Abstract
Mobility of toxic metals, originating from natural or anthropogenic sources, from soil to groundwater is a matter of utmost concern to human health. Remediation of the contaminated groundwater is of the highest priority as groundwater is an alternate source of freshwater that is used all over the world for drinking purpose. Hence, in the present study, Pteris vittata L. is used as a simple, biodegradable and efficient biosorbent of toxic metals in its non-living and pelletized form by employing organic binders and a simple manual pellet press. The capacity of the pelletized Pteris vittata L. to sequester the metals Cd(II) and Cr(VI) from an aqueous solution is determined through the study on the effect of operating conditions, isotherm and kinetic models. The metal removal capacity of the biosorbent pelletized using corn starch as the binder is 13.51 mg/g for Cd(II) at pH 6 and 1.66 mg/g for Cr(VI) at pH 2 as obtained from the Langmuir isotherm model. The diffusion of the metal ions into the micropores of the pellets aids its biosorption. Physical adsorption, ion exchange, covalent bonding and complexation are deduced to be few of the biosorption mechanisms involved. The findings contribute to the existing data in the biosorption technology. The novelty lies in the use of a weedy fern, Pteris vittata L., pelletized with desired structural characteristics as a potential low-cost biosorbent of toxic metals from groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A war over a source of freshwater is not far away from reality. The scarcity of clean water is increasing day by day. Growing population and industrialization have diminished the quality and quantity of potable water. About two-thirds of the global population and half the population of India are living under the conditions of water scarcity [1]. Besides surface freshwater, groundwater is an alternate source of freshwater that has continuously been subjected to the effect of human activities either directly or indirectly.
The leaching of the pollutants from the waste dump sites, pesticides of the application site soil of agricultural land and the wastewater applied to agrarian areas has led to groundwater pollution [2]. The leachates may contain organic and inorganic pollutants in them. However, the issue of grave concern today is the leaching of heavy metals into groundwater. Heavy metals have always been there, as a part of the environment, but overexploitation of resources and anthropogenic activities have increased their background concentration. Heavy metals over the past few decades have received considerable attention due to their severe effects on the living organisms [3, 4].
It is vital to understand the behaviour and transport of the heavy metals in the groundwater as it is strongly dependent upon the heavy metal adsorption/ desorption capacity of the soil and the properties of the metal ions. Studies have indicated cadmium and chromium to be the highly toxic common heavy metals present in the surface soil that gradually lead to groundwater pollution [5]. Cadmium is a non-essential metal present significantly in the fertilizer-applied surface soil, in effluents of the electroplating and Cd/Ni battery industries, and recycling sites [6, 7]. Chromium (Cr), on the other hand, exists as hexavalent [Cr(VI)] and trivalent [Cr(III)] chromium. Cr(VI) is the more toxic form of Cr. Cr(VI) compared to Cd(II) has high mobility in soil/ groundwater system because of its higher solubility and lesser adsorption by aquifer materials [5, 7, 8]. Heavy metals being highly mobile, non-biodegradable, persistent and toxic upon higher levels of exposure have to be removed from the groundwater before its consumption [8, 9].
Pteris vittata L., a small–medium-sized, non-edible pteridophyte with weedy potential and no seasonal limitations, was studied as a biosorbent of toxic metals in its non-living form. Untreated Pteris vittata L. pinnae tissue in its powdered form presented effective biosorption of Cd(II) and Cr(VI) with maximum removal capacities of 31.3 mg/g and 166.6 mg/g at 30 °C. Characterization studies conducted and reported in Prabhu et al. [10] proved the efficacy of the biomass as a biosorbent for Cd(II) and Cr(VI). Hence, in the present study, Pteris vittata L. powder has been immobilized to obtain a stable biosorbent by pelleting it. Batch-type sorption experiments have been conducted to screen the Cd(II) and Cr(VI) biosorption potential of the pristine Pteris vittata L. pinnae pellets and its application to remove the toxic metals of high initial concentrations.
