Abstract
Association of the cathodic operation of Ni(OH)2 doped water dispersible graphene (WDG) nanocomposite with three different electrolytes (NaHCO3, KHCO3, and NaCO3) with the help of carbon dioxide (CO2) electrochemical reduction is presented in this research. Graphene in short time is effectively synthesized and Ni(OH)2 doped on WDG by electrochemical method, structure, morphology and characterization analysis by X-ray diffraction, scanning electron microscope, transmission electron microscope and Raman spectroscopy. CO2 is effectively reduced to carbon monoxide (CO) at enhanced situation. Fourier transform infrared spectroscopy, which is used to exanimate integrated Ni(OH)2–WDG nanocomposite surfaces so as to uncover the carbonate ions and CO during reduction at three different electrolytes. The electron charge resistance of the nanocomposite is investigated by using electrochemical impedance spectroscopy analysis. Analysis of the Ni(OH)2–WDG nano composite was performed to decouple electrochemical reduction processes of CO2 to CO at different electrolytes by cyclic voltammetry and linear sweep voltammetry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Although, CO2 plays a significant part in producing greenhouse effect, it is accountable for the warming of the Earth [1,2,3]. CO2 is emanated, while performing human activities such as deforestation, fossil fuels combustion, and industrial processes. One must follow most of the valuable methods for reducing CO2 emissions, i.e.to diminish the consumption of fossil fuels. In current times, the ratio of atmospheric mixing of CO2 is higher than what it was in the last 800,000 years [4,5,6,7,8,9,10].In the nineteenth century, a chemical scheme of converting CO2 and hydrogen (H2) to methane was developed and later the same was transformed to liquid fuels [6].For the production of gases like carbon monoxide (CO)/H2 and other supportive organic compounds like methanol, methane, ethylene, and formic acid, CO2 was utilized as the C1 feedstock. The organic compounds have been merged with numerous chemical, photochemical, biochemical, and electrochemical procedures [8,9,10].
Owing to several reasons, the conversion of using CO2 electrochemical catalysis methods enticed a great deal of attention among researchers. Let us take a look at these reasons: (1) by means of electrode potentials and reaction temperature, the method is controllable; (2) the supporting electrolytes can be totally reprocessed, so as to minimize the whole chemical utilization to simply water (or) waste water; (3) the electricity utilized to drive the method can be attained without producing any new CO2-sources; and (4) The electrochemical reaction systems are compressed, modular, on-demand, and simple to scale-up [11]. In the past decades, the electrochemical CO2 reductions at metals (e.g. Hg, Cu, etc.) and related electrodes in addition to electrocatalytic CO2 reduction have been extensively examined in aqueous and non-aqueous Medias [12,13,14,15,16,17,18].
Carbon materials incorporates glassy carbon, graphite, boron-doped diamond (BDD), carbon black, carbon nano fiber, carbon tubes (CNTs), and these days graphene is extensively used as an electrode in the elementary innovations and application aspects of electrochemistry and electro analysis [19, 20]. Ni(OH)2/graphene composite has proven its application in energy storage applications like supercapacitor owing to its high specific capacitance of graphene, energy density, and good cyclic stability [21, 22].
In this paper, graphene is synthesised through electrochemical technique and then graphene is converted to WDG. In addition, Ni(OH)2 is doped on the surface on WDG with the help of electrochemical techniques. The cathodic performance of Ni(OH)2–WDG composite at three different electrolytes, namely, NaHCO3, KHCO3, and NaCO3 are investigated in detailed manner.
2 Experimental
2.1 Preparation of graphene
The graphene was made with the help of electrochemical process. The graphite rods were treated both as an anode and cathode by distributing at a distance of 2 cm in 3 M intensity of H2SO4 mixture. This mixture was used as an electrolyte. The graphene was washed from the graphite rod by escalating a potential of more than 7 V through DC electrical power supply. The acquired graphene was blended with the electrolyte mixture. The pure graphene was separated from the solution by several exfoliating with the help of double distilled water.
2.2 Preparation of water dispersible graphene
Graphene powder (0.1 g) was dissolved in 100 mL purified water and sonicated. Furthermore, 0.5 M NaOH is added to the solution drop wise and pH was altered to above 8. Additionally, the mixture was dehydrated at 80 °C to attain powdered mixture [23].
