Abstract
Aquatic plants are promising green energy feedstocks owing to their high rate of growth, photosynthesis, and CO2-fixing efficiency. They possess a paramount advantage of non-competitiveness with food crops over the first or second generation biofuel feedstocks. Specifically, low lignin content and higher concentrations of polysaccharides make these plants very attractive for biogas and liquid biofuel production. However, a regular supply of biomass is a limitation that can be overcome by employing harvesting techniques with sustainable measures, which ensure rapid regrowth of biomass for the next cycle. Harvesting of both aquatic macrophytes (weeds) as well as macroalgae is achieved by either manual or mechanical means. Following regular supply through effective harvesting, biofuel production can be further restricted due to their complex structural make-up. In order to improve the biofuel production, various pretreatment methods have been explored to disrupt the complex structure of aquatic weeds and macroalgae, thereby increasing the breakdown of biomass material more readily. This review examines traditional and modern techniques for biofuel production using aquatic weeds and macroalgae. It also discusses recent advancements in the harvesting and pretreatment techniques that improve overall efficiency. Choosing an effective pretreatment method can greatly influence biofuel recovery and production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fast growth rate of aquatic plants creates a major problem for freshwater bodies that have high nutrient levels. They form a thick canopy layer that can inhibit light penetration for suspended phytoplankton. This can lead to lower rates of photosynthesis, decreasing oxygen supply required by aquatic life. Uncontrolled growth of aquatic plants also poses a threat to hydroelectric dams, irrigation projects and aquaculture systems (Gusain and Suthar, 2017; Gupta et al. 2014). The major aquatic macrophytes around the world are Eichhornia crassipes, Typha spp., Ipomea carnea, Hydrilla verticillata, Salvinia spp. Alternanthera pheloxeroides, Ulva sp., Boergesenia sp., Padina sp., Crystoseira sp., and Grateloupia sp. (ICID 2002; Mantri et al. 2020). In case of macroalgae which are generally located in coastal areas, their accumulation poses environmental as well as aesthetical issues. It has been reported that to maintain eco-labels like Blue Flag Beach category for tourism, these macroalgae have been removed and disposed off by dumping in landfills (İnan and Özçimen 2019). Generally, millions of tons of macroalgae are discarded as waste throughout the world. This is also the case with aquatic macrophytes since they are often regarded as nuisance species when they become invasive and take over aquatic habitats. They are sometimes mechanically harvested and yield a massive amount of wet biomass. They are either burned after drying or disposed openly in wastelands, neither of which is an environmentally healthy practice (Gusain and Suthar 2017). Aquatic macrophytes and macroalgae have high carbohydrate, lipid and protein content, which makes them suitable for production of biofuels (Zhao et al. 2014; Ansari et al. 2017). Because of their fast growth rate, adaptability to adverse conditions, better chemical composition suited for biofuel production in comparison to lignocellulosic crops, and their suitability to generate valuable co-products, aquatic macrophytes are considered a viable bioenergy feedstock (Kaur et al. 2018). Macroalgae on the other hand, unlike microalgae and other lignocellulosic biomass, contain low lipid content, and high carbohydrate content, thereby, simplifying the stages of pretreatment (Daroch et al. 2013; John et al. 2011; Özçimen et al. 2012). They also contain substances like mannitol, carrageenan, laminarin and alginate, which can be further utilized in different sectors (İnan and Özçimen 2019).
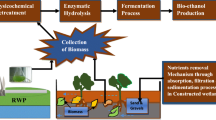
The primary step in the application of aquatic weed and macroalgae for the production of biofuel is ‘harvesting’. Harvesting can also be regarded as the removal of biomass from water body present in inland or coastland. The methods employed for the removal of biomass are mechanical, biological or chemical. Among these, one of the most efficient and effective method is mechanical harvesting which is used for the removal of invasive weeds as well as macroalgae (Gusain and Suthar 2017). Pretreatment is an essential process in biofuel production as it is necessary for the conditioning of biomass for further downstream processes of conversion like fermentation, enzymatic hydrolysis and transesterification. Pretreatment plays a crucial role in the overall cost as well as energy demand of biofuel production process (Zhang et al. 2015). The conversion technologies used for biofuel production from aquatic weed and macroalgae have been reported in the literature. However, scanty information is available on harvesting and pretreatment techniques used for the aquatic weeds and macroalgal biomass prior to biofuel production. In this review, harvesting and pretreatment techniques involved while producing biofuels from aquatic weeds and macroalgae are critically evaluated. Effect of these processing techniques on overall biofuel production process is also discussed.
Aquatic weeds
Problem causing aquatic vegetation may be present in both macroscopic and microscopic forms. Macroscopic vegetation includes aquatic plants like water hyacinth and macroalgae viz. Ulva spp., which are commonly called ‘aquatic weeds’ (Davis and Hirji 2003). Aquatic weeds are known to be troublesome and fast growing vegetation that grow and proliferate in water bodies (Aloo et al. 2013). They might be the result of deliberate introduction or simply through expansion by uncontrolled growth. These menace causing aquatic weeds have potential to create problem for any kind of economic and recreational activities in water bodies (Mondal 2018). Introduced aquatic plants can adapt very fast and can show uncontrollable growth due to absence of any natural predator whereas pre-existing or native weeds can suddenly become out of control due to favorable changes in physicochemical characteristics of water (Lembi 2009).
Aquatic weeds possess several exceptional qualities which makes them one of the major concerns of the present time, such as fast growth rate, exponential doubling rate, etc. when favorable conditions are met. Some of the aquatic weeds (e.g. water hyacinth) can have exceptional reproduction and doubling time of just few days and can double their biomass in just about 7–14 days through propagative reproduction (Hoevers 2011). The main factors which support the outbreak of aquatic weeds are associated with minimal requirements and adaptability. In the case of terrestrial plants, visually attractive appearance was the reason behind the wide spreading of weeds in tropical and non-tropical parts of the world. Especially during the nine-teenth and twentieth centuries, humans aided in the proliferation of weeds from one continent to another (Rooney et al. 2007; Aloo et al. 2013; Ogbaga et. al. 2019). Most of these weeds are limited due to nutrient availability and respond to increment in nutrients that are previously short in supply with impulsive growth. Thus, water bodies where large quantities of nutrients are being dumped regularly stay susceptible to invasion of aquatic weeds (Rey and Rutledge 2006). Availability of nutrients in the water body coupled with higher growth rate and stress helps aquatic weeds to effectively utilize these nutrients and enrich the biomass with compounds such as carbohydrates, lipids and proteins. Table 1 represents biochemical composition of some aquatic weeds. The carbohydrates, lipids and protein content of aquatic weed biomass makes them an attractive biofuel feedstock, as lipid fraction makes it potential biodiesel resource, whereas, sugar fraction can be used to produce other liquid biofuels such as bioethanol, biobutanol and biomethanol by fermentation. Aquatic weeds have also been proven effective in producing biomethane and biohydrogen (Mthethwa et al. 2018; O’Sullivan et al. 2010; Reddy et al. 2017).
Particularly in freshwater, aquatic weeds are mostly flowering plants (angiosperms) with ordinary leaves and roots (Asogwa and Asogwa 2018). These aquatic weeds can be grouped into various types based on varying habits and habitats. Various types of aquatic macrophytes include: i) emergent, ii) floating and iii) submerged (Fig. 1; Table 2). The type of aquatic macrophytes dictates the choice of harvesting and pretreatment technique during its application as biofuel feedstock.
Emergent macrophytes
Emergent weeds, also known as ‘shoreline’ or ‘marginal plants’ include grass-like and broad-leaved plants that mostly grow in shallow water. These weeds are found permanently in the regions where maximum and minimum water level is fixed due to seasonal rising and receding of water (Basak et al. 2015). These types of weed can have well developed root in the sediments with flowers and other principle photosynthetic parts above surface water (Mondal 2018). Emergent shoreline plants can effectively reduce soil erosion and also help in stabilizing the shore while supporting the fauna residing near shore with refuge, food, attachment, nesting ground, etc. (Gettys 2014; Rozas and Odum 1988; Stallings et al. 2015).
Floating weeds
Floating weeds grow and complete their whole life cycle in water. Some of these weeds float freely, whereas, some are rooted in the sediment of the water body and rest of the foliage and flowering parts float above the surface water. Therefore, floating weeds are further subdivided into two parts: (i) free floating weeds and ii) rooted floating weeds. The free floating weeds are mostly found in deep water (depth > 1 m) with their foliage above water and roots suspended underneath (Mondal 2018). These plants continuously float throughout the surface and may hinder the natural water flow. They are independent of depth or of any kind of substratum requirement and therefore, floating weeds are considered as most dangerous aquatic weeds (Aloo et al. 2013). Some of the most common free floating aquatic weeds are water hyacinth and water lettuce. The rooted floating weeds are similar to emergent weeds in most characteristics except all the foliage and flowering parts float on the surface. Unlike free floating weeds these are dominant in relatively shallow water (Lembi 2009). Some of the most common rooted floating weeds are water lily, lotus.