2 Materials and methods
The biomass, Pteris vittata L., grows resiliently in urban areas and is treated as an urban weed. As the biomass is abundantly available, Pteris vittata L. pinnae free of heavy metal contaminants were collected from Mangalore city and suburbs, India (coordinates: 12.87°N, 74.88°E). The powdered biosorbent was prepared as per the procedure mentioned in Prabhu et al. [10].
2.1 Preparation of Pteris vittata L. pinnae-based adsorbent pellet
Densification of the pristine biosorbent was conducted in a manual pellet press. It includes a cylindrical die (with a diameter of 12 mm, lengths of 34 mm and 13 mm in the presence of the pedestal) and a press (11.88 mm in diameter). Pellets were prepared using various organic binders such as polyvinyl alcohol (PVA) (HiMedia Laboratories Pvt. Ltd., Nashik, India), arrowroot starch (starch from Maranta arundinacea rhizome), potato starch (starch from Solanum tuberosum tuber) and cornstarch (starch from Zea mays grains) (AksharChem®, Gujarat, India) of concentrations 10% and 15%. The binders were specifically chosen because of their ease in availability and unvaried quality [11]. Biodegradability is the main advantage of a biosorbent. Hence, organic binders were chosen.
Initially, organic binders and the binder concentration required to yield stable pellets were determined. The powdered biosorbent of particle size ≤ 180 μm was selected for pelleting. For pelleting of the biosorbent, the powdered biosorbent was mixed thoroughly with the chosen binder (viscous) of suitable concentration at 1:1 ratio. The mixture was transferred into the pellet-press die. An approximate compaction pressure of 44.24 kPa was applied. The procedure was repeated thrice to examine the reproducibility in pellet stability. After pelleting, the pellets were dried in an electric oven at 70 °C for 48 h and stored in an airtight container until use.
2.2 Determination of pellet stability
2.2.1 Water stability test of the pellets under agitation
The stability of the pellets in water was determined by introducing the preweighed dried pellets into a conical flask containing 100 mL of distilled water. The pellets were continuously agitated at 150 rpm in an electrically thermostatic reciprocating shaker (Orbitek, Scigenics Biotech, Chennai, India). From the previously established results by Prabhu et al. [10], it was anticipated that the equilibrium would be attained by 8 h. Hence, the pellets were separated after 8 h, dried at 60 °C and weighed. The difference between the weight of the pellet before and after agitation gives the amount of biomass lost during the study. Pellets with a percentage stability of 80% and above were considered to be stable.
2.2.2 Pellet stability in pH-adjusted water
Stability of the pellets at pH 2–7 was determined by introducing the desired amount of pellets into 100 mL of pH-adjusted water. The study was conducted under continuous agitation speed of 150 rpm. The pellets were separated after 8 h, dried at 60 °C and weighed. The difference between the weight of the pellet before and after agitation gives the amount of biomass lost during the study.
2.2.3 Relaxed density (ρ r) and pellet expansion
The length and diameter of ten randomly selected pellets were measured using a screw gauge immediately after pelletization. The selected pellets were measured again after a week of storage. The density calculated then is the relaxed density.
2.3 General procedure for batch-scale experiments
In batch-scale biosorption experiments, the effect of contact time, initial metal ion concentration and the pellet dosage on metal biosorption was established. All the glassware used was washed with detergent, immersed overnight in 10% HNO3 and rinsed with distilled water several times to prevent cross-contamination. The biosorption of Cd(II) and Cr(VI) by the biomass pellets was conducted at pH 6 and 2, respectively, as previously established by Prabhu et al. [10]. Unless otherwise separately mentioned, all experiments were conducted with 20 g/L of the biosorbent dosage mixed with 100 mL of 100 mg/L metal solutions taken in stoppered Erlenmeyer flasks (250 mL) and agitated at 150 rpm in an electrically thermostatic reciprocating shaker (Orbitek, Scigenics Biotech, Chennai, India) for 120 min. After equilibration, the solution was filtered using Whatman No. 1 filter paper. The filtrate was refrigerated to prevent any bacterial or fungal activity until the completion of analysis. The residual metal concentration was analysed using Flame Atomic Absorption Spectrometer (GBC 932 plus, GBC Scientific Equipment Manufacturer PTY Ltd., Australia) at wavelengths of 428.9 and 228.8 nm for Cr(VI) and Cd(II), respectively. Controls composed of the metal solution with no biosorbent were maintained to determine whether any change in the initial metal concentration occurred.