2.3 Ni (OH)2 doped water dispersible graphene
Ni(OH)2 was effetely doped on WDG by using electrophoretic deposition (EPD). The conductive substrate such as stainless steel (SS) is used as negative electrode during the EPD procedure. The substrate was rinsed by ultrasonic vibrations caused in acetone, ethanol, and deionised water, correspondingly. The positive counter electrode was Pt with the same elements. Prior to EPD, the WDG and NiCl2 in 50 mL of deionised water were ultrasonically spread for 2 h. The electrodes were in the gap of 10 mm and immersed in the colloidal solution saturated with nitrogen gas. An applied potential of 2–10 V was used for 30–500 s to deposit. EPD was carried out on a current–voltage monitor and the current accuracy of 13 mA, when the applied voltage came to 10 V. After EPD, the nanocomposite film was dehydrated at 80 °C in an oven overnight.
2.4 Materials characterization
With the help of CuKα(λ = 1.5406Å) as a radiation source, the phase and crystalline nature of the synthesised samples were examined by X-ray diffractometer (XRD) (X’-Pert PRO, PANalytical, The Netherlands). The samples were examined over the 2θ range of 10–80 °C at room temperature (298 K). In relation to the reference diffraction data, the observed peak positions and the relative concentration of the powder pattern were indexed. The surface morphology of WDG and Ni(OH)2–WDG were decided by means of a scanning electron microscope coupled with energy dispersive X-ray analysis (SEM-EDAX) (JEOL JSM-6390LV, Japan) at 20 kV with a magnification of 10000× at 1 μm scale. Functional group information about powder surface was examined by means of FTIS (Spectrum 100; PerkinElmer, USA). The spectra were collected in the range of 4000–500 cm−1.
2.5 Electrodes preparation and characterization
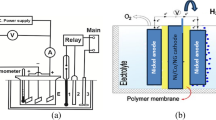
The SS plate was sliced into small pieces (16 cm2) and later on buffed delicately with the help of a solvent (acetone). The synthesized Ni(OH)2–WDG nanocomposites powder was assorted with a polyvinylidene difluoride (PVDF) at a ratio of 85:15 in a small quantity of N-Methyl-2-pyrrolidone as a solvent to make a paste. The paste substance was covered over the separate SS plates by means of a doctor blade [24]. The coating was done repetitively multiple times so as to attain a uniform thickness (10 mm) of electrodes on the SS plates. The Ni(OH)2–WDG nanocomposites electrodes were dehydrated at 85 °C in a hot-air oven for about 1 h and then used for further examination. The method of electrolysis was handled in the terylene diaphragm cell of 100-mL volume. The anode was an SS net (16 cm2) and cathode was an Ni(OH)2–WDG electrode (16 cm2). The gap between the anode and cathode was 2 cm. The electrical power was presented by a laboratory direct current power supply with a current–voltage monitor.
The nitrogen gas was passed into the electrolyte for 30 min so as to eliminate O2, and CO2 gas that was passed into the electrolyte for 30 min before the reaction takes place. NaHCO3 (1 M) was selected as the electrolyte at room temperature thereby keeping the voltage at 4 V. Similar procedure was done for another two different electrolyte (KHCO3 and NaCO3) at as prepared the two Ni(OH)2–WDG electrodes. During the preparative electrolysis, a sample was taken (in 10 min-periods). At a low current density of 8 mA/cm2, Ni(OH)2–WDG electrodes could function. A schematic diagram is given in Fig. 1.
2.6 Electrochemical measurements
The CV and EIS behavior of Ni(OH)2–WDG nanocomposites electrode was investigated with the help of a three-electrode cell set-up. In this set-up, the Ni(OH)2–WDG was used as a working electrode, where as platinum (Pt) and saturated calomel were majorly used as the counter and reference electrodes, correspondingly, for the carbonate-based electrolytes. The CV analysis was done in Ni(OH)2–WDG at different electrolytes in fixed scan rate by means of Autolab equipment (PGSTAT302 N, Metrohm Autolab, and the Netherlands). The applied potential window for CV study was − 1–1 V at 10 mV/s scan rate. The present performance of cathode was determined from the extrapolated data of the CV test.
3 Results and discussion
3.1 Structural characterizations
Figure 2a demonstrates XRD patterns of the WDG sheets, a sharp peak at 26.3 °C (002). The peak experimented in 26.3 °C is attributed to the crystal nature of the WDG [23].
