Abstract
Biological Soil Disinfestation (BSD) is used to control soil-borne phytopathogens like Ralstonia solanacearum by introducing fresh organic matter and covering the soil with thick, opaque plastic sheets to induce soil anaerobiosis. In this study, BSD was implemented for the first time in Egypt to manage R. solanacearum in five naturally-infested fields. To improve BSD’s efficiency, biocontrol agents, including Pseudomonas putida (PD3142), P. fluorescens (PD3339), Acinetobacter baumannii (PD3138), and Stenotrophomonas maltophilia (PD4560), or plant-animal compost were applied after removing the plastic. The pathogen was eliminated when the soil oxygen concentration was less than or equal to 1%. However, this method resulted in a decrease in potato production. Application of P. putida and P. fluorescens after BSD led to a significant increase in potato production. Furthermore, BSD led to a significant decrease in fungal biodiversity, measured by the Shannon index (H’). PCR-DGGE analysis showed that P. putida and S. maltophilia reduced bacterial biodiversity as indicated by species richness (S) and H’. Next generation sequencing assays showed Moraxellaceae and Pseudomonadaceae decrease in their abundance after BSD treatment, while Bacillaceae were less affected . Moreover, applying of compost following BSD was associated with an increase in beneficial bacterial genera such as Luteolibacter, Dyadobacter, Sphingobium, Flavobacterium, and Pseudomonas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Ralstonia solanacearum is a soil-borne pathogen that causes potato brown rot or bacterial wilt, which is a highly destructive disease resulting in significant crop losses, ranging up to 82%, depending on the race (García et al. 1999). The pathogen can persist in the soil for up to three years in the absence of host crops (Graham et al. 1979). Previous studies have shown that Biological Soil Disinfestation (BSD) can be effective in managing R. solanacearum (Messiha et al. 2007b; Momma 2008). BSD has been successful in eliminating the pathogen from “hiding” reservoirs such as volunteer-infected potato tubers, and from weed hosts such as Portulaca oleracea, Rumex dentatus, and Solanum nigrum (Graham et al. 1979; Elphinstone et al. 1998; Farag et al. 2004; Messiha et al. 2019).
Following the ban of methyl bromide fumigation due to its detrimental effects on human health and the horizon ozone layer, numerous alternatives for soil-borne disease management were applied (Messiha et al. 2021; Kumar et al. 2021, 2023), regardless of low economic feasibility (Kabir et al. 2005) and environmental safety. In Europe and Japan, soil sanitation using hot water or steam pumping was employed effectively for management (Takehara 2004; Takeuchi 2004); though, constraints raised about the high cost of fuel and the need for sophisticated equipment. Soil solarization and flooding were also tried, but not always as successful as chemical fumigation (Gamliel et al. 2000; Katan 2000). Blok et al. (2000) investigated BSD as a viable non-chemical alternative for phytopathogen management. The hypothesis is based on the fact that the soil is subjected to anaerobiosis for at least six weeks by increasing microbial respiration through the addition of fresh organic matter (easily digestible). Mulching with 0.135 mm opaque plastic sheets to restrict oxygen replenishment. The black plastic covering blocked off light, preventing the development of green plants that supplied oxygen via photosynthesis. In this regard, Shinmura (2004) used wheat bran or molasses. Many chemicals are being produced by soil microbes as a result of anaerobic growth, produce a variety of chemicals that include fatty acids, ammonia, methane, hydrogen sulphide, and nitrous oxide, which could be responsible for pathogen elimination (Momma et al. 2006). Other researchers then used the method to treat a variety of bacterial, fungal, and nematode infestations (Messiha et al. 2007b; Stremińska et al. 2014; Hewavitharana et al. 2019).
Various potential biocontrol agents such as Pseudomonas spp., Bacillus spp., Paenibacillus spp., Serratia spp., Streptomyces spp., and Trichoderma sp. have been identified by researchers (Biswal et al. 2018; Athira and Anith 2020; Kawicha et al. 2020; Rahman et al. 2023; Sharma 2023). However, their field efficiency was not as high as expected due to the complex rhizosphere, which could limit the establishment of biocontrol agents in soil. Therefore, repeated inoculation with a large inoculum was necessary (Angelopoulou et al. 2014). In the last 60 years, researchers have proposed inducing soil suppressiveness as an alternative to using biocontrol agents, as it offers a new tool for long-term soil-borne disease management. BSD was used to shift the microbial biodiversity towards a suppressive direction instead of direct biological control (Hewavitharana et al. 2019). To induce soil suppressiveness following BSD, either beneficial bioagents or suppressive compost could be used to generate long-term suppressive soil. The use of next-generation sequencing (NGS) facilitated the analysis of soil microbiomes and the characterization of general and specific soil suppressiveness (Schlatter et al. 2017).