The amount of metal uptake was calculated from the mass balance equation:
where V is the volume of the metal solution (L) and W is the mass of sorbent (g).
2.4 Preparation of synthetic metal solution
A metal stock solution of 1000 mg/L was prepared in 500 mL of the standard volumetric flask by dissolving 1.4288 g of potassium dichromate [K2Cr2O7] or 0.9137 g of cadmium chloride monohydrate [CdCl2 H2O] in deionized water, respectively. Metal ion solutions of desired concentrations were prepared by diluting the stock solutions.
2.5 Data evaluation
The applicability of the kinetic models on the time course data till the attainment of equilibrium was studied using pseudo-first-order, pseudo-second-order and intraparticle diffusion models. The data acquired from the biosorption studies were subjected to equilibrium modelling to have a better understanding of biosorption. Langmuir, Freundlich, Dubinin–Radushkevich and Temkin isotherm models were used in the present study to analyse the experimental data. Further details on the models are provided in Online Resource 1.
2.6 Statistical analysis
Batch experiments were repeated thrice, and the mean values and error bars representing standard deviation are presented. Data gathered were subjected to one-way analysis of variance (ANOVA) at 5% level of significance, to learn whether there were any statistically significant differences between the means of the independent groups. Since the sample size was small, one-way ANOVA option, Welch’s ANOVA, was applied to determine the significant difference [12]. Upon validation of all the assumptions, intergroup mean values were compared using post hoc Tukey’s test. If the assumption of equal variance was violated, Games-Howell test was applied to the data for multiple comparisons. Statistical Package for the Social Sciences (SPSS) for Windows software was used for statistical analysis.
3 Results and discussion
It is more suitable to use immobilized biosorbent than powdered biosorbent because they reduce the clogging of the column used for water treatment and have better resistance to attrition. Also, the usage of pellets results in localization of the remediated metal, which enables easy handling and removal of the heavy metal from the contaminated sources. Hence, pelletization of the biosorbent, Pteris vittata L., was proposed to be a suitable immobilization technique over the conventional methods, as the separation and multi-cycle application of the biosorbents would be more convenient. Initial studies to assess the feasibility of the immobilization technique, the stability of the pellets and their metal uptake efficiency were conducted to understand the applicability of the biosorbent pellets.
3.1 Standardization of biosorbent pellet preparation and its quality control
3.1.1 Binders and pellet stability
Table 1 displays the stability of the pellets in water excluding the pellets prepared with PVA because the pellets disintegrated upon their contact with water. Starch-based binders were proved to have differed efficiency as binders [13]. However, in the present study, statistical tests confirmed no significant difference (p > 0.05) in the stability of pellet offered by different binders at various concentrations.
The disintegration of the pellets prepared with polyvinyl alcohol (PVA) when put into water can be attributed to the high solubility of PVA in water at high turbulence. The stability of the pellets was affected by the binder and the treating temperature of the pellets. Temperature affects both the binder and the biomaterial. High temperature caused the melting of the components of the biomaterial. Upon cooling, solid bridges are formed between components that strengthen the pellet [14]. By the combined interaction between the moisture content and the biomass components (proteins, starch and/ or lignin) at high temperature and pressure, the proteins present in the biomass undergo plasticization/denaturation and the starch added as binders undergoes gelatinization that promotes better binding of the particles [14, 15]. Also, the waxes and lignins present in the biomass could have undergone a glass transition at 40–50 °C and 65–75 °C, respectively, as reported by Stelte et al. [11]. The combined mechanism of glass transition/plasticization of the biomaterial components and gelatinization of starch-based binder followed by hardening could have played an essential role in binding and improving the strength of the pellets, thus yielding non-significant differences in the stability of pellets offered by different starch-based binders.
