Abstract
Dichloro-diphenyl-trichloroethane (DDT) has been extensively used to control malaria during World War II as well as the other agricultural pests. DDT persists in the environment and accumulates in many living organisms. Also, the degradation products of DDT, which have also a similar chemical structure, could pose the same risk to the environment and human health. Measuring DDT and its degradation products in the environment could be challenging and costs money and time. Thus, modeling is an alternative method used by researchers to estimate the environmental relevant levels of the contaminants. The present study estimates the uptake levels of DDT and its degradation products i.e. o,p′-DDE and p,p′-DDE by crop-specific models (e.g. root, potato, leafy vegetables, cereal, fruit-tree models) in the root, potato, leaves, fruit, cereal, and water compartments by using the known environmental concentrations of DDT and its degradation products (µg kg−1) in soil sampled from Soke, Aydin. According to the crop-specific model results, predicted accumulation levels of DDT and its degradation products were found mainly in leafy vegetables, roots, cereals, and potato. The highest concentration levels were found in leafy vegetables in soil, followed by root, cereals, and potato respectively; however, leaves, fruits, and water had a low amount of accumulation of DDT and its degradation products. Further models are needed to evaluate risk assessment of the environmental compounds to human health and the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
DDT (dichloro-diphenyl-trichloroethane) is a well-known insecticide that was among the nine organochlorine pesticides which were named as “dirty dozen” and was classified as a persistent organic pollutants (POPs) in 2004 at the Stockholm Convention [3]. DDT was first used in 1939 and distributed to other countries around the 1940s [22, 28]. DDT was the most prominent insecticide applied in the study area (Soke, Aydin province, Turkey) throughout the years [27]. One of the main uses of DDT worldwide was to control mosquito, which has been used widely used in 75 countries [16]. Mosquito is one of the major vectors to transmit malaria to humans, which caused millions of deaths [14]. DDT was also used broadly to control insects in agriculture as well as tick control in farm animals i.e. cattle, sheep, goat, etc. [1, 15, 30, 32]. In the mid-1960s, approximately 80,000 tons of DDT was applied to the environment in the United States [20]. It has been stated that China was responsible for 20% of the total global production of DDT between 1950 and 1983 [11, 17, 33]. DDT was banned in the United States in the early 1970s, and 10 years later DDT was also banned in Turkey,however, DDT has been used in sub-Saharan Africa until recently [22, 27, 29]. Further, a study showed that commercially available Dicofol, an acaricide, formulations could contain 14.3% of the total DDT and its degradation product, which could suggest that DDT contamination could also occur from Dicofol application [27].
DDT primarily consist of 65–80% of p,p′-DDT, 15–21% of o,p′-DDT, 4% of p,p′-DDD, and 1.5% of 1-(p-chlorophenyl)-2,2,2-trichloroethanol [13]. DDT degradation products are the result of aerobic biotic degradation, abiotic dehydrochlorination, and photochemical decomposition [12, 22]. According to the U.S. Geological Survey report, the major degradation products were p,p′-DDE (60% in urban and 48% in rural areas), which followed by p,p′-DDD, p,p′-DDT, o,p′-DDD, o,p′-DDT, and o,p′-DDE [22]. Some degradation products of DDT are more persistent than DDT itself, including DDE. DDE was also found in soil 10 years after a DDT application [22].
DDT and its degradation products are persistent in the environment and can cause detrimental effects to humans, plants, and animals that contact with it in the soil, air, and water [26]. DDT can be taken up via plant roots and accumulate in lipophilic plant parts such as waxes and seeds [5, 6]. In addition, DDT and its degradation products have a high affinity for soil, water, and air particles and can move from one compartment to another [8]. DDT and its degradation products have a significant impact on plants, animals, and humans, as DDT accumulates in the food chain [34]. DDT has negative impacts on human health and can contribute to diseases and disorders in neurological, immunological, and reproductive systems and it is a known endocrine disrupter and carcinogen [2, 21]. In the 1960s, it was shown that DDT and its degradation products e.g. DDE and DDD (1,1-dichloro-2,2-bis(p-chlorophenyl)-ethane) detected in high concentrations in mammalian adipose tissues [22]. DDT and its degradation products accumulate in adipose tissues because of their chemical structures [7]. Studies have shown that DDT can be found in human milk, an important nutrient source for human infants,its presence in mother’s milk can have serious consequences for human infants [4, 31].