Submerged weeds
Submerged weeds germinate, grow and sprout with all their plant parts beneath the water surface. These weeds are mostly found in continuous flowing rivers and ditches. These weeds can cause serious problems in the water body as most of the time they are not visible inside the water and significantly impede the flow of the water (Asogwa and Asogwa, 2018). They can be further sub grouped as either rooted or non-rooted submerged weeds. Rooted submerged weeds are anchored weeds in the sediment and therefore are more efficient in nutrient exchange from the water body, e.g. Hydrilla spp. Non-rooted submerged weeds float freely beneath water surface. Some of aquatic weeds under this class include Ceratophyllum spp. and Utricularia spp. (Davis and Hirji 2003).
Macroalgae
Macroalgae are a wide group of photosynthetic, eukaryotic organisms thay may be found in either marine or freshwater. They are multicellular in nature possessing the characteristics of a plant. In different taxa, the common features and structures may vary. They may have forms that are leafy, long bladed, branched, or may form mats. They may be anchored with the help of a holdfast or may possess air bladder enabling them to freely float on the surface of water body. Macroalgae have a complex lifecycle displaying annual and perennial life histories, alternation of generation as well as asexual and sexual strategies. Macroalgae are classified on the basis of their pigment color. The green algae are known as ‘Chlorophyta’ and ‘Charophyta’, red algae as ‘Rhodophyta’ and brown algae as ‘Phaeophyta’ (Manilal et al. 2009). Green algae get its color from the pigments chlorophyll a and b. They require more light for undergoing the process of photosynthesis due to which are situated in the shallow parts of the water body. They contain starch, cellulose and pectin (Murdock and Wetzel 2009). The most commonly found species of green macroalgae belong to the genus Bryopsis, Chaetomorpha, Cladophora, Rhizoclonium, Ulva, Spirogyra, Chara, Nitella, Zygnema and Pithophora among many others (Caisova and Gabka 2009). Red algae get red or pink color from pigments phycocyanin and phycoerythrin allowing them to grow relatively deep in the water bodies. The carbohydrate compositions of this type of macroalgae are cellulose, glucan, and galactan (Samaraweera et al. 2012). Species belonging to the genus Gelidium and Polysiphonia are some of the most commonly found red algae (Al-Yamani et al. 2014). The brown algae derive color from the abundance of pigment fucoxanthin which masks the green color of chlorophyll. They are composed of carbohydrate laminarin, mannitol, fucoidan, alginate and cellulose (Jang et al. 2012; Kim et al. 2011). Species belonging to the genus Sargassum, Vaucheria and Sphacelaria are some of the most commonly found brown algae (Al-Yamani et al. 2014)). In macroalgae, the total polysaccharide concentration in dry weight can range from 4 to 76% (Holdt and Kraan 2011). Protein content is found to be higher in red (up to 47%) and green algae (up to 26%) while brown seaweed usually has low protein content, the highest ranging up to 10-15%. Table 3 represents biochemical composition of some macroalgae (Lafarga et al. 2020). The major classes of lipid found in macroalgae are phospholipids and glucolipids, which account for 1–5% of cellular composition (Chojnacka et al. 2012). Another important group of compound found in the macroalgae are phenolic compounds which are present in varying quantity in red, brown and green algae (Lordan et al. 2011). Macroalgae are also rich in elements like Ca, Mg, Na, P and K as well as trace elements like Zn, I and Mg (Lordan et al. 2011). The compounds found in macroalgae can be used as fodder, food as well as for biofuel production (Park et al. 2012). The high content of carbohydrate in macroalgae proves to be a good feedstock for the production of bioethanol and biobutanol (Panahi et al. 2019). The concentration of fatty acids in lipids vary considerably in green, brown and red algae. Hence, the yield of biodiesel is species dependent (Pereira et al. 2012).
Problems associated with aquatic weeds and macroalgae
Aquatic plants are termed as nuisance for water bodies when presence of these aquatic weeds or macroalgae starts creating problem and conflicts against its intended purpose like fishing, pisciculture and other recreational activities. Rapid growth and reproduction rate of these aquatic plants can severely affect the native species and if left unchecked have potential to eradicate these ecological and economically important native species (Adeniji 1979; Clayton 1996). Growth of aquatic plants can reach alarming stage especially in the water bodies near tropic areas where warm water favors their growth. Problems can even escalate further due to nutrient addition due to effluent discharge and poor land use practices (Aloo et al. 2013).
Excessive population of aquatic plants, especially free-floating and emergent plants have the tendency to block out sunlight, which severely affects the physical and chemical characteristics of the water ecosystem through reduction of photosynthesis (Kennish et al. 2008). Overpopulation and decomposition of aquatic weeds and macroalgae biomass also degrade the water quality by reducing dissolved oxygen, which may suffocate the fauna present in the water (Lembi 2009). Some water weeds and algae also possess offensive smell, color and foul taste causing water body to get polluted, discolored and smell like rotten eggs, adversely affecting fish population in the water (Asogwa and Asogwa 2018). Several other problems caused in water bodies include reduction in nutrient flux and effect on biodiversity, especially waterfowl population and obstruction to water flow affecting economic activities such as power generation and irrigation (Lembi 2009).
One of the most feasible solutions for management of aquatic weeds and macroalgae is their application in production of various biofuels. Biofuel production is a multistep process which includes biomass harvesting, pretreatment of biomass and conversion to biofuels. The harvesting and pretreatment techniques are important preceding steps which have influence on biofuel conversation in terms of yield, product quality and economic feasibility.
Harvesting techniques for aquatic weeds and macroalgae
Harvesting the aquatic plant biomass which is either grown for a particular purpose such as for production of biofuels or removing invasive aquatic plants are very similar activities, which are fundamentally done through physical control or physical harvesting methods. However, removing invasive aquatic plants from water bodies can have slight differences, as the natural water bodies face several uncontrolled conditions in comparison to removing cultivated biomass, as the latter are grown in strictly controlled conditions and cannot afford complete eradication of the plants. Therefore, sustainable production measures such as calculated cutting size and cutting cycles are necessary to ensure rapid regrowth of plants in case of cultivation. Harvesting of all aquatic plants including aquatic weeds and macroalgae are much similar however, some differences can be observed.
Manual harvesting
Excavating both aquatic weed and macroalgae from water bodies using machineries or manually has been a normal practice since several years throughout the world. Using manual harvesting methods is relatively cost efficient and low energy demanding and can involve aid of nets, wires, manual cutters, etc., and are mostly used in small water bodies like canals, small ponds, coastal areas, narrow streams, etc. (Gallagher and Haller 1990; Madsen 2000). Manual removal of aquatic weeds as well as macroalgae is a usual practice, but since macroalgae are mostly harvested from oceans and cultivated ponds, and are required in large quantities, mechanical harvesting is preferable (Datta 2009).
For effective manual removal, techniques can be divided to excavate floating and emergent weeds separately. As floating weeds can form dense mats, they can be harvested manually by cutting and separating into smaller parts, whereas, emergent weeds have to be dealt more cautiously as they are required for regrowth (Hoevers 2011). Generally, use of cutting tools such as blades or handheld motorized cutters can be employed, which can be then brought to shore by manually operated boats. However, considerable care has to be taken while performing these manual operations against parasites, venomous organisms, etc. This method however requires constant monitoring due to its temporary effect.
Mechanical harvesting
Mechanical harvesting employs the use of plant cutters/harvesters developed especially for removing aquatic flora more efficiently in deep and large water bodies and coastal waters where manual cleaning can be tedious and nearly impossible. Mechanical harvesting equipment can include amphibious vehicles, boats, land based long armed vehicle equipped with mechanical cutters, saws, choppers, mowing bar, vacuum suction apparatus to suck and collect small plants especially macroalgae, rake for collection, conveyer belts, trailers, loading cranes, etc. Removal of aquatic weeds using mechanical harvesting techniques is a common conduct when dealing with emergent and rooted submerged weeds in larger water bodies as manual removal can be inconvenient. However, in case of aquatic weeds, mechanical harvesters are more commonly designed according to the types of weeds and surroundings. In such an approach, ‘Kerela Agricultural University’ in India developed a harvester using conventional pump-sets as prime movers for harvesting Pistia stratiotes with an aim to reduce the harvesting cost. The harvester was equipped with a 10 HP engine and high capacity check device which could suck and then pump the weed to desired location to achieve a continuous harvesting rate of 16 tons/ha (Jayan and Satyanathan 2012).
Macroalgae are generally collected in large amount with mechanized harvesters (Roesijadi et al. 2008). As the biomass type of macroalgae is different from that of aquatic weeds, these mechanized harvesters are primarily equipped with suction apparatus, rotating mowers, dredgers etc. For a greater efficiency, chemicals like herbicides and flocculants are used for harvesting small sized biomass (Gupta et al. 2017, 2018; Sahoo et al. 2017). The mechanized harvesters are mostly fitted onto boats which may be operated from shores or in water body and once harvested, the macroalgae are pumped through pipe directly into dredges or nets from where they can be transported to the required locations (Potts et al. 2012; Roesijadi et al. 2010). The choice of harvesting method is directly related to the type of macroalgae in cultivation. The free-floating macroalgae harvesting may involve cutting, suction and dredging. Whereas, the seaweed grown attached to ropes, nets or lines are best harvested using rotating blades. Most of the times these attaching medium are just collected using collection boats, dredgers, and nets are transported to shore for processed harvesting (Peteiro and Freire 2012; Roesijadi et al. 2010).
