Abstract
Blastocystis sp. is more prevalent in mentally-retarded individuals, immunocompromised patients, and organ recipients. There are no inclusive studies in Iran evaluating the prevalence and molecular identification of Blastocystis sp. infection in schizophrenic patients. This study aimed to determine prevalence and subtype distribution of Blastocystis sp. in schizophrenic patients in Tehran province, Iran. This cross-sectional study was performed on 58 stool samples of schizophrenic male patients in Tehran province, Iran, using the sequence-tagged site (STS) and ST-specific primers. Overall, 58 stool samples were collected from hospitalized male patients with schizophrenia in Tehran province. After conventional PCR, positive samples were probed by ST-specific and STS primers. Following molecular evaluation, 32 samples (55.2%) were positive for Blastocystis sp. Which, 28 (87.5%) cases were ST3, 3 (9.4%) cases were found to be ST9, and one case (3.1%) was ST1.This study was the first report of the ST9 of Blastocystis in Iran. Blastocystis infection was more prevalent in asymptomatic patients (55.6%) compared with symptomatic patients (55%). There was a significant association between the duration of hospitalization and Blastocystis infection rate (0.002). The results of the present study represented a high prevalence and significance of Blastocystis sp. infection in schizophrenic patients. Patients with schizophrenia are unable to attend to their own care. Also, schizophrenic patients have a lack of perception that can result in poor hygiene, which needs more care, accuracy, prevention, and control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis sp. is a genetically diverse protozoan parasite, which has recently been categorized within stramenopiles phylogenetically [1]. It is recognized as the most common parasitic protist of the human intestinal tract [2]. It is expected that the fecal-oral route is the main transmission pathway, with cyst forms as the infective stages [3]. The prevalence rate of infection in developing countries (53.8%) is higher than in developed nations (3.3%) [4, 5]. Some risk factors such as contaminated food or water, poor hygiene, the host’s immune status, and close contact with animals would enhance the establishment of the infection [6]. Blastocystis sp. is more prevalent in mentally retarded individuals, immunocompromised patients, and organ recipients [7,8,9,10,11].
Based on small rRNA gene analysis (SSU rDNA), 17 Blastocystis subtypes (STs) were recognized [12], ten of which (ST1-ST9, and ST12) were found to infect humans [13]. Furthermore, ST1-ST4 are the most frequent isolated subtypes from human cases, with ST3 as the main causative agent of human infections [14, 15]. These common STs have also been identified in other vertebrate hosts such as birds, rodents, ungulates, as well as other primates [2]. It is demonstrated that ST9 is only confined to human cases [16]. Several factors influence the geographical distribution of human subtypes, including topographical and meteorological conditions, cultural practices, and contact with animals [4]. Microscopic examination and parasite-specific DNA detection are mostly used to detect Blastocystis sp. infection [17]. With respect to microscopic methods, Lugol’s iodine and trichrome staining procedures are frequently employed [18]. Also, following verification using STS primers is popular molecular approaches to reveal the genetic heterogeneities among isolates [16, 18, 19].
Schizophrenic patients have a very different perception of real life [20], and due to their hygiene condition, psychic patients are always at risk of opportunistic infectious diseases such as parasitic infections. Various studies have been reported a possible association between schizophrenia and infections by Human Herpesvirus 2, Borna Disease Virus, Human Endogenous Retrovirus, Chlamydophila pneumoniae, Chlamydophila psittaci, Toxoplasma gondii, and Toxocara spp. [21,22,23,24]. However, there is no comprehensive information on the prevalence and subtypes distribution of Blastocystis infection in schizophrenic patients.
We investigated the genetic diversity of Blastocystis sp. infection in schizophrenic patients in Tehran province, using microscopic examination, conventional PCR, seven pairs of sequence-tagged site primers, subtype-specific primers for ST8 and ST9, and sequencing.
Materials and Methods
Collecting Samples and Stool Examination
This cross-sectional descriptive study was conducted from September 2015 to February 2016. Overall, 58 stool samples were collected from schizophrenic male patients from Tehran Province, Iran. All samples underwent Lugol’s staining and microscopic examination. Subsequently, samples were maintained in 2.5% dichromate potassium at 4 °C for further molecular evaluations.