Models for environmental contaminants could save time and cost with providing invaluable information to understand the potential contamination levels and fate of compounds [3]. Environmental contaminants modeling have been extensively investigated and several models are available to estimate DDT and degradation product concentrations in the soil such as BETR and CliMoChem models and Monte Carlo simulations [3, 18, 19]. One of the modeling approaches that is exclusively used nowadays is crop-specific models which provide an estimation of the fate of chemicals i.e. degradation and uptake [10]. Legind and Trapp [10] used a new model framework to estimate the dietary intake of children (4–5 years old) and women (14–75 years old) by using crop-specific models. The study evaluated the intake of three environmental contaminants including benzo(a)pyrene, 2,3,7,8-TCDD, and dodecyl benzenesulfonic acid from their measured background concentrations in soil and air [10]. The present study aimed to evaluate the levels of DDT and its degradation products (i.e. o,p′-DDE and p,p′-DDE) contamination on root, potato, leaves, fruit, cereal, and water by applying crop-specific models including root, potato, leafy vegetables, cereal, fruit-tree models [10] on analyzed soil samples collected in Soke, Aydin, Turkey [26] where intensive agricultural practices and past DDT applications have taken place in the production of a broad range of products such as olive, wheat, cotton, and tomato.
2 Materials and methods
2.1 Input data
The chemical and physical properties of DDT permit it to highly accumulate in soils with lipophilic characteristics. In soils, environmental conditions support DDT degradation to its primary and secondary degradation products [26]. Turgut et al. [26] sampled soil samples from 74 locations in Soke, Aydin with different soil layers (depth) i.e. 0–30 cm, 30–60 cm, and 60–90 cm. The study results indicated that p,p′-DDT, p,p′-DDE, and o,p′-DDE levels were varied from 0.17–1.92 µg kg−1, 0.003–0.24 µg kg−1, and 0.018–0.48 µg kg−1 in different soil layers [26]. Sum values of the levels for p,p′-DDT, p,p′-DDE, and o,p′-DDE were used in crop-specific model which created by Legind and Trapp [10] to estimate plant uptake of p,p′-DDT, p,p′-DDE, and o,p′-DDE and their possible levels. The calculations were done by using a spreadsheet on Excel file and the parameters i.e. physical and chemical properties of p,p′-DDT, p,p′-DDE, and o,p′-DDE were obtained from EPI suite software (available online: https://www.epa.gov/oppt/exposure/pubs/episuitedl.htm). All data files can be obtained from Dr. Stefan Trapp at the Technical University of Denmark upon request. Also, all equation parameters and details are available and can be obtained from Legind and Trapp [10]. The physical and chemical properties of p,p′-DDT, p,p′-DDE, and o,p′-DDE are given in Table 1.
2.2 Model descriptions
2.2.1 Root model
Our work as presented in this paper is based on models published by Legind and Trapp [10]. In the study, the authors provided mathematical models for the partitioning of TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), dodecyl benzenesulfonic acid (linear alkylbenzene sulfonate, LAS) and benzo[a]pyrene (BaP) and their model predictions in the diet was calculated with plant roots, potato, leafy vegetables, cereals, and tree fruit models. We have calculated the concentration of DDT and its degradation products in the study area according to those models and presented the data here, following the descriptions of the mentioned models above. In the crop-specific model, the concentrations of chemicals in roots, potato, vegetables, fruits, and cereals can be estimated separately while some earlier models consider one generic plant model which consists of roots and leaves [10].
The uptake of DDT and its degradation products from the soil into plants was described with the root and soil equation from the model that Legind and Trapp [10] developed. The chemical equilibrium between soil water and wet bulk soil Kws is described as:
where Cw (mg L−1) is the concentration of the chemical in the soil water; Cs (mg kg−1) is the concentration of the chemical in wet bulk soil; pwet (kg L−1) is the density of the wet soil; OC (kg kg−1) is the fraction of organic carbon in the wet bulk soil; KOC (L kg−1) is the partition coefficient between organic carbon and water which estimated from the octanol–water coefficient (KOW). pdry (kg L−1) is the density of the dry soil, Ws and Gs (L L−1) are the volume fraction of water and gas in soil, and KAW (L kg−1) is the partition coefficient between air and water.