Figure 2b explains XRD patterns of Ni(OH)2–WDG composite, demonstrating that the Ni(OH)2–WDG compound has been well-synthesised, peaks at 12.1, 26.3, 33.3, and 54.4 °C relating to the (003), (002), (101), and (110) diffraction peaks correspondingly [25]. Figure 3 in Raman spectra, the D, G, and 2D bands are evidently seen in 1343, 1575, and 2704 cm−1, in that order, for the WDG sample. This is owing to the in-plane band stretching motion of pairs of sp2 hybridization. The aforementioned examination assures the presence of a graphene monolayer [26].
The morphology and structure of WDG and Ni(OH)2–WDG nanocomposites were explained with the help of SEM and TEM which are demonstrated through Fig. 4A and B. The SEM and TEM that gives a clear sign for the transparency of the WDG implies a thin film over the entire substrate and the Ni(OH)2 well doped on WDG surface.
3.2 FTIR of FeTiO3
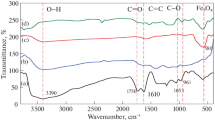
The typical FTIR spectra of Ni(OH)2–WDG exclusive of electro reduction and Ni(OH)2–WDG electrode surface after electro reduction at three different electrolytes is shown in Fig. 5a–d, respectively. Graph (a) clarifies synthesized Ni(OH)2–WDG had four important peaks at 538, 1347, 1452, and 1657 cm−1. The absorption peak 538 cm−1 is due to Ni–OH is stretching for Ni(OH)2, 1347, 1452, and 1657 cm−1owing to the C–H (rack), C–H (bend) and C=C for WDG [26, 27]. Graph (b), (c) and (d) elicited after CO2 reduction in three different electrolytes (NaHCO3), (KHCO3), and (NaCO3) Ni(OH)2–WDG electrodes surface. Graph (b) and (c) had four new peaks that were found (698,834, 996, and 1929 cm−1) due to the C–H, C–H (bend), O–H (carboxylic acid), (C=O) than graph (a) graph (d) for NaCO3 electrolyte after reduction Ni(OH)2–WDG surface was not clear, new peaks obtain than graph (a).After CO2 reduction, more sharp carbonyl functional group adsorption was found on Ni(OH)2–WDG in NaHCO3 and KHCO3 electrolytes than NaCO3 electrolyte.
3.3 Electrochemical impedance spectroscopy
The Nyquist plots of Fig. 6 explain the charge transfer resistance analysis of the Ni(OH)2–WDG nanocomposite at only electrolytes (1 M NaHCO3, KHCO3 and NaCO3). Red color line specifies NaHCO3, blue line indicates KHCO3, and green line denotes NaCO3. This graph gives a clear picture that NaHCO3 electrolyte medium favour high electron charge transfer that occurs on Ni(OH)2–WDG composite electrode surface than KHCO3 and NaCO3 electrolyte medium. The HCO-3and Na+ electrolyte medium favours the low resistance on Ni(OH)2–WDG mixture.
3.4 Cyclic voltammetry
The CV of Ni(OH)2–WDG at three different CO2 saturated electrolytes (1 M NaHCO3, KHCO3, and NaCO3) were done. Cathodic behaviour of as prepared Ni(OH)2–WDG nanocomposites were compared at three different CO2 saturated electrolytes. Calomel electrode was used as reference electrode and Platinum electrode was used as counter electrode. CV study of Ni(OH)2–WDG due to N2: CO2 saturated in three different electrolytes (1 M NaHCO3, KHCO3 and NaCO3) at 10 mV/s scan rate at room temperature was done. As shows in Fig. 7 CV for Ni(OH)2–WDG composite at CO2 saturate at three different electrolytes (1 M NaHCO3, KHCO3 and NaCO3). The clear reduction peaks were observed between − 0.5 and − 0.4 V, that should result from one-electron reduction of CO2, which generated an anion radical of CO2 [28].
This anion radical, however should react rapidly with the intermediate at the desired product. Graph (a) indicates CV for Ni(OH)2–WDG in CO2 saturated at 1 M NaHCO3 electrolyte, graph (b) shows CV for Ni(OH)2–WDG in CO2 saturated at 1 M KHCO3 electrolyte and graph (c) depicts CV for Ni(OH)2–WDG in CO2 saturated at 1 M NaCO3 electrolyte. In CV reduction peaks, positive site shifted depending on electrolyte serial (NaHCO3, KHCO3 and NaCO3). Increasing the cathodic behavior of Ni(OH)2–WDG in CO2 saturates NaHCO3 than KHCO3 and NaCO3 electrolytes. These CV results indicate that electrolyte plays an important role in CO2 electrochemical reduction and HCO−3 ions also offers great help in the CO2 reduction process.