DNA Extraction
Genomic DNA of each Blastocystis isolates was extracted directly from stool samples using cetyl trimethyl ammonium bromide (CTAB) method [25], with some modifications such as adding 1% KOH to each sample and incubation at 60 °C, washing with normal saline to exclude lipid, incorporating 8 M GuHCl followed by saline washing to eliminate fecal pigments. Lysis buffer and 10% sodium dodecyl sulfate (SDS) were added to make the DNA fragments more available, while proteinase K was used for solubilizing the protein components. Additionally, RNase (DNA Genotek) treatment was performed to remove RNA. The quantity and quality of the DNA was determined using spectrophotometry (Nanodrop, Thermo Fisher Scientific, Waltham, MA, USA) and gel electrophoresis.
Conventional PCR
All DNA from the stool specimens were tested by PCR for Blastocystis using bl1400ForC (5′-GGA ATC CTC TTA GAG GGA CAC TAT ACA T-3′) and bl1710RevC (5′-TTA CTA AAA TCC AAA GTG TTC ATC GGA C-3′), which amplify a 310-bp fragment of SSU rDNA gene [12, 26]. Each PCR reaction was done in a tube containing 7.5 μl of Taq DNA Polymerase Mix Red-Mgcl2 (Ampliqon©, Denmark), 1 μl of each primer (10 pmol), and 3 μl of DNA; 2.5 μl of DW also was added to reach the final volume of 15 μl. The PCR thermocycler condition was as follows: initial denaturation at 94 °C for 4 min and 35 cycles of denaturation at 94 °C, annealing at 60 °C, and extension at 72 °C, every level for 1 min. Ultimately, a final extension was set on 72 °C for 5 min (Mastercycler, Eppendorf®, Hamburg, Germany) [26]. PCR products were separated on 1.5% agarose gel and staining with CinnaGen DNA safe stain (Cat. No.: PR881603) for 45 min at 80 V, and then visualized by UV transilluminator (Uvitec, Cambridge, UK).
Subtype Analysis
In order to determine the Blastocystis sp. subtypes in contaminated samples, seven pairs of sequence-tagged site primers were used which amplify the RAPD product of interest. Along with these seven primer pairs, two additional ST-specific primer pairs were used that were able to identify ST8 and ST9 of Blastocystis, which were developed by “Yoshikawa and Iwamasa” (Table 1) [16, 27]. The PCR reaction was including 7.5 μl of Taq DNA Polymerase Mix Red-Mgcl2 (Ampliquon©, Denmark), 3 μl of extracted DNA, 1 μl of each primer (10 pmol), and 2.5 μl of sterile water also was added to reach the final volume of 15 μl. The PCR condition for ST1-ST9 was performed by Yoshikawa and Scanlan’s method [16, 27, 28]. At last, each PCR product was run on 1.5% agarose gel and visualized with CinnaGen DNA safe stain (Cat. No.: PR881603) staining under UV transilluminator (Uvitec, Cambridge, UK).
Sequencing
Totally, PCR products of three positive samples were purified by using QIAquick® Gel Extraction Kit (QIAGEN, Hilden, Germany) and sequenced on both ends by an ABI-3730XL capillary machine (Macrogen© Inc., South Korea) using sequence-tagged site primer pairs (SB83 and SB227) and ST-specific primer pairs (ST9). The raw nucleotide sequences and chromatograms of both forward and reverse directions were viewed and analyzed using the Chromas program as implemented in the software BioEdit version 7.2.5 [29], and consensus sequences were assembled and aligned using Clustal W, as implemented in this software. The final aligned sequences are converted in FASTA format for further analysis and also making matrix of divergence or similarity. Consensus sequences were compared with homologous sequences in the GenBank database using the BLAST algorithm [30]. Each subtype (ST1, ST3, and ST9) was approved by closest similarity or an exact match with recorded sequence data for Blastocystis sp. Genetic divergence between each Blastocystis sp. sequences and also those downloaded from GenBank were calculated according to Kimura 2-parameter model by MEGAX software [31].
Nucleotide Sequence Accession Numbers
Three samples were submitted to GenBank (accession numbers MF774610–12).
Statistical Analysis
To statistically assess the relationship between Blastocystis sp. prevalence, clinical symptoms, and level of education, the chi-square test was exerted using SPSS software (version 20, IBM Inc., USA). Also, the independent t test was performed to demonstrate a possible correlation between estimated prevalence of infection and age as well as the period of hospitalization.