Legind and Trapp [10] described the phase equilibrium between roots and water as KRW (L kg−1), and modeled it according to the following equation:
where CR (mg (kg fresh weight) −1) is the concentration of the pesticide in the root; CW (mg L−1) is its concentration in water; WR (L kg−1) is the water content of the root; LR (kg kg−1) is the lipid content of the root, KOW is the octanol–water coefficient, GR (L kg−1) is the gas content of the root; KAW is the partition coefficient between air and water (air phase could be neglected unless high KAW observed, b (for roots) is equal to 0.77, and a is equal to p octanol−1 (L kg−1) is equal to 1.22.
2.2.2 Potato model
Legind and Trapp [10] provided a model that describes the diffusion process of environmental contaminants from soil to potatoes:
where CP (mg kg−1) is the concentration in plant, Cw (mg L−1) is equal to the chemical concentration in water; W (L kg−1) is the water content of potato, fCH (L kg−1) is the fraction of solids in the potato that are carbohydrates; KCH is the partition coefficient of carbohydrates to water L (kg kg−1) is the lipid content of potato; ɑ is equal to 1/poctanol, which is equal to 1.22 L kg−1; b, the (difference between root lipids and n-octanol is equal to 0.77; GP (L kg−1) is the gaseous content of potato, and KAW is the partition coefficient between air and water.
2.2.3 Leafy vegetables model
Legind and Trapp [10] provided a model that describes the diffusion rate of the environmental contaminants from soil to leafy vegetable, which we applied to DDT and its degradation products:
where L is the index for leaves; W is the index for soil water; A is the index for air; Q is the transpiration stream (L d−1); TSCF is the transpiration stream concentration factor (L L−1) CW is the concentration of the chemical of interest in the soil water (mg L−1); g is the conductance for diffusive transfer between leaves and air (m d−1); CA is the concentration of the chemical in air (mg m−3); KLA is the partition coefficient between leaves and air (m3 kg−1); p is the density of leaves (kg m−3); km is the first-order rate of degradation (d−1); ML is the mass of chemicals in leaves (mg); fP is the fraction of the chemical adsorbed on soil particles, and vdep is the deposition velocity of particles on leaves (m d−1).
2.2.4 Cereal model
The uptake of chemicals and its degradation products was modeled by using the Legind and Trapp [10] cereal model:
where CC is the concentration of the chemical of interest in cereal (mg kg−1 fresh weight); CA is its concentration in air (mg m−3); WC (L kg−1), LC (kg kg−1), GC (L kg−1) and CHC (kg kg−1) are respectively the water, lipid, air and carbohydrate concentrations in cereals; ɑ is 1/poctanol is equal to 0.22 L kg−1; KOW is the partition between octanol and water; b for cereal is the same as for leaves and is equal to 0.95; KAW is the partition coefficient between air and water, and KCH is the partition coefficient of carbohydrates to water.
2.2.5 Fruit tree model
The differential equation for fruit concentration was calculated by Legind and Trapp [10] fruit tree model:
where F is the index for fruits; QF is the sum of the phloem and xylem flow (L d−1); KFW is the partition coefficient between fruits and water (L kg−1); PF is the permeability for exchange between fruits and air (m d−1).
3 Results and discussion
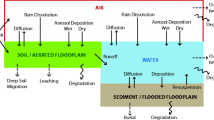
DDT and its degradation products were detected in soil samples collected from the Soke region of Aydin, Turkey [26]. The crop-specific models result showed that DDT and its degradation products primarily accumulate in the root, potato tuber, leafy vegetables with soil and cereals compared to leaves fruits, and water. Thus, we infer that soil plays a crucial role in the accumulation of DDT and its degradation products in plants. DDT and its degradation products accumulated in plant parts where the soil is in direct contact, as compared to plant parts held above the soil, such as fruits and leaves Fig. 1. Legind et al. [9] estimated methomyl insecticide uptake and translocation on pepper through drip irrigation. Study results indicated that modeled estimations by crop-specific model demonstrated quite similar results compared to measured levels of methomyl in pepper fruit [9]. This could strongly indicate that uptake models i.e. crop-specific models can be used to predict and estimate contaminant levels in food including cereal, potato, fruit, vegetables. In addition, crop-specific models could be used to estimate the potential exposure of chemicals to adults and children in the environment through food intake [10]. The study concluded that a crop-specific model was found to be more relevant compared to TGD (the Guidance Document, a risk assessment procedure) in estimating the dietary exposure of TCDD, BaP, and LAS for both children and adults [10]. Trapp et al. [25] conducted a study to test the diffusion of PAHs i.e. naphthalene, phenanthrene, anthracene, and fluoranthene in carrot and potato by using potato model. A study indicated that mass transfer of the chemicals occurs mainly via pore water and the mass transfer ratio between plant tissue and water does not depend on the hydrophobicity of the chemical [25]. In addition to that model, results were in agreement with the field results and suggested that methods were convenient to estimate non-volatile organic chemicals in various plants [25]. Trapp [24] first introduced the fruit tree model (a mathematical model for uptake of organic compounds from soil and air) and the study indicated that model consists of eight compartments including two soil compartments, fine roots, thick roots, stem, leaves, fruits, and air that this model could be useful to predict the accumulation of environmental contaminants into plants. For this reason, we assumed that using a crop-specific model would be an appropriate approach to estimate the levels of DDT and its degradation products p,p′-DDE and o,p′-DDE in the present study.