The current study represents the first attempt to test the procedure in Egyptian conditions. The study aimed to examine the effect of directly inoculating soil with either beneficial microbes or compost after BSD on disease control, microbial community, and crop yield.
Materials & methods
General outlines
Five experiments were conducted at four sites in Bani Suef governorate and one site in Kafr Shukr, Qaliobia governorate, with varying seasonal conditions to evaluate the method under different conditions. Anaerobic conditions were created for a minimum of three weeks to eliminate the pathogen. This was achieved by adding fresh organic matter that was easily digestible and stimulated microbial activity, leading to a decrease in soil oxygen levels. The oxygen replenishment was prevented by covering the soil with thick black Hermatex plastic, which also prevented the growth of green weeds that replenish oxygen through photosynthesis. The oxygen levels in the soil were measured using an oxygen meter (Servomex, Servoflex MiniMp 5200, UK). The method started by burying two diffusion chambers, which were made of PVC tubes with rubber stoppers and extended above the soil, to a depth of 30 cm in each plot. The rubber stopper was removed, and the tube was connected to the oxygen meter to measure the oxygen levels once or twice a week. The edges of the plastic cover were buried at a depth of one meter, creating a tunnel that was filled with water to maintain a constant oxygen barrier.
The details of each experiment are briefly described:
Location 1 (Sids Agricultural Research Station, ARC)
The experiment was conducted in a naturally infested site with R. solanacearum. Potato plants were planted in the soil along with clover and cabbage, and the total green matter was approximately 5–6 kg/ m2. The soil was covered with 0.150 mm thick black plastic for four weeks (from 21 March to 16 April), after which four antagonists were separately applied. The strains of the antagonistic bacteria used included P. putida (PD3142), P. fluorescens (PD3339), A. baumannii (PD3138), and S. maltophilia (PD4560). For tracing their survival under diverse treatments, spontaneous rifampicin- and chloramphenicol-resistant mutants have been adapted and cultivated in the laboratory (Messiha et al. 2007a). The antagonistic potential of each mutant strain was assessed and compared to that of its original parents.
Every 2 plots were inoculated with one antagonist, and the survival of the antagonists was evaluated in the soil of each plot. After one month, cowpea was planted in the soil to prepare the soil for the potato season and enhance the survival of the biocontrol agent. The antagonists’ survival in the cowpea soil was evaluated before planting potato tubers (cv. Lady Rosette), and the inoculum density of the pathogen was determined in different plots.
Location 2 (Naeem village 1, Bani Suef)
The experiment was repeated on a larger scale in Naaeem district, Bani Suef governorate, which covered a total area of 624 m2 (8 × 78 m2) using plastic. Two trials were conducted using different types of fresh organic matter. The first trial used clover, while the other used fresh leaves of cabbage. The soil was ploughed with the green matter at the rate of 5 kg/m2 during the winter season and then covered with plastic for one month. The potato (cv. Lady Rosette) was planted from February 1st to April 29th. Eight plots were designated for each trial. Two plots received treatment with either P. fluorescens, S. maltophilia, Biogene (Azotobacter spp), and two plots were left without further treatment (BSD only). Untreated control plots were also considered where no BSD was applied. The biocontrol agents were prepared on 1% CMC to ensure the standard densities of the previous experiment. At the end of the experiment, pathogen density in the soil was determined, along with crop yield, disease suppression, and pathogen reduction in the soil.
Location 3 (Naeem village 2, Bani Suef)
The third round of BSD was conducted in May at another location in Naeem village, Bani Suef at the end of spring. The total area covered was 360 m2 (3.6 × 100 m2) and black plastic sheets with a thickness of 0.135 mm were used.
Location 4 (Hoad El-Remal Area, Bani Suef governorate)
Barnyard grass was the fresh organic stuff utilized. The total surface area was 144 m2 (8 × 18 m2). The experiment was carried out in mid-August (summer season). The total area was divided into 3 equal plots. After removing the plastic, one plot was inoculated with a mixture of P. fluorescens and S. maltophilia (Messiha et al. 2007a), the second was mixed with three Streptomyces spp. (MT509554.1, MT509556.1 and MT509569.1) (Messiha et al. 2019) and the third plot was inoculated with Biogine: Azotobacter spp. from ARC.
Location 5 (Kafr Shukr, Kaliobia)
The experiment was extended from mid-August till the end of September at the area of 500 m2. The method was applied directly before planting the potato. The green young corn plants were used as a green matter and preparations were followed as previously mentioned for 6 weeks followed by the application of the plant-animal compost with the following characteristics: (pH7.7, EC 7.43 ds/m, and 20.4% OM C/N ratio was 13/1, percentage of N, P, K were 1.4, 0.28, 1.05 respectively. Anions (ml/L), HCO3− (8.36), Cl− (632.2), SO4 − 2 (67), Cations (ml/L) Ca++ (106.06), Mg++ (62.77), Na+ (347.04) and K+ (185.82)). Potato (cv. Spunta) was grown to evaluate the method.