Results
Microscopic Examination and Conventional PCR
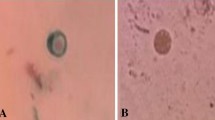
Out of 58 stool samples, 22 cases (37.9%) were positive microscopically, whereas 32 cases (55.2%) were shown 310-bp band and to be positive with the PCR method (Fig. 1). In the detection of Blastocystis positive cases, the molecular method was more sensitive compared with the microscopic method. Twenty-two positive samples in direct microscopy were also PCR positive.
Subtyping and Sequencing
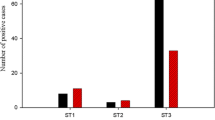
Subtyping of positive Blastocystis samples using ST-specific primer pairs, sequence-tagged site (STS) primers, and sequencing demonstrated that 28 cases (87.5%) were ST3, 3 cases (9.4%) were ST9, and one case (3.1%) was ST1, there were no reports of ST2, ST4-ST8, and mixed subtypes among examined samples (Table 2). ST3 was reported as a dominant subtype among schizophrenic patients in the present study.
Nucleotide Sequence Similarity and Genetic Diversity
Three nucleotide sequences were submitted to GenBank, so that the sequences of ST1 obtained in the present study with accession number MF774610 showed 99.7% identity to a published sequence from Iran (AB714500). Kimura 2-parameter (K2P) Mean genetic divergence between these sequences was 0.3%. The sequences of ST3 with accession number MF774611 showed 98.7% identity to other published sequence from Iran (JX483861) with 1.3% mean K2P genetic divergence. Furthermore, the sequences of ST9 obtained in the present study with accession number MF774612 showed 99.13 and 98.65% identity to the sequence with accession number KC138681 from Denmark and to the sequence with accession number KT438703 from Japan, respectively. These ST9 sequences also showed 98.36% identity to two published sequences from Japan (AF408425–26). The degree of genetic divergence (Kimura 2-parameter) between the Blastocystis sp. sequences of the ST9 is presented in Table 3. Mean genetic diversity within the ST9 sequences from Iran, Denmark, and Japan was 1%.
Blastocystis Infection, Demographic Data, and Gastrointestinal Symptoms
Demographic characteristics of schizophrenic patients with Blastocystis sp. infection is presented in (Table 4). Following statistical analysis of recorded demographic data, there was no statistically significant correlation between age (P = 0.2) and level of education (P = 0.5) with Blastocystis sp. infection. Although, the period of hospitalization was significantly correlated to the Blastocystis sp. infection (P = 0.002). There was no significant difference between frequency of Blastocystis subtypes in symptomatic and asymptomatic schizophrenic patients. Also, no associations were found between Blastocystis positive rate in symptomatic patients and asymptomatic patients (P = 0.969) (Table 5). Blastocystis infection was more prevalent in asymptomatic patients (55.6%) compared with symptomatic patients (55%) (Table 5).
Discussion
Regarding diverse genetic variations among Blastocystis sp. isolates, sophisticated molecular methods are preferred for subtyping the parasite. For this aim, two molecular tests are frequently used, including sequencing of small subunit rRNA gene and PCR on subtype-specific sequence-tagged-sites (STSs) [27]. Until now, Blastocystis sp. subtyping in Iran has not yet been accomplished by seven pairs of sequence-tagged site primers along with ST-specific primers. So, we sought to determine the prevalence of Blastocystis sp. infection and its dedicated human subtypes in schizophrenic patients in Tehran Province, Iran. Schizophrenic patients encompass 1 % of the world’s population [32]. Totally, 37.9% rate of prevalence was obtained in microscopy, while the sensitive PCR yielded a 55.2% prevalence rate. All positive samples in microscopy were confirmed for the infection with PCR method. Some limitations of microscopy such as low sensitivity, requiring expert technicians and incapability in the detection of Blastocystis sp. subtypes, confine its utilization for diagnostic purposes [33]. Moreover, some studies indicate that the parasite culture is appropriate for routine diagnosis and epidemiologic studies. Although some parasite isolates in axenic cultures do not grow or grow slowly, hence the PCR technique is the method of choice for subtyping the parasite [5, 34,35,36].