The removal of aquatic plant biomass for biofuel production can have several beneficial effects such as reduction in organic carbon load of water body. This results in decrease in methane production from the water surface bodies (Dalal et al. 2008). However, extensive care is required during the mechanical harvesting as the process is non-selective and can therefore remove important non-target species such as economic plants, fish, frogs, snails, etc.
Pretreatment of aquatic weed and macroalgae biomass for biofuel production
One of the most crucial step in the production of biofuel from any kind of lignocellulosic biomass, like that of aquatic weed and macroalgae is the selection of pretreatment method as it can largely effect the overall economic cost of production. Selection process depends upon the kind of biomass and the proportion of the polymers viz. lignin, cellulose, hemicelluloses and others constituents. Pretreatment basically helps in solubilizing and separation of these compounds, thus, improving the decomposition of biomass material more readily (Ansari et. al. 2017). A good pretreatment method should help improve the sugar recovery, reduce the loss of valuable compounds and most importantly reduce the cost and energy demand in the biofuel production process. Pretreatment methods are classified as physical pretreatment, chemical pretreatment and biological pretreatment (Fig. 2). There are also combined pretreatment methods which employ more than one technique (Fig. 2).
Physical pretreatment
Like any other lignocellulosic biomass, aquatic weeds and macroalgae are required to go through the preprocessing steps before biofuel production. Physical pretreatment basically changes structure of the biomass through the application of mechanical shear, without adding any kind of chemical or biological reagent (Harun et al. 2011). This can be thoroughly done by employing physical pretreatment methods, which include coarse size reduction through copping or blending, irradiation (gamma radiation, microwave radiation), ultrasound, etc. This is a very important step as it may determine the final particle size, cellulose crystallinity and degree of polymerization (Sun and Cheng 2002).
Size reduction
Size reduction using methods like chopping, milling, grinding, and beating, are used to enhance the conversion of carbohydrate to sugar by increasing the surface area to volume ratio of the biomass (Wang et al. 2011; Wei et al. 2013). Effectiveness of chopping in increasing the surface area of the biomass feedstock was proven by Haug (2018) where chopping of water hyacinth increased the biogas production. This was attributed to enhancement of the access area for the microbes to act upon the chopped water hyacinth. In another instance, Amriani et al. (2016) pretreated water hyacinth biomass through a combination of physical methods such as chopping the biomass, drying it at 105 °C, and thereafter grinding it to the size range of 0.1–1.0 mm. The researchers also compared the physical pretreatment with biophysical pretreatment done with Aspergillus niger and concluded that physical pretreatment produced a higher cellulose content as compared to biophysical pretreatment.
Mechanical size reduction for macroalgae, however, depends upon the type of macroalgae. Researchers have found that the same milling technique applied on different lignocellulosic biomass might not have similar effect on some macroalgae and might even have negative effect on sugar extraction (Adams et al. 2017). Research has shown that beneficial value of mechanical size reduction largely depends on the macroalgal cell wall structure, as more fibrous cell wall shows greater effect of mechanical treatment on sugar production (Nielsen and Heiske 2011). Milling of Laminaria digitata, which is a large flat blade brown algae had no effect on surface area or glucose release (Manns et al. 2016). Similarly, Montingelli et al. (2016) showed that milling of Laminaria sp. reduced methane yield. On the other hand, Oliveira et al. (2014) showed that maceration of Gracilaria vermiculophylla resulted in considerable increase in methane production which was mainly attributed to the increased surface area.
Thermal treatment
Thermal pretreatment is the application of heat on the biomass to effectively disrupt the chemical bonds, resulting in degradation of lignin and hemicellulose, cleavage of cell walls and rupture of cell membrane (Kavitha et al. 2014). The temperature application varies from 50 to 270 °C and can be divided into three types: low temperature thermal pretreatment, hydrothermal pretreatment and steam explosion thermal pretreatment (Passos et al. 2014; Ananthi et al. 2019). Low temperature thermal pretreatment involves heat below 100 °C. Hydrothermal pretreatment involves heat above 100 °C at subsequently higher pressure; and the steam explosion thermal pretreatment involves explosion of a high pressure steam (temperature more than 150 °C) on the biomass and then reducing the pressure to let the biomass undergo decompression (Rodriguez et al. 2015). Different type of treatment can be chosen according to the type of biomass. Schultz-Jensen et al. (2013) reported that autoclaving of Chaetomorpha linum for 10 min at atmospheric temperature increased the glucan yield by 25.7%. In another study, Ruiz et al. (2013) investigated the effect of hydrothermal process on the extraction of agar and carragenaans from red algae at temperature above 85 °C and reported considerable increase in the agar yield. Vivekanand et al. (2012) reported 20.2% increase in biogas production upon pretreating Saccharina latissima by steam explosion method at 130 °C. Similarly, efficacy of steam explosion for aquatic weed E. crassipes was reported by Ganguly et al. (2018) where the authors stated steam explosion to be effective in lignin transformation and hemicellulose degradation, which increases the cellulose hydrolysis potential. Thermal pretreatment is mostly classified as a simple method which can be performed with general equipment, but can release toxic compounds, such as levulinic acid, furfural, and 5-hydroxymethylfurfural (Martı́n et al. 2002).
Microwave pretreatment
Researchers consider microwave pretreatment to be one of the most suitable pretreatment methods for macroalgae and aquatic weeds due to high moisture content which helps in rapid temperature and pressure rise, facilitating the cell wall rupture, which further enhances the biofuel production (Vázquez-Delfín et al. 2014; Ansari et al. 2018). In case of macroalgae, microwave pretreatment is majorly used to extract high-value products like agar, carageenan, fucoidan, etc. Yuan and Macquarrie (2015) reported enhancement of ethanol production from macroalgae Ascophyllum nodosum employing microwave pretreatment by 60.7% in comparison to the theoretical yield. Similar to this study, Romagnoli et al. (2017) investigated microwave pretreatment of Fucus vesiculosus and showed 92% increment in methane production in comparison to untreated biomass. Quitain et al. (2013) compared microwave pretreatment with other thermal pretreatment methods on macroalgae Undaria pinnatifida and reported that microwave pretreatment was more effective in the extraction of polysaccharide and fucoidan compounds.
Microwave pretreatment for water hyacinth was investigated by Liang et al. (2019), where they analyzed the effect of microwave on the biomass structure for the production of pyrolysis products. They reported that microwave pretreatment was effective in changing and destroying the smooth surface structure of the water hyacinth which positively affected the pyrolysis behavior towards the weed biomass, thereby, increasing the pyrolysis product’s yield. In case of aquatic weeds, microwave pretreatment is usually performed in combination or in sequel to some other pretreatment techniques. Similarly, Thi et al. (2017) investigated the cellulose recovery of water hyacinth by comparing the three physical pretreatment methods (waterbath, microwave and ultrasound). The performances were compared on the basis of yield of composition and structure of water hyacinth biomass using Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM). The result showed that microwave pretreatment had highest yield of cellulose and hemicellulose among the studied pretreatment methods. Microwave pretreatment method can also be effectively used for intensifying the lipid extraction process using organic solvents. Microwave pretreatment has the capacity to minimize the sugar degradation due to its rapid heating and also reduces the production of toxic inhibitory products such as levulinic and (5-HMF) 5-hydroxymethylfurfural, usually formed during thermal pretreatment methods.
Ultrasonication
Ultrasound pretreatment technique, commonly known as sonication, is the supply of compression and depression cycle of sonic waves at a very fast rate which results in continuous formation and collapse air cavities which causes breaking of cell envelope (Kavitha et al. 2016). Sonication improves the biofuel production by change in biomass morphology increasing the access of microbes to the fermentable sugars (Passos et al. 2014). Researchers have reported sonication to be an effective technique to extract compounds such as fucoidan, laminarin and other phytochemicals (Kadam et al. 2015). However, sonication pretreatment for macroalgae was reported to be unfit by Karray et al. (2015), where upon comparison with three other pretreatment methods, ultrasonication resulted in least amount of sugar yield. Similarly, Mittal et al. (2017) reported ultrasonication to be unsatisfactory when employed alone, and despite optimization resulted in minimal phycobili proteins extraction, whereas, ultrasonication in combination with different primary pretreatment methods resulted in higher extraction that was mainly attributed to the synergistic effect.
Kist et al. (2018) studied the performance of sonication pretreatment and its efficacy on removal of lag time along with enhancement of methane production for three aquatic weeds (E. crassipes, P. stratiotes and Salvinia molesta). ‘Biomethane potential’ test was conducted to analyze the decomposition of aquatic weeds and was reported that ultrasonication could reduce the lag time and simultaneously increased the methane production. However, the results were not found to be similar for all the weeds since E. crassipes and P. stratiotes showed greater methane production after the ultrasonication pretreatment but on the contrary S. molesta showed better methane production for the untreated biomass.