The K+ ions, increasing the cathodic behavior and increasing reaction intermediated for CO2 to CO [29], this CV results represents that the Na+ ions increases the cathodic behavior than K+ ions in electrolyte medium. The Na+ ions are well coordinated to the intermediate components of the CO2 electrochemical reduction.
3.5 Linear sweep voltammogram
LSV is one of the electrochemical studies for the reduction of CO2. Figure 8 gives a clear explanation that the mixture of Ni(OH)2–WDG nanocomposite on reduction of CO2 at different electrolytes (1 M NaHCO3, KHCO3 and NaCO3), high efficiency reduction peak can be seen between − 0.5 and − 0.4 V. A, blue line specified NaHCO3 electrolyte peak appear near to − 0.4 V, red line demonstrates KHCO3 electrolyte peak show near to − 0.5 V and green line signifies NaCO3 electrolyte peak that appear above − 0.5 V. The outcome specified to well evidence that NaHCO3 electrolyte is of great help to CO2 reduction of Ni(OH)2–WDG nanocomposite. Figure 9 tafel plot for LSV of Ni(OH)2–WDG nanocomposite reduction of CO2 at different electrolytes (1 M NaHCO3, KHCO3, and NaCO3). This figure gives a clear view of Ni(OH)2–WDG composite reduction peak that occurs near − 0.4 V and better CO2 reduction behavior than Ag, Pt, and Ru doped TiO2 and Cd, Co (− 0.75) [30].
4 Conclusions
The electrolysis researches were transmitted out in an undivided cell under mild conditions; prevent the adding up of toxic solvents and catalysts. The electrical power was provided by a laboratory direct current power supply with a current–voltage monitor. Ni(OH)2–WDG mixture could be functioned at low current density 8 (mA/cm2) attained with the help of electrode for CO2 reduction at three different electrolytes. XRD specifies good crystalline peaks that exist by effective fusion of WDG. When doped Ni(OH)2 on WDG, it has fully reduced the crystalline structure of WDG and it is well indicated in the XRD for Ni(OH)2–WDG. Raman, SEM, and TEM images signifies WDG had monolayer by effectively blended WDG, another SEM and AFM images epitomizes the white portion point to the Ni(OH)2 fine doped on WDG. FTIR indicates adsorbed carbonyl functional groups on Ni(OH)2–WDG at three different electrolytes (NaHCO3, KHCO3, and NaCO3) after CO2 reduction, increased concentration peaks appear NaHCO3 than KHCO3 and NaCO3 electrolytes on Ni(OH)2–WDG electrode surface. The results of CV and LSV specifies the good cathodic behavior of Ni(OH)2–WDG composite in CO2 reduction than Cd, Co, and Cu (− 0.75), this consequence epitomises Ni(OH)2–WDG has better CO2 reduction behavior (− 0.4 V) in NaHCO3 than the KHCO3 and NaCO3, Na+ and HCO−3 ions augments the CO2 reduction cathodic behavior of Ni(OH)2–WDG electrode.