In the present study, the PCR procedure was employed for accurate detection of Blastocystis sp. subtype analysis which demonstrated that 28 cases (87.5%) were infected to ST3, which was probably anticipated due to long-term hospitalization of patients and low contact with outdoors. Based on previous studies, the human-to-human transmission is presumably the routine pathway of infection for ST3. This subtype is the most frequent isolate in symptomatic and asymptomatic individuals [33, 37, 38]. In Iran, different studies with various detection methods have been used for Blastocystis sp. subtyping. Motazedian and colleagues used restriction fragment length polymorphism (RFLP) to identify subtypes, and hence they recognized 7 ribodemes [39]. Some other studies employed STS primers such as Badparva et al., Moosavi et al., and Khademvatan et al., suggesting ST3 as a dominant subtype, followed by other isolates, consisting of ST1, ST2, ST4, ST5, and ST7 [35, 40, 41]. Yoshikawa et al.’s primers were used to detect ST9 in the current study [16], which possessed high-grade sensitivity to reveal this subtype. The recorded ST9 in our study is very uncommon globally, with only four isolates worldwide and no reports from Iran [4, 16]. This is the first documentation of this rare subtype in Iran. In a study by Yoshikawa and colleagues, 32% of examined isolates were ST6 and ST7 as well as an unknown isolate, which was later recognized as ST9 [27]. One of the four reported isolates of ST9 was identified in a patient with gastrointestinal symptoms in Denmark [42]; the other three isolates were distinguished by Yoshikawa in Japan. Since ST9 has not yet been isolated from non-human sources, its origin still is open to question [16].
However, molecular genetic analysis of the SSU rDNA gene conducted using the Kimura 2-parameter model showed that Blastocystis sp. sequences of ST9 obtained in the present study was more close to the sequences of ST9 from Denmark with a genetic divergence of 0.5% while mean genetic divergence between ST9 sequences from Iran (present study) and Japan was 1.1 to 1.2% (Table 3). Mean genetic diversity within the ST9 sequences from these countries was 1% that can be clinically important for this rare subtype because it only has been detected in humans.
The STS primers only amplify Blastocystis sp. DNA; hence they possess high specificity that also detects mixed subtype infections and obviates the need for sequencing. Our knowledge about the target regions of the STS gene, the amount of conservation, and the level of difference among them is limited. Owing to the fact that there are few sequences of STS products, we are not aware of the variation degree in various loci of different subtypes. For instance, ST3 is less conserved and it’s even more difficult when describing the variation within genotypes of ST3 [19]. Few studies have indicated a high genetic variation in the STS region. The sample with this alteration in the current study supports this hypothesis with 98% identity to the registered sample by Moosavi et al. [35]. The statistically significant correlation between the period of hospitalization and Blastocystis sp. infection (P = 0.002) highlights the prominent role of the fecal-oral route. However, there wasn’t any statistically significant correlation between age and level of education with the prevalence of infection.
In the current study, similar to the previous study [7], there was no significant difference between frequency of Blastocystis subtypes in symptomatic and asymptomatic schizophrenic patients. Also, no associations were found between Blastocystis positive rate in symptomatic patients and asymptomatic patients. Although there was no significant difference in the prevalence and subtype distribution of Blastocystis in symptomatic and asymptomatic schizophrenic patients, similar to the previous study [43], Blastocystis infection was more prevalent in asymptomatic patients (55.6%) compared with symptomatic patients (55%).
Although parasitic diseases have declined in recent decades, however, these diseases are prevalent in developing countries. On the other hand, patients with schizophrenia because of having mental problems, lack of control over personal behaviors, and failure to comply with individual health, self-contamination, and continuation of the infection are susceptible to various infections, one of which can be parasitic infections. Self-contamination in these patients results in the persistence of infection and increased parasitic burden of Blastocystis, which can lead to digestive problems and inflammation, as well as a suitable area for other infectious diseases. These events will worsen the conditions of psychotic illness in these people and endanger their health. Because of the sensitivity of the physical, health, and mental conditions of schizophrenic patients and pathogenic subtypes of Blastocystis sp., diagnosis, and control of Blastocystis sp., infection can be very helpful in improving the health and hygienic conditions of patients with schizophrenia. Therefore, from a logical point of view, it was necessary to consider the prevalence and subtype distribution of Blastocystis sp. in schizophrenic patients in Tehran Province, Iran.