Physical methods are relatively costly and energy intensive and therefore, they are not opted for large scale biofuel production. On top of that several of the mentioned pretreatments are ineffective alone and require to be combined with other forms of pretreatments which again requires extra input cost and energy (Amriani et al. 2016; Kumari and Singh 2018; Menon and Rao 2012).
Chemical pretreatment
Chemical pretreatment involves using various chemicals such as alkali, organic solvents, acids, carbon dioxide and other similar compounds. The basic motive of this pretreatment is to improve the degradability of the compounds present in the lignocellulosic biomass of aquatic weeds by decreasing the degree of polymerization and crystallinity of the cellulosic compound (Pirzadeh and Ghoreyshi 2014; Swatloski et al. 2002). This is the most studied pretreatment technique in comparison to other methods as there is constant research going on to find a better and more suitable compounds capable of degrading the weed biomass.
Alkali pretreatment
Pretreatment using alkali such as hydroxides of sodium, potassium and calcium has been fairly popular in recent times. Alkali reacts with biomass to cause salvanation and saponification through increase in the surface area of the biomass, degrading cell wall by disrupting the structure and compounds of lignin and hemicellulose. Simultaneously, it causes swelling of cellulosic compounds which results in reduction of crystallinity and decrease in degree of polymerization (Banu et al. 2018; Chandra et al. 2007). Alkali pretreatment is known to enhance biofuel production by reducing pH during the acidogenesis process. Earlier studies have shown that alkali pretreatment is usually considered unattractive due to high processing cost. However, it has been used in the pretreatment of high lignin biomass like aquatic weed and macroalgae. Dilute NaOH (0.5%) and ammonia are the most commonly used compounds for pretreatment of water hyacinth and overall delignification is around 50–70%. Similar to this, alkaline pretreatment of Gracilarial emaneiformis with 5% (w/v) NaOH at 85 °C for 2 h increased the gel strength by six times in comparison to control (Li et al. 2008). The major advantages of alkali pretreatment over acid pretreatment is the requirement of comparatively lower temperature and lower reactor cost. However, on the negative side, the chemicals involved may be costlier and there are higher chances of generation of toxic residual compounds (Mosier et al. 2005).
Acid pretreatment
Acid pretreatment has been one of the major choices of treatment and its efficiency has been fairly investigated by several researchers using dilute and concentrated acids such as sulphuric acid, nitric acid, hydrochloric acid, acetic acid, and others. Sulfuric acid is most commonly used among these. Satyanagalakshmi et al. (2011) studied the effect of acid pretreatment using hydrochloric acid, sulfuric acid, acetic acid and formic acid on the bioethanol production through separate hydrolysis and fermentation. The study showed that among all the organic and mineral acids used for pretreatment, sulfuric acid resulted in highest amount of sugar production. The performance of sulfuric acid was followed by hydrochloric acid and it was further concluded that mineral acids outperformed organic acids in the production of bioethanol. Sarto et al. (2019) studied varying effect of different concentrations of sulfuric acids on biogas production from water hyacinth. Different concentrations ranging from 0–5% v/v with different time (0, 30, 45, 60, 75, 90 min) were taken in the experiment. The results showed that pretreatment successfully altered the cellulose content along with glucose, chemical oxygen demand (COD) and COD/Nitrogen ratio. This enhanced the biogas production. The authors concluded that a 5% (v/v) sulfuric acids with 60 min residence time increased the biogas production by 131.45% in comparison to the untreated biomass. Similarly, a brown algae Macrocystis pyrifera was pretreated using 2 vol% sulfuric acid, water and three different ionic liquids (Ravanal et al. 2016). Sulfuric acid pretreatment strategy, which was followed by saccharification of cellulose along with mixture of cellulases at pH value 5.2 for 4 h showed better results in comparison to other methods for glucose production.
Acid pretreatment has been preferred over alkali pretreatment methods as the former is more effective in degrading hemicellulose content and delignifying aquatic weeds and macroalgal biomass. Dwivedi and Dwivedi (2018) pretreated the samples of water hyacinth using different alkalis (sodium hydroxide and potassium hydroxide) and compared them with acids (hydrochloric acid, sulphuric acid and formic acid) at different concentrations of 1, 2, 3, 4 and 5% v/v each. The results showed that the sulphuric acid at the treatment concentration of 4% v/v showed the best result for sugar conversion. In similar kind of study, Bhetalu and Patil (2012) studied the efficacy of different pretreatment methods for three types of aquatic weeds (water hyacinth, cattail, and duckweed) for bioethanol production. Pretreatment methods included dilute acid pretreatment, concentrated acid pretreatment, and alkali pretreatment at varying concentrations for all three aquatic weeds. The result showed that among all three methods, dilute sulfuric acid (3% v/v) produced maximum fermentable sugar for both water hyacinth and cattail, however, for duckweed, the maximum fermentable sugar was produced by using nitric acid (3% v/v).
Although chemical mode of pretreatment are considered to be one of the best pretreatment methods, certain negative impact in the form of toxicity and corrosiveness cannot be overlooked. Hence, there should be extra attention given to acid concentration, reaction time, and other safety measures to reduce formation of harmful inhibitor compounds (Ajayi and Adefila 2012; Kumari and Singh 2018). Also, it is highly advised to use the pretreatment in combination of more than one mode as this improves the enzymatic hydrolysis and biofuel production considerably (Sindhu et al. 2016). Mood et al. (2013) suggested that alkali pretreatment can be effectively used with acid pretreatment or even with other physical pretreatment methods for better delignification.
Biological pretreatment
Biological pretreatment is safest and an environment friendly processing step. This mostly involves the use of microorganisms and biological enzymes that possess the ability to degrade various compounds like lignin, hemicellulose, and other polyphenols in order to extract energy easily. Most commonly used biological pretreatment methods include usage of fungi, bacteria, or enzymes.
Fungal pretreatment of lignocellulosic biomass viz. aquatic weeds is a relatively new process to improve the digestibility of the biomass (Sinegani et al. 2005). White-, soft- and brown-rot fungi are most commonly used in degrading lignocellulosic compounds present in aquatic weeds. Out of these, brown-rots mainly degrade cellulosic compounds, whereas, white- and soft- rot fungi degrade both lignin and cellulosic compounds by production of several enzymes such as lignin peroxidases, laccases, maganesse-dependent peroxidases, and polyphenol oxidases (Agbor et al. 2011). Upon comparison, white-rot has been reported to be most effective for the biological pretreatment of lignocellulosic biomass (Sun and Cheng 2002). Fungi species such as Trichoderma sp. (Pérez et al. 2002), Pichiastipis sp. (Pothiraj et al. 2014), Aspergillus terreus (Emtiazi et al. 2001), Penicillium camemberti (Taseli 2008) are commonly used in biological pretreatment. Barua et al. (2018) studied biological pretreatment using three bacterial lignocellulose degrading strains which were isolated from soil (Bordetella muralis), from the gut of silverfish (Citrobacter werkmanii) and from millipede (Paenibacillus sp.) to enhance the production of biogas from water hyacinth. The study concluded that microbial pretreatment efficiently enhanced the biodegradability of the water hyacinth biomass. Among all the three strains, pretreatment of C. werkmanii was more effective in comparison to other two and successfully increased the biogas production. Whereas, biological pretreatment of Mexican Caribbean macroalgae consortiums using Bm-2 strain of Trametes hirsuta, isolated from decaying wood was investigated by Tussell et al. (2018) and it showed 20% increment in the methane production. The authors suggested that application of fungal strains which are easily available in nature can be a solution to problems of sustainable energy. Similarly, Singh et. al. (2021) corroborated the biological pretreatment of water hyacinth with white-rot fungus Alternaria alternata strain AKJK-2 for large scale utilization in bioenergy production.
Enzymatic pretreatment involves addition of cellulose, hemicellulose, and starch degrading enzymes or mixture of enzymes to enhance the degradation of biomass. Some of these enzymes are already present in the digester, especially during anaerobic digestion produced by the added microorganisms (Bohutskyi and Bouwer 2013). Enzymatic pretreatment can be used as an alternative to mechanical and chemical methods which are much more energy demanding and costly. Perez et al. (2018) studied the enzymatic pretreatment of Sargassum spp. using laminarinase and cellulase. The result showed that solo treatment with either enzyme resulted in low yield of ethanol implied to poor saccharification, whereas, the pretreatment using combination of both resulted in subsequent higher ethanol production which was attributed to the synergistic effect between both enzymes. The main disadvantage of enzymatic pretreatment is related to the long reaction and residence time in comparison to other pretreatment methods, which usually requires 10–14 days (Agbor et al. 2011).