3.1.2 Pellet stability in pH-adjusted water
To study the applicability of the pellets at a wide range of water pH, the pellets prepared with 15% corn starch binder were tested for stability from pH 2 to 6. The solution pH influenced the stability of the pellets. As the pH was increased from 2 to 6, the stability of the pellets increased from 65.46 ± 5.06 to 86.53 ± 0.87% at pH 6 (Online Resource 2, Fig. S1). Welch’s ANOVA was conducted to compare the effect of pH on the stability of the pellets. The effect was significant with *p < 0.05, Welch’s F (4, 4.79) = 12.515. Tukey’s post hoc comparisons indicated the pellet percentage stability at pH 2 to be significantly lower (p = 0.000) (M = 65.46, SD = 5.06) than at pH 3 (M = 88.43, SD = 0.65), 4 (M = 86. 3, SD = 1.01), 5 (M = 86.93, SD = 0.47) and 6 (M = 86.53, SD = 0.87).
3.1.3 Relaxed density and pellet expansion
Table 2 presents the compressed and relaxed density of the pellets after a week’s storage. A slight increase in the relaxed pellet length was observed upon storing. However, the change was insignificant (p = 0.626) compared to compressed pellet length. Though there was a significant difference (*p = 0.027) observed in the compressed density and relaxed density, the lower relaxed density could be attributed to the loss of moisture (wt %) content of the pellets during the heat treatment of the pellets before storage. The pellets thus prepared were suitable to be used as a biosorbent.
During the storage of the pellets, the pellets could expand, having a substantial impact on their quality as a biosorbent. The elasticity of the fibres in the biomass causes the spring back of the pellets resulting in volume expansion and reduced density of the pellets. Si et al. [16] explain the probability of longitudinal expansion or increased length of the pellet over diametric expansion. The axial pressure applied to the biomass during pelleting is assumed to be the reason.
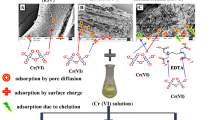
Our previous work [17] inferred amide (–C=O), carboxyl (–COO) and alcohol/ester alkoxy (–CO) to be the functional groups present in the pelletized biosorbent based upon ATR-FTIR analysis. However, amide (–C=O) and alcohol/ester alkoxy (–CO) groups were concluded to be the active functional groups that biosorbed Cd(II) and Cr(VI) ions [10]. In the present work, to obtain maximum uptake of Cd(II) and Cr(VI) on the pelletized biosorbent, the effect of various operating conditions was ascertained.
3.2 Determining the effect of operating conditions to obtain the maximum Cd(II) and Cr(VI) uptake by pelletized biosorbent
The metal biosorption on pelletized biosorbent was tested using only those pellets that passed the stability tests. The effect of operating conditions on the metal biosorption was studied at a temperature of 30 °C. The controls maintained with a predetermined amount of cornstarch powder showed no changes in the initial metal concentration after the experiments, indicating the inability of the binder to remove heavy metals.
3.2.1 Effect of pellet dosage on biosorption of Cd(II) and Cr(VI)
Figure 1 displays a decrease in the equilibrium biosorption amount (mg/g) of Cd(II) and Cr(VI) on the pelletized biosorbent with an increase in the pellet dosage. Figure 2, on the other hand, shows an increase in the biosorption efficiency of the pellets at equilibrium. The uptake of Cd(II) and Cr(VI) was gradual on pelletized Pteris vittata L pellets. The decrease in the metal uptake with an increase in dosage is attributed to unsaturation of the binding sites through adsorption reaction. On the other hand, the higher availability of the exchangeable sites results in increased percent removal of heavy metals. The results are in agreement with the findings of Gavrilescu [18], Suguna and Kumar [19] and Tsekova et al. [20].