Conclusion
Based on the results of the current study, the microscopic examination has low sensitivity, and hence molecular techniques are recommended to determine the Blastocystis sp. infection. The Blastocystis sp. prevalence in schizophrenic patients in Tehran province was reported to be 55.2%. Blastocystis infection was more prevalent in asymptomatic patients (55.6%) compared with symptomatic patients (55%). The higher abundance of ST3 and significant relationship between the duration of hospitalization and the infection emphasize the possibility of human to human transmission, suggesting the low hygiene practices among the examined population. However, there was no significant difference between frequency of Blastocystis subtypes in symptomatic and asymptomatic schizophrenic patients. Also, no associations were found between Blastocystis positive rate in symptomatic patients and asymptomatic patients. This study was the first report of the ST9 of Blastocystis sp. in Iran. Therefore, it’s highly recommended to perform further studies in order to clarify the exact prevalence status of this rare subtype in the country. On the other hand, the prevalence of Blastocystis sp. was very high in our study population among schizophrenic patients. Due to health conditions and lack of awareness, these patients seem to be susceptible to many other infections that need more care, accuracy, prevention, and control.
References
Silberman JD, Sogin ML, Leipe DD, Clark CG. Human parasite finds taxonomic home. Nature. 1996;380(6573):398.
Alfellani MA, Taner-Mulla D, Jacob AS, Imeede CA, Yoshikawa H, Stensvold CR, et al. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164(4):497–509.
Suresh K, Smith H, Tan T. Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Appl Environ Microbiol. 2005;71(9):5619–20.
Alfellani MA, Stensvold CR, Vidal-Lapiedra A, Onuoha ESU, Fagbenro-Beyioku AF, Clark CG. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013;126(1):11–8.
Roberts T, Barratt J, Harkness J, Ellis J, Stark D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am J Trop Med Hyg. 2011;84(2):308–12.
Li L-H, Zhou X-N, Du Z-W, Wang X-Z, Wang L-B, Jiang J-Y, et al. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol Int. 2007;56(4):281–6.
Asghari A, Zare M, Hatam G, et al. Molecular identification and subtypes distribution of Blastocystis sp. isolated from children and adolescent with cancer in Iran: evaluation of possible risk factors and clinical features. Acta Parasitol. 2020. https://doi.org/10.2478/s11686-020-00186-2.
Bednarska M, Jankowska I, Pawelas A, Piwczyńska K, Bajer A, Wolska-Kuśnierz B, et al. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res. 2018;117(9):2869–79.
Rao K, Sekar U, Iraivan KT, Abraham G, Soundararajan P. Blastocystis hominis—an emerging cause of diarrhoea in renal transplant recipients. J Assoc Physicians India. 2003;51(1):719–21.
Mohammadi-Meskin V, Hamedi Y, Heydari-Hengami M, Eftekhar E, Shamseddin J, Sharifi-Sarasiabi K. Intestinal parasitic infections in mental retardation Center of Bandar Abbas, southern Iran. Iran J Parasitol. 2019;14(2):318–25.
Shehata AI, Hassanein F. Intestinal parasitic infections among mentally handicapped individuals in Alexandria, Egypt. Ann Parasitol. 2015;61(4).
Stensvold CR, Suresh GK, Tan KS, Thompson RA, Traub RJ, Viscogliosi E, et al. Terminology for Blastocystis subtypes–a consensus. Trends Parasitol. 2007;23(3):93–6.
Parkar U, Traub RJ, Vitali S, Elliot A, Levecke B, Robertson I, et al. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet Parasitol. 2010;169(1):8–17.
Clark CG, van der Giezen M, Alfellani MA, Stensvold CR. Recent developments in Blastocystis research. Adv Parasitol. 2013;82(1):32.
Özyurt M, Kurt Ö, Mølbak K, Nielsen HV, Haznedaroglu T, Stensvold CR. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int. 2008;57(3):300–6.
Yoshikawa H, Iwamasa A. Human Blastocystis subtyping with subtype-specific primers developed from unique sequences of the SSU rRNA gene. Parasitol Int. 2016;65(6):785–91.
Asghari A, Sadraei J, Pirestani M, Mohammadpour I. First molecular identification and subtype distribution of Blastocystis sp. isolated from hooded crows (Corvus cornix) and pigeons (Columba livia) in Tehran Province, Iran. Comp Immunol Microbiol Infect Dis. 2019;62:25–30.
Stensvold CR, Arendrup MC, Jespersgaard C, Mølbak K, Nielsen HV. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn Microbiol Infect Dis. 2007;59(3):303–7.
Stensvold CR. Comparison of sequencing (barcode region) and STS PCR for Blastocystis subtyping. J Clin Microbiol. 2012;02541–12.
Williamson P. Mind, brain, and schizophrenia. Oxford: University press; 2006.
Khademvatan S, Khajeddin N, Izadi S, Yousefi E. Investigation of anti-Toxocara and anti-toxoplasma antibodies in patients with schizophrenia disorder. Schizophr Res Treat. 2014;2014:1–7.