Biological pretreatment for lignocellulosic biomass is a very promising technique with various advantages viz. low energy input, no or minimal chemical requirement and environment friendly working procedures (Salvachúa et al. 2011). There are several disadvantages of biological pretreatment as well which make them less attractive for large scale and commercial production of bioenergy (Rodriguez et al. 2015). These include slow process, making it unsuitable for industrial purpose, requirement of large place, high enzyme cost, and low enzyme-substrate specificity. Apart from this, biological pretreatment may produce some inhibitory enzymes and some part of the carbohydrate is consumed by microorganisms itself resulting in a low biofuel yield (Agbor et al. 2011; Chandra et al. 2007; Shi et al. 2008). Due to these, biological pretreatment faces barriers to be used at commercial scale. To overcome these difficulties, biological pretreatment can be used in combination with other pretreatment methods, efficiency of which has been proven at several instances. Ma et al. (2010) evaluated the effect of combinations of biological pretreatment with mild acid pretreatment. Biological pretreatment was carried out with a white-rot fungus specie Echinodontium taxodii and brown rot fungus Antrodia sp. and 0.25% v/v sulfuric acid was used in chemical pretreatment. The result concluded that the white-rot fungus E. taxodii in combination with sulfuric acid was more effective in boosting the enzymatic hydrolysis process and production of ethanol from water hyacinth biomass. The combination enhanced the bioethanol production to 1.13–2.11 times than that with the chemical or biological pretreatment alone (Ma et al. 2010).
Combined pretreatment method
Combined pretreatment is complex but has been proven to be highly effective in enhancing the productivity of lignocellulosic biomass. Several pretreatment combinations are vastly being explored as alternative for conventional pretreatment techniques such as combinations of ball milling and microwave irradiation (Peng et al. 2013), alkali pretreatment with high pressure homogenization (Fang et al. 2014), washing with torrefication (Zhang et al. 2018), acid treatment with ionization radiation (Yang and Wang, 2018), combined thermo-chemo-ozone pretreatment (Kannah et al. 2017), ozonation with ultrasonication (Dastpak et al. 2020) and others. Effectiveness of combined pretreatment methods was investigated by Patil et al. (2011), where performance of different pretreatment methods and some along with combinations viz. water hyacinth in four forms as (i) chopped, dried, and ground (ii) NaOH, dried, and ground (iii) ground, water hyacinth combined with poultry waste (iv) ground, water hyacinth combined with primary sludge while using only water hyacinth as control. Water hyacinth combined with primary sludge showed better biogas production potential than any other mode of pretreatment. Following this pretreatment method, NaOH treated dried and grounded water hyacinth had better results in comparison to dried and grounded alone. Several other combined methods for aquatic weeds and macroalgae are mentioned in Table 4.
Influence of harvesting and pretreatment on biofuel production
The manual harvesting of cultivated as well as naturally grown macroalgae is very common. However, manual harvesting is a time taking and labor intensive process and its use for the production of biofuel is inefficient. The manpower requirement gets reduced by the mechanization of harvesting technique. Ships and boats are required for the operation of these mechanical harvesters which are equipped with cutters, movers as well as pumps (Burton et al. 2009; Ugarte and Sharp 2001). The bulk harvest derived by the application of mechanical harvesters unlike manual harvesting is apt for biofuel generation. The harvesting of macroalgae is more economical than harvesting of microalgae. The issue with cultivating macroalgae or aquatic weeds is that, these are frequently damaged by yachts and commercial ships which reduce total biomass collection. Macroalgae and aquatic weeds need to be prepared and transported after harvest to convert to target fuel. They need to be dried as it prevents the growth of microorganisms as well as inhibits enzymatic reactions leading to deterioration. Sun drying is the most conventional and least expensive method used for drying which is a volume and weather dependent process (Valderrama et al. 2014). Dewatering of the algal and weed biomass by pressing or centrifugation increases its shelf life and reduces the water content in biomass by 20–30%, thereby reducing transportation cost (Burton et al. 2009). An alternative method of preservation is ensiling, which converts water-soluble carbohydrate to organic acids, which in turn decreases the pH of moist biomass (Ashbell and Weinberg 2005). Ensiling is done by bacteria present on the crop, which enables anaerobic lactic acid fermentation. It has been reported by FAO that this method can preserve the harvested energy by > 90% of the original biomass (Kreuger et al. 2011). The biomass after harvest consists of impurities which need to be removed. This is done by washing which removes debris, salt and other minerals. In the chemical and thermochemical processes, the algal biomass gets affected by the effluent production, water usage and energy input requirements, thus influencing the biofuel production.
Aquatic weeds and macroalgae contain high carbohydrate, cellulose and hemicellulose with low lignin content. However, due to complex and recalcitrant structure of these biomass, digestion for any kind of biofuel production is rather difficult. Recalcitrant structure hinders the hydrolysis and inhibits enzymatic action on the biomass. Hence, additional process of pretreatment is essential in increasing the biofuel production from these biomass by breaking of cellulose, hemicellulose and lignin to facilitate hydrolysis (Sindhu et al. 2017). As mentioned earlier, various pretreatment techniques have been investigated for different types and structure of aquatic weeds and macroalgae biomass for an efficient and low-cost biofuel extraction (Barua and Kalamdhad 2017). Alkali or acid pretreatment, which are rather effective in hydrolysing polymer and swelling biomass fibers may produce inhibitory compounds. Washing, which is termed effective in removing inhibitory salts may have varying or no effect on some of the biomass. Hence, effectiveness of these pretreatment methods and techniques strictly depends upon the chemical composition and physical structure of biomass and therefore, one pretreatment technique may be highly efficient on one type and may have a negative effect on another (Sindhu et al. 2016). Efficacy of different pretreatment methods on biogas and bioethanol production from different types of aquatic weeds and macroalgae are mentioned in Table 5.
Future prospects and conclusion
Currently, there is growing renewable energy demand which can be fulfilled by biofuels. Aquatic weeds and macroalgal biomass being third generational feedstock have the potential to meet these demands and therefore are being studied extensively. For aquatic weeds and macroalgae based biofuel to compete with the crude oil in present times, the major bottleneck is processing and production economics, harvesting, pretreatment, hydrolysis, and fermentation. Aquatic weeds and macroalgae based biorefinery is anticipated to rise considerably in coming future, attributed to its economic and ecological advantages. There is a need to investigate and develop new technologies for more efficient and eco-friendly harvesting, pretreatment and fermentation techniques. Since the harvesting location and time greatly influence the chemical composition of the aquatic plants, an extensive research is required to extend the knowledge of distribution of these resources, designing of superior harvesting techniques, and choice of pretreatment to bridge the economic gap between aquatic plant biomass and other conventional biofuel feedstock. Mechanical harvesting techniques need to be developed while avoiding their disruptive effect on ecology because of nonselective nature. Use of a bioflocculant instead of harmful chemical based flocculants should be encouraged. Biological pretreatments are eco-friendly over conventional chemical and physical pretreatment of biomass. Emphasis should be given on development and advancement of biological pretreatment methods for cell disruption and extraction of constituents from biomass. From the techno-economic evaluation, various combined pretreatment techniques seem to have the potential to show synergistic effect cancelling each other's drawbacks and negative environmental impacts. Life cycle assessment and environmental risk assessment studies should be carried out for developed processing techniques before implementation at commercial scale. In conclusion, aquatic plant biomass possess the potential to outclass first and second generation biofuel feedstocks in the future and therefore, a major effort is required to develop efficient, environmentally friendly and cost effective processing steps for producing advanced biofuels as sustainable energy source.