Methodology in relation to pathogen density in soil
To detect the presence of the pathogen in the trials, several methods were employed. These included selective medium (SMSA) as described by Anonymous (1998), conventional PCR with specific primers (RS-1-F and RS-1-R, 718 bp) following the guidelines of PM 7/21 (3) (2022), and serological assay using the indirect fluorescent antibody staining (IFAS) technique as described by Janse (1988). Polyclonal antiserum for R. solanacearum (from goat) (catalogue number 07356/01) was provided by Loewe Biochemica GmbH, and rabbit anti-goat conjugated with FITC (ab6737) was provided by Abcam. Confirmation of the presence of the pathogen was achieved through real-time PCR analysis of both potato tubers and plant DNA extracts. These analyses were conducted at the central laboratories of the Faculty of Agriculture at Cairo University using the Biorad cfx96 machine, with specific primers (RS-I-F and RS-II-R) and a probe (RS-P, FAM) as described by Weller et al. (2000).
Soil analysis and NPK content
Four soil samples were collected from different locations and mixed thoroughly. The samples were taken from a depth of 15–20 cm. The organic matter content was determined using the Walkley and Black 1934 method as described in ICARDA (2013. Aqua regia (a mixture of hydrochloric and nitric acid at a ratio of 3:1) was used to digest the total elements (P and K) at a temperature of 150o C for two hours. The AOAC micro-Kjeldahl method was used to determine the soil nitrogen content (AOAC 1990). Upon the experiment’s completion, potato leaves’ NPK content was measured and compared to the untreated control.
Effect of the suggested program on soil biodiversity
Soil samples were collected and stored at -30 °C for DNA extraction and microbial analysis using PCR-DGGE analysis. The DNA was extracted using NucleoSpin®Soil kits with slight adjustments to the manufacturer’s protocol. The yield and purity of the extracted DNA were checked using a NanoDrop spectrophotometer, and DNA with a purity between 1.5 and 2 at 260 / 280 was used for 16 S rDNA amplification. For 16 S rDNA extraction, only 30ng of pure DNA per reaction was needed. PCR-DGGE analysis was conducted using the protocol described by Heuer and Smalla (1997), with modifications. Mytaq HS Red Mix, 2x, was used as the master mix, and a reagent control was included in every PCR run. The PCR products were examined by gel electrophoresis to confirm product integrity and estimate yield. The expected product size was approximately 450 bp for 16 S Eubacteria and 390 bp for 18 S rDNA.
Effect of the suggested program on abundance of bacterial community
To extract DNA, the DNeasy® PowerSoil® kit from Qiagen GmbH in Germany (Cat. No. 12888-100) was used. The metagenome 16S amplicon was sequenced by Macrogen in South Korea using an Illumina system. The sequencing platforms used were MiSeq 300 bp PE; 30%PhiX > 16s (v3-v4), Bakt 341F: CCTACGGGNGGCWGCAG, and Bakt 805R: GACTACHVGGGTATCTAATCC. The total number of reads ranged from 150,000 to 200,000, and the read is paired-end with a length of 301 bases. The library kit used for the Illumina system was the Herculase II Fusion DNA Polymerase Nextera XT Index V2 Kit, and the library protocol was 16S Metagenomic Sequencing Library Preparation Part # 15044223 Rev. B. The samples were sequenced on the Illumina platform after being prepared according to the NGS library preparation workflow. To construct libraries, DNA extracts that met quality control standards were used. The DNA was randomly fragmented before being ligated with 5’ and 3’ adapters. After PCR amplification of the adapted ligated fragments, the primer anneals to the end of each adapter, and the library templates undergo quality control and quantification. Each fragment is then amplified into a variety of clonal clusters using bridge amplification. The templates are then ready for sequencing. The raw data files generated by the Illumina sequencers (Binary Base Call (BCL) files) were converted into FASTAQ files using the bcl2fastaq package provided by Illumina, with the adapters left in place. For raw data statistics, the total number of bases, reads, GC (%), Q20 (%), and Q30 (%) were calculated for all samples.
Statistical analysis
The microbial diversity in soil samples was determined by species richness (S) and as the Shannon–Wiener index of bacterial diversity, (H’) for the developed bands on PCR-DGGE polyacrylamide gel (van Diepeningen et al. 2006; Eichner et al. 1999). Tukey multiple comparisons (Tukey HSD) of means 95% confidence level were conducted to compare S and H’ for soil following different treatments. Crop production was compared using Tukey HSD on R version 4.2.1.