Arias I, Sorlozano A, Villegas E, de Dios LJ, McKenney K, Cervilla J, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136(1–3):128–36.
Fuglewicz AJ, Piotrowski P, Stodolak A. Relationship between toxoplasmosis and schizophrenia: a review. Adv Clin Exp Med. 2017 Sep 1;26(6):1033–8.
Kaplan M, Kalkan A, Kuk S, Demirdag K, Ozden M, Kilic SS. Toxocara seroprevalence in schizophrenic patients in Turkey. Yonsei Med J. 2008;49(2):224–9.
Pınar A, Akyön Y, Alp A, Ergüven S. Adaptation of a sensitive DNA extraction method for detection of Entamoeba histolytica by real-time polymerase chain reaction. Mikrobiyol Bul. 2010;44(3):453–9.
Stensvold R, Brillowska-Dabrowska A, Nielsen HV, Arendrup MC. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J Parasitol. 2006;92(5):1081–7.
Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IKM, Hossain MB, et al. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res. 2004;92(1):22–9.
Scanlan PD, Stensvold CR, Cotter PD. Development and application of a Blastocystis subtype-specific PCR assay reveals that mixed-subtype infections are common in a healthy human population. Appl Environ Microbiol. 2015;81(12):4071–6.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. InNucleic acids symposium series 1999 Jan 1 (Vol. 41, No. 41, pp. 95–98). London: Information Retrieval Ltd. c1979–c2000.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC bioinformatics. 2009;10(1):421.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9.
Messias EL, Chen C-Y, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin N Am. 2007;30(3):323–38.
Santín M, Gómez-Muñoz MT, Solano-Aguilar G, Fayer R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol Res. 2011;109(1):205–12.
Tungtrongchitr A, Manatsathit S, Kositchaiwat C, Ongrotchanakun J, Munkong N, Chinabutr P, et al. Blastocystis hominis infection in irritable bowel syndrome patients. 2004.
Moosavi A, Haghighi A, Mojarad EN, Zayeri F, Alebouyeh M, Khazan H, et al. Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitol Res. 2012;111(6):2311–5.
Parkar U, Traub R, Kumar S, Mungthin M, Vitali S, Leelayoova S, et al. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology. 2007;134(3):359–67.
Stensvold CR, Alfellani M, Clark CG. Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect Genet Evol. 2012;12(2):263–73.
Jantermtor S, Pinlaor P, Sawadpanich K, Pinlaor S, Sangka A, Wilailuckana C, et al. Subtype identification of Blastocystis spp. isolated from patients in a major hospital in northeastern Thailand. Parasitol Res. 2013;112(4):1781–6.
Motazedian H, Ghasemi H, Sadjjadi S. Genomic diversity of Blastocystis hominis from patients in southern Iran. Ann Trop Med Parasitol. 2008;102(1):85–8.
Badparva E, Sadraee J, Kheirandish F, Frouzandeh M. Genetic diversity of human blastocystis isolates in Khorramabad, Central Iran. Iran J Parasitol. 2014;9(1):44–9.
Khademvatan S, Masjedizadeh R, Yousefi-Razin E, Mahbodfar H, Rahim F, Yousefi E, et al. PCR-based molecular characterization of Blastocystis hominis subtypes in southwest of Iran. J Infect Public Health. 2017.
Stensvold C, Lewis H, Hammerum AM, Porsbo LJ, Nielsen S, Olsen K, et al. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol Infect. 2009;137(11):1655–63.
Dullaert-de Boer M, Schuurs TA, Vermeer M, Ruijs GJ, van der Zanden AG, Weel JF, et al. Distribution and relevance of Dientamoeba fragilis and Blastocystis species in gastroenteritis: results from a case-control study. Eur J Clin Microbiol Infect Dis. 2020 Jan 1;39(1):197–203.
Acknowledgments
The authors thank the Vice Chancellor for Research of Tarbiat Modares University for support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All stages of the present study were confirmed by the Ethics Committee of Tarbiat Modares University, Tehran, Iran.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Sheikh, S., Asghari, A., Sadraei, J. et al. Blastocystis sp. Subtype 9: as the First Reported Subtype in Patients with Schizophrenia in Iran. SN Compr. Clin. Med. 2, 633–639 (2020). https://doi.org/10.1007/s42399-020-00285-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00285-1