References
Adams JMM, Bleathman G, Thomas D, Gallagher JA (2017) The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. J Appl Phycol 29:2463–2469
Adelakun KM, Kehinde AS, Amali RP, Ogundiwin DI, Omotayo OL (2016) Nutritional and phytochemical quality of some tropical aquatic plants. Poultry, Fish Wildl Sci 1–4
Adeniji HA (1979) Framework document on special problem of man-made lakes In: SIL Workshop on African Limnology, UNEP Head-Quarters, Nairobi, Kenya
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29:675–685
Ajayi OA, Adefila SS (2012) Methanol production from cow dung. J Environ Earth Sci 2:948–2225
Aloo P, Ojwang W, Omondi R, Njiru JM, Oyugi D (2013) A review of the impacts of invasive aquatic weeds on the bio-diversity of some tropical water bodies with special reference to Lake Victoria (Kenya). Biodivers J 4:471–482
Al-Yamani FY, Polikarpov I, Al-Ghunaim A, Mikhaylova T (2014) Field guide of marine macroalgae (Chlorophyta, Rhodophyta, Phaeophyceae) of Kuwait. Kuwait Inst Sci Res Kuwait
Amamou S, Sambusiti C, Monlau F, Dubreucq E, Barakat A (2018) Mechano-enzymatic deconstruction with a new enzymatic cocktail to enhance enzymatic hydrolysis and bioethanol fermentation of two macroalgae species. Molecules 23:174
Amriani F, Salim FA, Iskandinata I, Khumsupan D, Barta Z (2016) Physical and biophysical pretreatment of water hyacinth biomass for cellulase enzyme production. Chem Biochem Eng Q 30:237–244
Ananthi V, Prakash GS, Chang SW, Ravindran B, Nguyen DD, Vo D-VN, La DD, Bach Q-V, Wong JWC, Gupta SK (2019) Enhanced microbial biodiesel production from lignocellulosic hydrolysates using yeast isolates. Fuel 256:115932
Ansari FA, Wahal S, Gupta SK, Rawat I, Bux F (2017) A comparative study on biochemical methane potential of algal substrates: implications of biomass pre-treatment and product extraction. Bioresour Technol 234:320–326
Ansari FA, Gupta SK, Nasr M, Rawat I, Bux F (2018) Evaluation of various cell drying and disruption techniques for sustainable metabolite extractions from microalgae grown in wastewater: a multivariate approach. J Clean Prod 182:634–643
Asogwa VC, Asogwa JN (2018) Aquatic weeds in fish culture: prevention and control practices. Am J Mar Res Rev 1:001–009
Banu JR, Kannah RY, Kavitha S, Gunasekaran M, Kumar G (2018) Novel insights into scalability of biosurfactant combined microwave disintegration of sludge at alkali pH for achieving profitable bioenergy recovery and net profit. Bioresour Technol 267:281–290
Banu JR, Tamilarasan K, Chang SW, Nguyen DD, Ponnusamy VK, Kumar G (2020) Surfactant assisted microwave disintegration of green marine macroalgae for enhanced anaerobic biodegradability and biomethane recovery. Fuel 281:118802
Barua VB, Kalamdhad AS (2017) Effect of various types of thermal pretreatment techniques on the hydrolysis, compositional analysis and characterization of water hyacinth. Bioresour Technol 227:147–154
Barua VB, Goud VV, Kalamdhad AS (2018) Microbial pretreatment of water hyacinth for enhanced hydrolysis followed by biogas production. Renew Energy 126:21–29
Basak SK, Ali MM, Islam MS, Shaha PR (2015) Aquatic weeds of Haor area in Kishoregonj district, Bangladesh: availability, threats and management approaches. Int J Fish Aquat Stud 2:151–156
Bhetalu A, Patil S, Ingole N (2012) Studies on generation of power alcohol as a non-conventional energy source from aquatic macrophytes- a critical review. J Eng Res Stud 3:9–17
Bohutskyi P, Bouwer E (2013) Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In: Lee J (ed) Advanced biofuels and bioproducts. Springer, New York, NY, pp 873–975
Burton T, Lyons H, Lerat Y, Stanley M, Rasmussen MB (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sustainable Energy Ireland. pp 1–88
Caisova L, Gąbka M (2009) Charophytes (Characeae, Charophyta) in the Czech Republic: taxonomy, autecology and distribution. Fottea 9:1–43
Chakraborty S, Santra SC (2008) Biochemical composition of eight benthic algae collected from Sunderban. Indian J Mar Sci 37:329–332
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? In: Olsson L (ed) Biofuels advances in biochemical engineering/biotechnology, vol 108. Springer, Berlin, Heidelberg, pp 67–93
Chen Q, Jin Y, Zhang G, Fang Y, Xiao Y, Zhao H (2012) Improving production of bioethanol from duckweed (Landoltia punctata) by pectinase pretreatment. Energies 5:3019–3032
Cheng J, Xia A, Su H, Song W, Zhou J, Cen K (2013) Promotion of H2 production by microwave-assisted treatment of water hyacinth with dilute H2SO4 through combined dark fermentation and photofermentation. Energy Convers Manag 73:329–334
Chojnacka K, Saeid A, Witkowska Z, Tuhy L (2012) Biologically active compounds in seaweed extracts-the prospects for the application In: The Open Conference Proceedings Journal
Clayton JS (1996) Aquatic weeds and their control in New Zealand Lakes. Lake Reserv Manag 12:477–486
Dalal RC, Allen DE, Livesley SJ, Richards G (2008) Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant Soil 309:43–76
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feedstocks. Appl Energy 102:1371–1381
Das SP, Ravindran R, Ghosh A, Deka D, Das D, Jawed M, Fontes CMGA, Goyal A (2014) Efficient pretreatment for bioethanol production from water hyacinth (Eichhornia crassipes) involving naturally isolated and recombinant enzymes and its recovery. Environ Prog Sustain Energy 33:1396–1404
Das SP, Gupta A, Das D, Goyal A (2016) Enhanced bioethanol production from water hyacinth (Eichhornia crassipes) by statistical optimization of fermentation process parameters using Taguchi orthogonal array design. Int Biodeterior Biodegradation 109:174–184
Dastpak H, Pasalari H, Jafari AJ, Gholami M, Farzadkia M (2020) Improvement of co-composting by a combined pretreatment Ozonation/Ultrasonic process in stabilization of raw activated sludge. Sci Rep 10:1–7
Datta S (2009) Aquatic weeds and their management for fisheries. Aquat Weeds Their Manag Fish, pp 1–22
Davis R, Hirji R (2003) Management of Aquatic Weed, Technical Note G.4 In: Water Resource Management
Dwivedi M, Dwivedi AK (2018) Water hyacinth feedstock: a renewable source for bio-ethanol production. Cellulose 18:35
Emtiazi G, Naghavi N, Bordbar A (2001) Biodegradation of lignocellulosic waste by Aspergillus terreus. Biodegradation 12:257–261
Fang Z-F, Liu K-L, Chen F-S, Zhang L-F, Guo Z (2014) Cationic surfactant-assisted microwave-NaOH pretreatment for enhancing enzymatic hydrolysis and fermentable sugar yield from peanut shells. BioResources 9:1290–1302
Gallagher JE, Haller WT (1990) History and development of aquatic weed control in the United States. Rev Weed Sci 5:115–192
Ganguly P, Gangwar C, Mishra A, Rani R, Awasthi S, Singh RK, Bhatnagar T (2018) Effect of Saccharification Methods on Bioethanol Production by Thermophiles from Eichhornia crassipes. Int J Curr Microbiol App Sci 7:3595–3603
Ge X, Zhang N, Phillips GC, Xu J (2012) Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Bioresour Technol 124:485–488
Gettys LA (2014) Aquatic weed management: control methods. SRAC Publication—Southern Regional Aquaculture Centre
Gupta SK, Chabukdhara M, Kumar P, Singh J, Bux F (2014) Evaluation of ecological risk of metal contamination in river Gomti, India: a biomonitoring approach. Ecotoxicol Environ Saf 110:49–55
Gupta SK, Ansari FA, Nasr M, Rawat I, Nayunigari MK, Bux F (2017) Cultivation of Chlorella sorokiniana and Scenedesmus obliquus in wastewater: fuzzy intelligence for evaluation of growth parameters and metabolites extraction. J Clean Prod 147:419–430
Gupta SK, Kumar NM, Guldhe A, Ansari FA, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68
Guragain YN, De Coninck J, Husson F, Durand A, Rakshit SK (2011) Comparison of some new pretreatment methods for second generation bioethanol production from wheat straw and water hyacinth. Bioresour Technol 102:4416–4424
Gusain R, Suthar S (2017) Potential of aquatic weeds (Lemna gibba, Lemna minor, Pistia stratiotes and Eichhornia sp.) in biofuel production. Process Saf Environ Prot 109:233–241
Harun MY, Radiah ABD, Abidin ZZ, Yunus R (2011) Effect of physical pretreatment on dilute acid hydrolysis of water hyacinth (Eichhornia crassipes). Bioresour Technol 102:5193–5199
Haug R (2018) The practical handbook of compost engineering. Routledge, Abington
Hoevers R (2011) Aquatic biofuels for local development. FACT
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
ICID.CIID International Commission on Irrigation and drainage (2002) Aquatic Weeds & their Management. Water 65.