Effect of biosorbent dosage on the uptake of Cd(II) and Cr(VI) by the pelletized biosorbent. Conditions: initial metal concentration of 100 mg/L at 30 °C with an agitation speed of 150 rpm. Values are expressed as mean ± SD. ‡‡‡p = 0.000 compared to Cd(II) sorption at 20 g/L, ###p = 0.000 compared to Cd(II) sorption at 20 g/L, †††p = 0.003 compared to Cr(VI) sorption at 15 g/L
3.2.2 Effect of initial metal concentration on biosorption of Cd(II) and Cr(VI) on pelletized biosorbent
The effect of initial metal concentrations was studied from 50 to 200 mg/L with a biosorbent dosage of 20 g/L. The factor of initial metal concentration is vital to understand the sensitivity of the pelletized biosorbent to lower metal concentrations. Figure 3 displays the decrease in biosorption efficiency of Cd(II) and Cr(VI) on the pelletized biosorbent with increasing initial metal concentrations. The decrease in the biosorption efficiency of the pellet at higher concentrations of Cd(II) and Cr(VI) is associated with the saturated active sites. From Table 3, it is observed that the equilibrium metal uptake amount of Cd(II) and Cr(VI) increases with the increase in its concentration. The pellets displayed significant (**p < 0.005) Cr(VI) biosorption at 100 and 150 mg/L compared to 50 mg/L. The increase in equilibrium uptake amount of Cd(II) and Cr(VI) with increasing initial metal concentration is attributed to the necessary driving force provided at higher metal concentrations that helps overcome the mass transfer resistances of the molecules present between the aqueous and the solid phases.
Sağ and Aktay [21] and Pokethitiyook and Poolpak [22] in their work have linked the reduced diffusion of the metal ions in the boundary layer of the biosorbent at higher metal concentrations to the decrease in the biosorption efficiency of the pellet at higher initial metal concentrations. Malkoc and Nuhoglu [23], on the other hand, have associated the increase in metal uptake with the greater driving force provided at higher metal concentrations that help overcome the mass transfer resistance.
3.3 Biosorption kinetic studies
In the present study, the controlling mechanism of metal biosorption was investigated by fitting adsorption reaction models, pseudo-first-, pseudo-second-order and intraparticle diffusion models, to the experimental data. The models were accepted only when the correlation coefficient was close to unity.
A lower correlation coefficient was obtained for the pseudo-first-order kinetic model (Online Resource 3, Table S1) compared to pseudo-second-order kinetic model. Table 4 presents the parameters of pseudo-second-order kinetic model for the sorption of Cd(II) and Cr(VI) on the pelletized biosorbent. From Table 4, it is seen that the experimental and theoretical qe values in pseudo-second-order model were very close. The slight difference is due to the heterogeneous nature of the biosorbent. The low correlation coefficient obtained for the pseudo-first-order kinetic model suggests the biosorption of Cd(II) or Cr(VI) on pelletized biosorbent not to occur onto one site per ion. However, high correlation coefficients for the pseudo-second-order kinetic model indicate the rate-limiting step to be chemisorption. Ho and McKay [24] have reported the pseudo-first-order model to be applicable for an initial period only, whereas the pseudo-second-order model to be applicable for an extended period.
Intraparticle diffusion plays a crucial role in biosorption on a porous biosorbent. Hence, the kinetic data were fitted into the intraparticle diffusion model. Intraparticle diffusion model studies indicated the metal biosorption to be influenced by macro-, meso- and micropore diffusion. Table 5 shows the significance of intraparticle diffusion in determining the uptake of Cd(II) and Cr(VI) on the pelletized biosorbent at various pellet dosages. Upon comparing the Kid values (Table 5), it is evident that micropore diffusion is the rate-determining step as the constants, Kid2 (micropore diffusion constant) were lower than those for the Kid1 (macropore diffusion constant). Both Kid1 and Kid2 constants were in the following order: 10 > 15 > 20 g/L. Lower Kid value represented slower diffusion process. Hence, internal diffusion of Cd(II) and Cr(VI) was fast at pellet dosage of 10 g/L and slowest at 20 g/L, thus justifying the trend obtained for metal uptake amount at various pellet dosages.
The Kid2 constants at all pellet dosages were the lowest for Cd(II). With a molecular weight of 112.41 g/mol, Cd(II) exhibited slow micropore diffusion as indicated by the lowest micropore diffusion constant (Kid2) compared to Cr(VI) of molecular weight 51.99 g/mol, thus indicating the influence of the molecular weight on micropore diffusion. The boundary layer effect was higher at the micropore diffusion stage (C2) than at the macropore stage (C1) as C2 > C1.