Estimated levels of DDT (p,p′-DDT) and its metabolites (i.e. o,p′-DDE, p,p′-DDE) in plant parts including root, potato, leaves with soil, leaves, fruit, cereals, and water (mg kg−1). Sum of p,p′-DDT, o,p′-DDE, and p,p′-DDE is given as Total DDT. Accumulation of DDT and its metabolites primarily occurred in the root, potato, leaves with soil and cereals and levels in leaves, fruit, and water was found minimal
The results lead us to affirm that leafy vegetables produced in the area with a prior history of DDT application may contain reduced concentrations of DDT and its degradation products. p,p′-DDT (2.0 × 10–5 mg kg−1) accumulates in leaves with soil more compared to other degradation products p,p′-DDE (2.7 × 10−6 mg kg−1) and o,p′-DDE (7.0 × 10−6 mg kg−1). Furthermore, the results show a reduction of the concentration of DDT and its degradation products in roots with concentration of 2.0 × 10–6 mg kg−1 for p,p′-DDT followed by o,p′-DDE (1.3 × 10–6 mg kg−1) and p,p′-DDE (2.0 × 10–7 mg kg−1). Also, the concentration in potato tuber was found lower than the root concentration of the compounds. The concentration of p,p′-DDT in potato was 1.0 × 10–6 mg kg−1; however, o,p′-DDE was found higher than p,p′-DDT with a concentration of 1.3 × 10–6 mg kg−1. We attribute this reduction to the feeding of the potato tuber by the phloem; perhaps much of the DDT and its degradation products are partitioned by the potato root tissues or leaves before they reach the tubers. DDT and its degradation products accumulated less in cereals compared to leaves with soil and root. Babu et al. [1] found a similar concentration ratio on basmati rice. Soil concentrations of p,p′-DDE and p,p′-DDT was higher compared to plant compartments which followed as husk, straw, grain and root [1]. The difference in the degree of accumulation in different plants and plant parts is related to the chemical structures of these compounds as well as the physiology of the plants and the properties of the soils analyzed. Note the apparent role that soil plays in the compartmentalization of the studies compounds in the various plant parts. Leaves contaminated with soil showed greater concentrations than roots, and roots showed greater concentrations than the phloem-fed potato tubers. The concentration of the DDT degradation products changed in different models o,p′-DDE was higher than other degradation products in root and potato models, while p,p′-DDT was found higher in leaves with soil and cereals. Also, the study indicated that polar, non-volatile compounds transported from soil to fruits while lipophilic compounds accumulate from air into fruit based on the fruit tree model [24]. This could be the reason that the present study results indicated very low accumulation levels into fruit and leaves. However, the deposition through the air can be neglected [9] due to low levels of DDT in the air because DDT has not been used since the 1980s [27]. Another study suggested that very lipophilic compounds such as DDT have a very low diffusion rate and they mostly remain in the peel or root of the vegetables [23]. This could be the reason the small traces of DDT and its degradation products were seen in root and potato compartments in the present study.
4 Conclusion
The present study summarizes the uptake of DDT and its degradation products (p,p′-DDE and o,p′-DDE) from contaminated soil into plant compartments i.e. leaves, fruit, roots, cereal, potato, and water via crop-specific models. The concentrations of DDT and its degradation products in soil were obtained from Turgut et al. [26] and estimated levels were calculated by using the crop-specific model [10]. Model outputs indicated that o,p′-DDE, p,p′-DDE, and p,p′-DDT primarily accumulated in the leafy vegetables in soil, root, cereal, and potato. However, estimated levels of DDT and its degradation products in leaves, fruit, and water were minimal and negligible. Also, it is important to know the contamination levels of DDT and its degradation products to understand the potential impact on the environment and human health via the consumption of plants from the areas where DDT has been applied. Thus, further studies are needed to estimate the exposure of DDT and its degradation products through diet in such areas as previously highlighted [10].