İnan B, Özçimen D (2019) A comparative study of bioprocess performance for improvement of bioethanol production from macroalgae. Chem Biochem Eng Q 33:133–140
Jang J-S, Cho Y, Jeong G-T, Kim S-K (2012) Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess Biosyst Eng 35:11–18
Jard G, Dumas C, Delgenes JP, Marfaing H, Sialve B, Steyer JP, Carrère H (2013) Effect of thermochemical pretreatment on the solubilization and anaerobic biodegradability of the red macroalga Palmaria palmata. Biochem Eng J 79:253–258
Jayan PR, Sathyanathan N (2012) Aquatic weed classification, environmental effects and the management technologies for its effective control in Kerala, India. Int J Agric Biol Eng 5:76–91
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Jung H, Kim J, Lee C (2016) Continuous anaerobic co-digestion of Ulva biomass and cheese whey at varying substrate mixing ratios: different responses in two reactors with different operating regimes. Bioresour Technol 221:366–374
Kadam SU, Álvarez C, Tiwari BK, O’Donnell CP (2015) Extraction of biomolecules from seaweeds. In: Jones W (ed) Seaweed sustainability. Elsevier, Amsterdam, pp 243–269
Kannah RY, Kavitha S, Banu JR, Yeom IT, Johnson M (2017) Synergetic effect of combined pretreatment for energy efficient biogas generation. Bioresour Technol 232:235–246
Karray R, Hamza M, Sayadi S (2015) Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatments on anaerobic digestion of Ulva rigida for biogas production. Bioresour Technol 187:205–213
Kaur M, Kumar M, Sachdeva S, Puri SK (2018) Aquatic weeds as the next generation feedstock for sustainable bioenergy production. Bioresour Technol 251:390–402
Kaur M, Kumar M, Singh D, Sachdeva S, Puri SK (2019) A sustainable biorefinery approach for efficient conversion of aquatic weeds into bioethanol and biomethane. Energy Convers Manag 187:133–147
Kavitha S, Jayashree C, Kumar SA, Kaliappan S, Banu JR (2014) Enhancing the functional and economical efficiency of a novel combined thermo chemical disperser disintegration of waste activated sludge for biogas production. Bioresour Technol 173:32–41
Kavitha S, Banu JR, Kumar JV, Rajkumar M (2016) Improving the biogas production performance of municipal waste activated sludge via disperser induced microwave disintegration. Bioresour Technol 217:21–27
Kennish MJ, Haag SM, Sakowicz GP (2008) Seagrass demographic and spatial habitat characterization in Little Egg Harbor, New Jersey, using fixed transects. J Coast Res. https://doi.org/10.2112/SI55-0013.1
Kim N-J, Li H, Jung K, Chang HN, Lee PC (2011) Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour Technol 102:7466–7469
Kist DL, Cano R, Sapkaite I, Pérez-Elvira SI, Monteggia LO (2020) Macrophytes as a digestion substrate. Assessment of a sonication pretreatment. Waste Biomass Valor 11(5):1765–1775
Korzen L, Pulidindi IN, Israel A, Abelson AGA (2015) Single step production of bioethanol from the seaweed Ulva rigida using sonication. R Soc Chem Adv 5:16223–16229
Kreuger E, Prade T, Escobar F, Svensson S-E, Englund J-E, Björnsson L (2011) Anaerobic digestion of industrial hemp–Effect of harvest time on methane energy yield per hectare. Biomass Bioenergy 35:893–900
Kumar MD, Kaliappan S, Gopikumar S, Zhen G, Banu JR (2019) Synergetic pretreatment of algal biomass through H2O2 induced microwave in acidic condition for biohydrogen production. Fuel 253:833–839
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sustain Energy Rev 90:877–891
Lafarga T, Acién-Fernández FG, Garcia-Vaquero M (2020) Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res 48:101909
Lembi CA (2009) Identifying and managing aquatic vegetation, Aquatic Plant Manage. Purdue Extension APW-3-W.
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Li H, Kjerstadius H, Tjernström E, Davidsson Å (2013) Evaluation of pretreatment methods for increased biogas production from macro algae. Sven Gastek Cent AB, SGC Rapp 278
Liang J, Yu Z, Chen L, Fang S, Ma X (2019) Microwave pretreatment power and duration time effects on the catalytic pyrolysis behaviors and kinetics of water hyacinth. Bioresour Technol 286:121369
Lordan S, Ross RP, Stanton C (2011) Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs 9:1056–1100
Ma F, Yang N, Xu C, Yu H, Wu J, Zhang X (2010) Combination of biological pretreatment with mild acid pretreatment for enzymatic hydrolysis and ethanol production from water hyacinth. Bioresour Technol 101:9600–9604
Madsen JD (2000) Advantages and disadvantages of aquatic plant management techniques. Environmental Laboratory, Vicksburg, MS
Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, Lipton AP (2009) Antimicrobial potential and seasonality of red algae collected from the southwest coast of India tested against shrimp, human and phytopathogens. Ann Microbiol 59:207–219
Manns D, Andersen SK, Saake B, Meyer AS (2016) Brown seaweed processing: enzymatic saccharification of Laminaria digitata requires no pre-treatment. J Appl Phycol 28:1287–1294
Mantri VA, Kavale MG, Kazi MA (2020) Seaweed biodiversity of India: reviewing current knowledge to identify gaps, challenges, and opportunities. Diversity 12:13
Martı́n C, Galbe M, Wahlbom CF, Hahn-Hägerdal B, Jönsson LJ (2002) Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb Technol 31:274–282
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci 38:522–550
Miranda AF, Biswas B, Ramkumar N, Singh R, Kumar J, James A, Roddick F, Lal B, Subudhi S, Bhaskar T (2016) Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol Biofuels 9:221
Mittal R, Tavanandi HA, Mantri VA, Raghavarao K (2017) Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason Sonochem 38:92–103
Mondal D (2018) The utilization of aquatic weeds in an environmental friendly way of fish feed formulation- a review. Int Res J Environ Sci 7:60–66
Montingelli ME, Benyounis KY, Stokes J, Olabi A-G (2016) Pretreatment of macroalgal biomass for biogas production. Energy Convers Manag 108:202–209
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Mthethwa NP, Nasr M, Bux F, Kumari S (2018) Utilization of Pistia stratiotes (aquatic weed) for fermentative biohydrogen: electron-equivalent balance, stoichiometry, and cost estimation. Int J Hydrogen Energy 43:8243–8255
Murdock JN, Wetzel DL (2009) FT-IR microspectroscopy enhances biological and ecological analysis of algae. Appl Spectrosc Rev 44:335–361
Nielsen HB, Heiske S (2011) Anaerobic digestion of macroalgae: methane potentials, pre-treatment, inhibition and co-digestion. Water Sci Technol 64:1723–1729
O’Sullivan C, Rounsefell B, Grinham A, Clarke W, Udy J (2010) Anaerobic digestion of harvested aquatic weeds: water hyacinth (Eichhornia crassipes), cabomba (Cabomba caroliniana) and salvinia (Salvinia molesta). Ecol Eng 36:1459–1468
Ogbaga CC, Bajhaiya AK, Gupta SK (2019) Improvements in biomass production: learning lessons from the bioenergy plants maize and sorghum. J Environ Biol 40:400–406
Oliveira JV, Alves MM, Costa JC (2014) Design of experiments to assess pre-treatment and co-digestion strategies that optimize biogas production from macroalgae Gracilaria vermiculophylla. Bioresour Technol 162:323–330
Özçimen D, Gülyurt MÖ, İnan B (2012) Algal biorefinery for biodiesel production. Biodiesel-Feedstocks production and application. Rijeka, Intech, pp 25–57
Panahi HKS, Tabatabaei M, Aghbashlo M, Dehhaghi M, Rehan M, Nizami A-S (2019) Recent updates on the production and upgrading of bio-crude oil from microalgae. Bioresour Technol Rep 7:100216
Park J-H, Hong J-Y, Jang HC, Oh SG, Kim S-H, Yoon J-J, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Passos F, Uggetti E, Carrère H, Ferrer I (2014) Pretreatment of microalgae to improve biogas production: a review. Bioresour Technol 172:403–412
Patil JH, AntonyRaj M, Gavimath CC (2011) Study on effect of pretreatment methods on biomethanation of water hyacinth. Int J Adv Biotechnol Res 2:143–147
Peng H, Li H, Luo H, Xu J (2013) A novel combined pretreatment of ball milling and microwave irradiation for enhancing enzymatic hydrolysis of microcrystalline cellulose. Bioresour Technol 130:81–87
Pereira H, Barreira L, Figueiredo F, Custódio L, Vizetto-Duarte C, Polo C, Rešek E, Engelen A, Varela J (2012) Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar Drugs 10:1920–1935
Pérez J, Munoz-Dorado J, De la Rubia T, Martinez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Perez CMT, Pajares IG, Alcantara VA, Simbahan JF (2018) Bacterial laminarinase for application in ethanol production from brown algae Sargassum sp. using halotolerant yeast. Biofuel Res J 5:792–797
Peteiro C, Freire Ó (2012) Outplanting time and methodologies related to mariculture of the edible kelp Undaria pinnatifida in the Atlantic coast of Spain. J Appl Phycol 24:1361–1372
Pirzadeh K, Ghoreyshi AA (2014) Phenol removal from aqueous phase by adsorption on activated carbon prepared from paper mill sludge. Desalin Water Treat 52:6505–6518
Pothiraj C, Arumugam R, Gobinath M (2014) Sustaining ethanol production from lime pretreated water hyacinth biomass using mono and co-cultures of isolated fungal strains with Pichia stipitis. Bioresour Bioprocess 1:27
Potts T, Du J, Paul M, May P, Beitle R, Hestekin J (2012) The production of butanol from Jamaica bay macro algae. Environ Prog Sustain Energy 31:29–36