3.4 Adsorption isotherms
In the present study, the adsorption isotherms were determined by varying the metal ion concentrations. Langmuir, Freundlich, Dubinin–Radushkevich and Temkin isotherm models in linear forms were tested for the experimental data. Table 6 presents the parameters of the isotherm models. Langmuir isotherm with correlation coefficients of 0.936 and 0.911 was shown to be the best fit to the experimental data compared to Freundlich model. The findings indicate the biosorption of Cd(II) and Cr(VI) on pelletized biosorbent at 30 °C to be monolayered. The maximum Cd(II) and Cr(VI) biosorption capacity (Qo) of the pelletized biosorbent was 13.51 mg/g and 1.66 mg/g. Furthermore, the RL values for Cd(II) and Cr(VI) biosorption showed a favourable biosorption process.
The D-R isotherm model plot (lnqe vs ε2) was directed towards chemical sorption of Cd(II) and Cr(VI) on pelletized biosorbent at 30 °C as free energy, E > 16 kJ/mol. The mean free energy E was calculated to be 256.49 kJ/mol for Cd(II) and 170.01 kJ/mol for Cr(VI). The findings are in agreement with the mean free energy range given by Argun et al. [25], Cavas [26] and Sarı and Tuzen [27]. From Table 6, the value of the heat of adsorption obtained from the Temkin isotherm data signifies the uptake of Cd(II) and Cr(VI) on pelletized biosorbent to be an exothermic process.
4 Conclusions
The focus of the present research was to explore an efficient biosorbent for the removal of heavy metals from the contaminated groundwater for its reuse. Pteridophytes having no direct role in the food chain are treated as weeds because of their rapid reproduction through spores. With no known application, pteridophytes were the suitable candidates for biosorbents of heavy metals. Abundant availability of the pteridophyte Pteris vittata L. in the location of research made the biomaterial more efficient. The best operating conditions required for an effective uptake of heavy metals on the biosorbent were studied by conducting batch-scale experiments. Corn starch of 15% concentration helped in binding the particles of Pteris vittata L. pinnae powder through the plasticization mechanism of the binder, yielding stable pellets. The quality of the pellets was unhampered even upon storage. The pellets, when applied as a biosorbent of Cd(II) and Cr(VI) from an aqueous system, proved to be effective. At a pellet dosage of 20 g/L, the biosorption of Cd(II) and Cr(VI) occurred with maximum efficiencies of 93.62% and 86.77% at 840 min and 1020 min, respectively. Langmuir isotherm model indicated the biosorption of Cd(II) and Cr(VI) on the pelletized biosorbent to be monolayered with maximum biosorption capacities of 13.51 mg/g and 1.66 mg/g. The Cr(VI) removal capacity at the pH of 2 was lower and challenging compared to Cd(II) at pH 6 due to the instability of the biosorbent pellets at the acidic pH. However, the lower molecular weight of Cr(VI) aided its diffusion into micropores of the pellets resulting in the biosorption of Cr(VI) to a certain extent. Physical adsorption, ion exchange, covalent bonding and complexation were deduced to be few of the sorption mechanisms involved. The results obtained in the research work have contributed to the existing data in biosorption technology that can be an alternative treatment method in groundwater treatment of heavy metals. Thus, the pelletized Pteris vittata L. is a potential biosorbent form that can be used efficiently for the removal of a metal pollutant from a near-neutral aqueous system.