Models are useful tools to predict the fate of the environmental contaminants as well as providing invaluable information for potential outcomes to the human population [3, 10, 18]. In addition, some models could be precise to predict the environmental levels of certain contaminants [9]. Furthermore, models could be used for practical purposes including application rates, pre-harvest intervals [9], in addition to estimating the environmental relevant levels of compounds and how to design remediation and removal practices. For this reason, using the models could help us to understand better about the environmental contaminants and could also save time, money, and effort regarding analytical measurements for environmentally relevant concentrations. Furthermore, the uptake of compounds is mainly depending on several parameters and conditions, which could potentially result in large variations and uncertainty [9]. This could tell us that there is a need to improve the model certainties with further studies and improved models that would give us strengthen outcome. Furthermore, this model can also help to remediate the contaminated soils by rotating the crops variety to minimize the uptake of the environmental contaminants.
References
Babu GS, Farooq M, Ray RS, Joshi PC, Viswanathan PN, Hans RK (2003) DDT and HCH residues in basmati rice (Oryza sativa) cultivated in Dehradun (India). Water Air Soil Pollut 144:149–157
Bian XS, Liu HB, Gan JL, Li R, Yang J (2009) HCH and DDT residues in bivalves Anodonta woodiana from the Taihu Lake, China. Arch Environ Con Tox 56:67–76
Camenzuli L, Scheringer M, Hungerbuhler K (2016) Local organochlorine pesticide concentrations in soil put into a global perspective. Environ Pollut 217:11–18
Cok I, Mazmanci B, Mazmanci MA, Turgut C, Henkelmann B, Schramm KW (2012) Analysis of human milk to assess exposure to PAHs, PCBs and organochlorine pesticides in the vicinity Mediterranean city Mersin, Turkey. Environ Int 40:63–69. https://doi.org/10.1016/j.envint.2011.11.012
Feng K, Yu BY, Wang XL, Ge DM, Wang XZ, Wong MH, Cao ZH (2004) Distribution of organo-chlorine pesticides (DDT and HCH) between plant and soil system. Environ Geochem Health 26:253–258
Gao JP, Garrison AW, Hoehamer C, Mazur CS, Wolfe NL (2000) Uptake and phytotransformation of o,p'-DDT and p,p'-DDT by axenically cultivated aquatic plants. J Agric Food Chem 48:6121–6127
Jaga K, Duvvi H (2001) Risk reduction for DDT toxicity and carcinogenesis through dietary modification. J R Soc Promo Health 121:107–113. https://doi.org/10.1177/146642400112100212
Jan MR, Shah J, Khawaja MA, Gul K (2009) DDT residue in soil and water in and around abandoned DDT manufacturing factory. Environ Monit Assess 155:31–38
Legind CN, Kennedy CM, Rein A, Snyder N, Trapp S (2011) Dynamic plant uptake model applied for drip irrigation of an insecticide to pepper fruit plants. Pest Manag Sci 67:521–527
Legind CN, Trapp S (2009) Modeling the exposure of children and adults via diet to chemicals in the environment with crop-specific models. Environ Pollut 157:778–785
Liu WB et al (2008) Distribution of DDT in a typical DDT waste contaminated site B. Environ Contam Tox 80:280–282
Megharaj M, Jovcic A, Boul HL, Thiele JH (1997) Recalcitrance of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) to cometabolic degradation by pure cultures of aerobic and anaerobic bacteria. Arch Environ Contam Tox 33:141–146
Metcalf RL (1995) Insect control technology. In: Kroschwitz J, Howe-Grant M (eds) Kirk-Othmer encyclopedia of chemical technology, vol 14. Wiley, New York, pp 524–602
Nayyar GML, Breman JG, Newton PN, Herrington J (2012) Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis 12:488–496
Ozcan S, Aydin ME (2009) Organochlorine pesticides in urban air: concentrations, sources, seasonal trends and correlation with meteorological parameters. Clean Soil Air Water 37:343–348
Perez-Maldonado IN et al (2010) Assessment of DDT levels in selected environmental media and biological samples from Mexico and Central America. Chemosphere 78:1244–1249
Pham T, Lum K, Lemieux C (1996) Seasonal variation of DDT and its metabolites in the St Lawrence River (Canada) and four of its tributaries. Sci Total Environ 179:17–26