References
Gao J, Wu Y, Jia C, Zhong Z, Gao F, Yang Y, Liu B (2016) Controllable synthesis of α-MoC1 − x and β-Mo2C nanowires for highly selective CO2 reduction to CO. Catal Commun 84:147–150
Socolow RH, Pacala SW (2006) A plan to keep carbon in check. Sci Am 295:50–57
Raval A, Ramanathan V (1989) Observational determination of the greenhouse effect. Nature 342:758–761
Frölicher TL, Joos F (2010) Reversible and irreversible impacts of greenhouse gas emissions in multi-century projections with the NCAR global coupled carbon cycle-climate model. Clim Dyn 35:1439–1459
Andersen LL, Osterstrøm FF, Nielsen OJ, Andersen MPS, Wallington TJ (2014) Atmospheric chemistry of (CF3) 2CFOCH3. Chem Phys Lett 607:5–9
Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, Jaramillo TF (2014) Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. Am Chem Soc 136:14107–14113
Grünewald H (1968) Elsevier, vol 80, pp 52–52
Whipple DT, Kenis PJA (2010) Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J Phys Chem Lett 1:3451–3458
Appel AM, Bercaw JE, Bocarsly AB, Dobbek H, DuBois DL, Dupuis M, Ferry JG, Fujita E, Hille R, Kenis PJA, Kerfeld CA, Morris RH, Peden CHF, Portis AR, Ragsdale SW, Rauchfuss TB, Reek JN, Seefeldt LC, Thauer RK, Waldrop GL (2013) Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem Rev 113:6621–6658
Kondratenko EV, Mul G, Baltrusaitis J, Perez Ramırez LJ (2013) Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ Sci 6:3112–3135
Qiao J, Liu Y, Hong F, Zhang J (2010) Energy Environ Sci 3:1311–1315
Chaplin RPS, Wragg AA (2003) Effects of process conditions and electrode material on reaction pathways for carbon dioxide electroreduction with particular reference to formate formation. Appl Electrochem 33:1107–1123
Hori Y, Costas VG, Ralph WE, Maria G-AE (2008) Springer, NY, vol 42, pp 89–189
Jhong HRM, Ma S, Kenis PJA (2013) Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr Opin Chem Eng 2:191–199
Costentin C, Robert M, Saveant JM (2013) Catalysis of the electrochemical reduction of carbon dioxide. Chem Soc Rev 42:2423–2436
Qiao J, Liu Y, Zhang J (2014) A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem Soc Rev 43:631–675
Lu X, Leung YC, Dennise H Wang, Leung KH, Michael J Xuan (2014) Electrochemical reduction of carbon dioxide to formic acid. ChemElectroChem 1:836–849
Jones JP, Prakash GKS, Olah GA (2014) Electrochemical CO2 reduction: recent advances and current trends. Isr J Chem 54:1451–1466
Mc Creery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687
Yang N, Swain GM, Jiang X (2016) Nanocarbon electrochemistry and electroanalysis: current status and future perspectives. Electroanalysis 28:27–34
Zhang H, Zhang X, Zhang D, Sun X, Lin H, Wang C, Ma Y (2013) One-step electrophoretic deposition of reduced graphene oxide and Ni (OH) 2 composite films for controlled syntheses supercapacitor electrodes. J Phys Chem B 117:1616–1627
Wang H, Casalongue HS, Liang Y, Dai H (2010) Ni (OH) 2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J Am Chem Soc 132:7472–7477
Saminathan K, Mayilvel Dinesh M, Selvam M, Srither SR, Rajendran V, Kaler KVIS (2015) Water soluble graphene as electrolyte additive in magnesium–air battery system. J Power Sources 276:32–38
Selvam M, Saminathan K, Siva P, Saha P, Rajendran V (2016) Corrosion behavior of Mg/graphene composite in aqueous electrolyte. Mater Chem Phys 172:129–136
Yan J, Fan Z, Sun W, Ning G, Wei T, Zhang Q, Zhang R, Zhi L, Wei F (2012) Advanced asymmetric supercapacitors based on Ni (OH) 2/graphene and porous graphene electrodes with high energy density. Adv Funct Mater 22:2632–2641
Selvam M, Sakthipandi K, Suriyaprabha R, Saminathan K, Rajendran V (2013) Synthesis and characterization of electrochemically-reduced graphene. Bull Mater Sci 36:1315–1321
Dubal DP, Fulari VJ, Lokhande CD (2012) Effect of morphology on supercapacitive properties of chemically grown β-Ni (OH) 2 thin films. Microporous Mesoporous Mater 151:511–516
Yang H, Gu Y, Deng Y, Shi F (2002) Electrochemical activation of carbon dioxide in ionic liquid: synthesis of cyclic carbonates at mild reaction conditions. Chem Commun 3:274–275
Liu M, Pang Y, Zhang B, Luna PD, Voznyy O, Xu J, Zheng X, Dinh CT, Fan F, Cao C, Pelayo García de Arquer F, Safaei TS, Mepham A, Klinkova A, Kumacheva E, Filleter T, Sinton D, Kelley SO, Edward Sargent H (2016) Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537:382–386
Shibata M, Furuya N (2001) Electrochemical synthesis of urea at gas-diffusion electrodes: part VI. Simultaneous reduction of carbon dioxide and nitrite ions with various metallophthalocyanine catalysts. J Electroanal Chem 507:177–184
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palanisamy, S., Srinivasan, S. Electrochemical reduction of CO2 on Ni (OH)2 doped water dispersible graphene under different electrolyte conditions. SN Appl. Sci. 1, 837 (2019). https://doi.org/10.1007/s42452-019-0846-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0846-0