References
Mekonnen MM, Hoekstra AY (2016) Four billion people facing severe water scarcity. Sci Adv. https://doi.org/10.1126/sciadv.1500323
Baharudin F, Mohd Tadza MY, Mohd Imran SN, Jani J (2018) Removal of iron and manganese in groundwater using natural biosorbent. Earth Environ Sci IOP Conf Ser 140(1):1–6
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology. Springer, New York, pp 133–164
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Dong D, Zhao X, Hua X, Liu J, Gao M (2009) Investigation of the potential mobility of Pb, Cd and Cr(VI) from moderately contaminated farmland soil to groundwater in Northwest, China. J Hazard Mater 162(2–3):1261–1268
Kachenko AG, Singh B, Bhatia NP (2007) Heavy metal tolerance in common fern species. Aust J Bot 55(1):63–73
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20
Ashraf A, Bibi I, Niazi NK, Ok YS, Murtaza G, Shahid M et al (2016) Chromium(VI) sorption efficiency of acid– activated banana peel over organo-montmorillonite in aqueous solutions. Int J Phytoremediation 19(7):605–613
Sharma A, Sachdeva S (2015) Cadmium toxicity and its phytoremediation: a review. Int J Sci Eng Res 6(9):395–405
Prabhu SG, Srinikethan G, Hegde S (2019) Spontaneous Cr(VI) and Cd(II) biosorption potential of native pinnae tissue of Pteris vittata L., a tropical invasive pteridophyte. Int J Phytoremediation 21(4):380–390
Stelte W, Sanadi AR, Shang L, Holm JK, Ahrenfeldt J, Henriksen UB (2012) Recent developments in biomass pelletization—a review. BioResources 7(3):4451–4490
McDonald J (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing, Baltimore
Ståhl M, Berghel J, Frodeson S, Granström K, Renström R (2012) Effects on pellet properties and energy use when starch is added in the wood-fuel pelletizing process. Energy Fuels 26(3):1937–1945
Tabil LG (1996) Binding and pelleting characteristics of Alfalfa. Dissertation, University of Saskatchewan
Tumuluru JS, Conner CC, Hoover AN (2016) Method to produce durable pellets at lower energy consumption using high moisture corn stover and a corn starch binder in a flat die pellet mill. J Vis Exp 112:1–13
Si Y, Hu J, Wang X, Yang H, Chen Y, Shao J et al (2016) Effect of carboxymethyl cellulose binder on the quality of biomass pellets. Energy Fuels 30(7):5799–5808
Prabhu S, Srinikethan G, Hegde S (2019) Efficient biosorption of Pb(II) on Pteris vittata L. from aqueous solution using pulsed plate column technique. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1675702
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4(3):219–232
Suguna M, Kumar NS (2013) Equilibrium, kinetic and thermodynamic studies on biosorption of lead(II) and cadmium(II) from aqueous solution by polypores biomass. Indian J Chem Technol 20:57–69
Tsekova K, Christova D, Todorova D, Ivanova S (2011) Removal of Cu(II), Co(II) and Fe(III) ions from ternary solution by free and entrapped in PVA-Hydrogel biomass of Penicillium cyclopium. Biotechnol Biotechnol Equip 25(Sup 1):41–46
Sağ Y (2001) Biosorption of heavy metals by fungal biomass and modeling of fungal biosorption: a review. Sep Purif Rev 30(1):1–48
Pokethitiyook P, Poolpak T (2016) Biosorption of heavy metals from aqueous solutions. In: Ansari AA, Gill SS, Gill R, Langa GR, Newman L (eds) Phytoremediation: management of environmental contaminants. Springer International Publishing, Cham, pp 113–141
Malkoc E, Nuhoglu Y (2007) Potential of tea factory waste for chromium (VI) removal from aqueous solutions: thermodynamic and kinetic studies. Sep Purif Technol 54(3):291–298
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J Hazard Mater 141(1):77–85
Cavas L (2008) Comment on Equilibrium sorption isotherm studies of Cd(II), Pb(II) and Zn (II) ions detoxification from waste water using unmodified and EDTA-modified maize husk. Electron J Biotechnol 11(2):1–3
Sarı A, Tuzen M (2008) Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 160(2–3):349–355
Acknowledgements
The authors acknowledge Aishwarya Sanjeev Kumar, Bharath Goud Ediga and Uday Krishna Devara, undergrad students, Department of Chemical Engineering, National Institute of Technology Karnataka, India, for providing help during the research. The authors are thankful to National Institute of Technology Karnataka, Surathkal, Karnataka, India, for the financial assistance provided in the form of Institute Research Fellowship to Smruthi G. Prabhu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prabhu, S.G., Srinikethan, G. & Hegde, S. Pelletization of pristine Pteris vittata L. pinnae powder and its application as a biosorbent of Cd(II) and Cr(VI). SN Appl. Sci. 2, 132 (2020). https://doi.org/10.1007/s42452-019-1906-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1906-1