Schenker U, Scheringer M, Hungerbuhler K (2008) Investigating the global fate of DDT: model evaluation and estimation of future trends. Environ Sci Technol 42:1178–1184. https://doi.org/10.1021/es070870h
Schenker U, Scheringer M, Sohn MD, Maddalena RL, McKone TE, Hungerbuhler K (2009) Using information on uncertainty to improve environmental fate modeling: a case study on DDT. Environ Sci Technol 43:128–134. https://doi.org/10.1021/es801161x
Sericano JL, Wade TL, Sweet ST, Ramirez J, Lauenstein GG (2014) Temporal trends and spatial distribution of DDT in bivalves from the coastal marine environments of the continental United States, 1986–2009. Mar Pollut Bull 81:303–316
Tao S, Li BG, He XC, Liu WX, Shi Z (2007) Spatial and temporal variations and possible sources of dichlorodiphenyltrichloroethane (DDT) and its metabolites in rivers in Tianjin, China. Chemosphere 68:10–16
Thomas JE, Ou LT, Al-Agely A (2008) DDE remediation and degradation. Rev Environ Contam Toxicol 194:55–69
Trapp S (2002) Dynamic root uptake model for neutral lipophilic organics. Environ Toxicol Chem 21:203–206
Trapp S (2007) Fruit Tree model for uptake of organic compounds from soil and air. Sar Qsar Environ Res 18:367–387
Trapp S, Cammarano A, Capri E, Reichenberg F, Mayer P (2007) Diffusion of PAH in potato and carrot slices and application for a potato model. Environ Sci Technol 41:3103–3108. https://doi.org/10.1021/es062418o
Turgut C, Cutright TJ, Mermer S, Atatanir L, Turgut N, Usluy M, Erdogan O (2013) The source of DDT and its metabolites contamination in Turkish agricultural soils. Environ Monit Assess 185:1087–1093
Turgut C, Gokbulut C, Cutright TJ (2009) Contents and sources of DDT impurities in dicofol formulations in Turkey. Environ Sci Pollut Res 16:214–217
Turusov V, Rakitsky V, Tomatis L (2002) Dichlorodiphenyltrichloroethane (DDT): Ubiquity, persistence, and risks. Environ Health Perspect 110:125–128
van den Berg H, Manuweera G, Konradsen F (2017) Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malaria J 16:401
Van Zwieten L, Ayres MR, Morris SG (2003) Influence of arsenic co-contamination on DDT breakdown and microbial activity. Environ Pollut 124:331–339
Voigt K et al (2013) Evaluation of organochlorine pesticides in breast milk samples in Turkey applying features of the partial order technique. Int J Environ Health Res 23:226–246
Wang F et al (2007) Organochlorine pesticides in soils under different land usage in the Taihu Lake region, China. J Environ Sci China 19:584–590
Yang XL, Wang SS, Bian YR, Chen F, Yu GF, Gu CG, Jiang X (2008) Dicofol application resulted in high DDTs residue in cotton fields from northern Jiangsu province, China. J Hazard Mater 150:92–98
Yao FX, Yu GF, Bian YR, Yang XL, Wang F, Jiang X (2007) Bioavailability to grains of rice of aged and fresh DDD and DDE in soils. Chemosphere 68:78–84
Acknowledgements
This study was prepared as a part of the requirements of DTU summer school 12906 Modeling of Plant uptake which conducted by Professor Dr. Stefan Trapp, Department of Environmental Engineering, Technical University of Denmark, Denmark. We would like to thank Linda J. Brewer, Ms, Department of Horticulture at Oregon State University, USA and Khawar Jabran, Faculty of Agriculture and Natural Sciences, Duzce University, Duzce, Turkey for reviewing and editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
The present manuscript was submitted to 2nd International Congress of Agriculture, Environment and Health which was held in Aydin, Turkey and it was accepted by the scientific committee to publish the data on the abstract book. This text is present on a website which can be accessed on https://icaeh.com/gorseller/files/icaeh-2018-tam-metin.pdf. This manuscript is not published nor is under publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mermer, S., Yalcin, M. & Turgut, C. The uptake modeling of DDT and its degradation products (o,p′-DDE and p,p′-DDE) from soil. SN Appl. Sci. 2, 761 (2020). https://doi.org/10.1007/s42452-020-2577-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2577-7