Quitain AT, Kai T, Sasaki M, Goto M (2013) Microwave–hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Ind Eng Chem Res 52:7940–7946
Ramaraj R, Unpaprom Y (2019) Enzymatic hydrolysis of small-flowered nutsedge (Cyperus difformis) with alkaline pretreatment for bioethanol production. Maejo Int J Sci Technol 13:110–120
Ravanal MC, Pezoa-Conte R, von Schoultz S, Hemming J, Salazar O, Anugwom I, Lienqueo ME (2016) Comparison of different types of pretreatment and enzymatic saccharification of Macrocystis pyrifera for the production of biofuel. Algal Res 13:141–147
Reddy K, Nasr M, Kumari S, Kumar S, Gupta SK, Enitan AM, Bux F (2017) Biohydrogen production from sugarcane bagasse hydrolysate: effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ Sci Pollut Res 24:8790–8804
Rey JR, Rutledge R (2006) Seagrass beds of the Indian River lagoon. Accessed http://edis.ifas.ufl.edu/in189
Rodriguez C, Alaswad A, Mooney J, Prescott T, Olabi AG (2015) Pre-treatment techniques used for anaerobic digestion of algae. Fuel Process Technol 138:765–779
Roesijadi G, Copping AE, Huesemann MH, Forster J, Benemann JR (2008) Techno-economic feasibility analysis of offshore seaweed farming for bioenergy and biobased products, Battelle Pacific Northwest Division Report Number PNWD-3931
Roesijadi G, Jones SB, Snowden-Swan LJ, Zhu Y (2010) Macroalgae as a biomass feedstock: a preliminary analysis. Pacific Northwest National Lab.(PNNL), Richland, WA (United States). Doi: https://doi.org/10.2172/1006310
Rohani-Ghadikolaei K, Abdulalian E, Ng W-K (2012) Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J Food Sci Technol 49:774–780
Romagnoli F, Pastare L, Sabūnas A, Bāliņa K, Blumberga D (2017) Effects of pre-treatment on Biochemical Methane Potential (BMP) testing using Baltic Sea Fucus vesiculosus feedstock. Biomass Bioenerg 105:23–31
Rooney WL, Blumenthal J, Bean B, Mullet JE (2007) Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod Biorefining 1:147–157
Rozas LP, Odum WE (1988) Occupation of submerged aquatic vegetation by fishes: testing the roles of food and refuge. Oecologia 77:101–106
Ruiz HA, Rodríguez-Jasso RM, Fernandes BD, Vicente AA, Teixeira JA (2013) Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: a review. Renew Sustain Energy Rev 21:35–51
Sahoo NK, Gupta SK, Rawat I, Ansari FA, Singh P, Naik SN, Bux F (2017) Sustainable dewatering and drying of self-flocculating microalgae and study of cake properties. J Clean Prod 159:248–256
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour Technol 102:7500–7506
Samaraweera AM, Vidanarachchi JK, Kurukulasuriya MS (2012) Industrial applications of macroalgae. Handbook of Marine macroalgae. Wiley, Amsterdam, pp 500–521
Sarto S, Hildayati R, Syaichurrozi I (2019) Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew Energy 132:335–350
Satyanagalakshmi K, Sindhu R, Binod P, Janu KU, Sukumaran RK, Pandey A (2011) Bioethanol production from acid pretreated water hyacinth by separate hydrolysis and fermentation
Schultz-Jensen N, Thygesen A, Leipold F, Thomsen ST, Roslander C, Lilholt H, Bjerre AB (2013) Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol–comparison of five pretreatment technologies. Bioresour Technol 140:36–42
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99:6556–6564
Sinbuathong N (2019) Predicting the increase of methane yield using alkali pretreatment for weeds prior to co-digestion. Energy Sour A 41:1124–1131
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass–An overview. Bioresour Technol 199:76–82
Sindhu R, Binod P, Mathew AK, Abraham A, Gnansounou E, Ummalyma SB, Thomas L, Pandey A (2017) Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Bioresour Technol 242:146–151
Sinegani AAS, Emtiazi G, Hajrasuliha S, Shariatmadari H (2005) Biodegradation of some agricultural residues by fungi in agitated submerged cultures. Afr J Biotechnol 4
Singh A, Bishnoi NR (2013) Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind Crops Prod 44:283–289
Singh JK, Chaurasia B, Dubey A, Noguera AM, Gupta A, Kothari R, Upadhyaya CP, Kumar A, Hashem A, Alqarawi AA, Abd Allah EF (2021) Biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eicchornia crassipes) biomass hydrolysis. Sustainability 13:245
Song W, Ding L, Liu M, Cheng J, Zhou J, Li YY (2020) Improving biohydrogen production through dark fermentation of steam-heated acid pretreated Alternanthera philoxeroides by mutant Enterobacter aerogenes ZJU1. Sci Total Environ 716:134695
Stallings KD, Seth-Carley D, Richardson RJ (2015) Management of aquatic vegetation in the southeastern United States. J Integr Pest Manag 6:3
Suleiman M, Khadija AY, Nasiru Y, Garba AA, Alhassan M, Bello HJ (2020) Proximate, minerals and anti-nutritional composition of water hyacinth (Eichhornia crassipes) grass. Earthline J Chem Sci 3:51–59
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124:4974–4975
Syaichurrozi I, Villta PK, Nabilah N, Rusdi R (2019) Effect of sulfuric acid pretreatment on biogas production from Salvinia molesta. J Environ Chem Eng 7:102857
Tantayotai P, Mutrakulchareon P, Tawai A, Roddecha S, Sriariyanun M (2019) Effect of organic acid pretreatment of water hyacinth on enzymatic hydrolysis and biogas and bioethanol production. IOP Conf Ser Earth Environ Sci 346:12004
Tapia-Tussell R, Avila-Arias J, Domínguez Maldonado J, Valero D, Olguin-Maciel E, Pérez-Brito D, Alzate-Gaviria L (2018) Biological pretreatment of mexican caribbean macroalgae consortiums using Bm-2 strain (Trametes hirsuta) and its enzymatic broth to improve biomethane potential. Energies 11:494
Taseli BK (2008) Fungal treatment of hemp-based pulp and paper mill wastes. Afr J Biotechnol 7
Thi BTN, Thanh LHV, Lan TNP, Thuy NTD, Ju Y-H (2017) Comparison of some pretreatment methods on cellulose recovery from water hyacinth (Eichhornia crassipe). J Clean Energy Technol 5:274–279
Tibbetts SM, Milley JE, Lall SP (2016) Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J Appl Phycol 28:3575–3585
Tipnee S, Ramaraj R, Unpaprom Y (2015) Nutritional evaluation of edible freshwater green macroalga Spirogyra varians. Emergent Life Sci Res 1:1–7
Ugarte RA, Sharp G (2001) A new approach to seaweed management in eastern Canada: the case of Ascophyllum nodosum. Cah Biol Mar 42(1/2):63–70
Valderrama D, Iyemperumal S, Krishnan M (2014) Building consensus for sustainable development in aquaculture: a delphi study of better management practices for shrimp farming in India. Aquac Econ Manag 18:369–394
Vanegas CH, Hernon A, Bartlett J (2015) Enzymatic and organic acid pretreatment of seaweed: effect on reducing sugars production and on biogas inhibition. Int J Ambient Energy 36:2–7
Vázquez-Delfín E, Robledo D, Freile-Pelegrín Y (2014) Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J Appl Phycol 26:901–907
Vivekanand V, Eijsink VGH, Horn SJ (2012) Biogas production from the brown seaweed Saccharina latissima: thermal pretreatment and codigestion with wheat straw. J Appl Phycol 24:1295–1301
Wang X, Liu X, Wang G (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation F. J Integr Plant Biol 53:246–252
Wei N, Quarterman J, Jin Y-S (2013) Marine macroalgae: an untapped resource for producing fuels and chemicals. Trends Biotechnol 31:70–77
Yan J, Wei Z, Wang Q, He M, Li S, Irbis C (2015) Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour Technol 193:103–109
Yang G, Wang J (2018) Pretreatment of grass waste using combined ionizing radiation-acid treatment for enhancing fermentative hydrogen production. Bioresour Technol 255:7–15
Yazdani P, Zamani A, Karimi K, Taherzadeh MJ (2015) Characterization of Nizimuddinia zanardini macroalgae biomass composition and its potential for biofuel production. Bioresour Technol 176:196–202
Yin Y, Wang J (2018) Pretreatment of macroalgal Laminaria japonica by combined microwave-acid method for biohydrogen production. Bioresour Technol 268:52–59
Yuan Y, Macquarrie DJ (2015) Microwave assisted acid hydrolysis of brown seaweed Ascophyllum nodosum for bioethanol production and characterization of alga residue. ACS Sustain Chem Eng 3:1359–1365
Zhang S, Guo H, Du L, Liang J, Lu X, Li N, Zhang K (2015) Influence of NaOH and thermal pretreatment on dewatered activated sludge solubilisation and subsequent anaerobic digestion: focused on high-solid state. Bioresour Technol 185:171–177
Zhang Q, Wei Y, Han H, Weng C (2018) Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour Technol 251:358–363
Zhao Y, Fang Y, Jin Y, Huang J, Bao S, Fu T, He Z, Wang F, Zhao H (2014) Potential of duckweed in the conversion of wastewater nutrients to valuable biomass: a pilot-scale comparison with water hyacinth. Bioresour Technol 163:82–91
Acknowledgements
Mr. Shahrukh Nawaj Alam and Ms. Zaira Khalid are grateful to Central University of Jharkhand, Ranchi, Jharkhand, India for receiving UGC University fellowship. Dr. Abhishek Guldhe is thankful to Department of Biotechnology, Govt. of India for the award of Ramalingaswami Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alam, S.N., Khalid, Z., Guldhe, A. et al. Harvesting and pretreatment techniques of aquatic macrophytes and macroalgae for production of biofuels. Environmental Sustainability 4, 299–316 (2021). https://doi.org/10.1007/s42398-021-00178-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-021-00178-6