The raw reads were processed and a table of the operational taxonomic unit (OTU) was generated, using the locally installed QIIME2, version: 1.9.1. Statistical analysis and visualization were carried out on a locally installed SHAMAN server. The heatmap was created in R software, version 4.2.1, to visualise hierarchical clustering. Data values were then transformed to a colour scale, for the most abundant bacterial community as related to the experimental stage.
In order to conduct a comparative analysis of the occurrence of bacterial families, genera, and species across various treatments, stacked bar graphs and a heat map were produced utilizing the Chart Builder feature within IBM SPSS Statistics version 23.
Results
BSD in relation to pathogen density
Location 1
At the start of the experiment, the oxygen level under the plastic cover was 20.6%. However, after three days of covering the plots, the oxygen level had decreased to 1–2% (Fig. 1A). A slight cut in the plastic resulted in some oxygen leakage in two plots, which caused the observed oxygen concentration to increase for a week before being sealed with waterproof stickers. No R. solanacearum was detected in any plot except for the two plots that had experienced short-term anaerobiosis, which showed a concentration of 2.95 ± 1.94 CFU/g soil compared to 3.07 ± 2.75 CFU/g soil for the untreated control. The typical colonies were observed via SMSA plating, and the exact identity was determined using real-time PCR.
Location 2
A significant decline in oxygen content after one week after covering the soil, but only for the clover treatment, contrary to cabbage and beets. The pathogen density in untreated soil was 3.53 ± 3.13 CFU/g and 2.95 ± 1.76 CFU/g for poor BSD, and it was below the detection limit only when the soil oxygen content was less than 1%. (Fig. 1B; Table 1).
Location 3
the pathogen density was 2.25 ± 1.82 CFU/g. The BSD proved to be successful since anaerobic conditions with 1% oxygen concentration could be sustained for 7 weeks, and the pathogen was undetectable in the BSD-treated soil. (Fig. 1C).
Location 4
The fourth application of BSD was less successful because the anaerobic conditions could not be maintained for more than 18 days (Fig. 1D). The initial pathogen density was 2.20 ± 1.50. One plot had an oxygen leak, and the density was 2.50 ± 2.07 in that plot, whereas the pathogen was below detection in the plots subjected to anaerobiosis for 18 days.
Location 5
The pathogen density was 4.11 ± 2.76 CFU/g soil initially, but it became undetectable in soil treated with BSD. The anaerobic conditions were maintained for 6 weeks. Real-time PCR was performed on potato tubers grown in BSD-treated soil and in the adjacent control. The results showed that the pathogen was recovered (CT = 17.26) in the untreated field, while it remained undetectable in tubers grown in BSD-treated soil. (Data not shown).
The density of the applied biocontrol agents
The inoculum density of P. putida (PD3142) was found to be 4.14 ± 3.05 CFU/g soil, P. fluorescens (PD3139) was 3.85 ± 2.94 CFU/g soil, A. baumanii (PD3138) was 3.31 ± 2.53 CFU/g soil, and S. maltophilia (PD4560) was 4.45 ± 3.19 CFU/g soil after planting cowpea and before planting potato. The antagonistic potential of the mutant strains was assessed and found to be similar to the original strains, (Supp. Figure 1.A and 1.B).
Effect of BSD on soil chemicals and potato yield
Location 1
BSD-treated soil experienced a considerable reduction in potato yield of 23% (P = 0.01) due to the application of BSD. However, the introduction of P. putida to the BSD-treated soil resulted in a significant improvement in potato yield of 75% (P = 0.001) when compared to the BSD-only treatment. In contrast, when A. baumanii and S. maltophilia were applied to the BSD-treated plots, there was a significant decrease in potato yield compared to the untreated controls by 30.8% and 30.1%, respectively (P = 0.001). This decrease in yield was comparable to that observed in the BSD-only treatment. Moreover, applying P. fluorescens to plots with unsuccessful BSD treatment due to oxygen leakage resulted in a decrease in potato yield (P = 0.02) (Table 2).
Location 2
The increase in EC and Na after cabbage incorporation may be attributed to sulphur and other minerals of this plant. BSD didn’t show any influence on most minerals, though the reported decrease in N content (Table 3). The crop yielding was higher for the untreated control, followed by plots where potato seeds were treated with P. putida and fluorescens just before planting. The least productivity was recorded for BSD amended with S. maltophilia which was similar to the BSD control (Table 1).
Location 5
Potato grown in BSD-treated soil amended with compost showed about a 34% decrease in yield compared to neighbouring untreated control amended with compost only (Table 4). BSD treatment had enhanced N uptake over that of untreated control, and a low OM ratio was recorded for BSD-treated soil. An increase in Cu, Fe and Zn treatment after BSD, was reported to that before BSD. Moreover, a decrease in Na + and K + was shown (Table 4).
Effect of the protocol on soil biodiversity
PCR-DGGE analysis
Location 1
A significant decrease in soil bacterial diversity as expressed by the H’ index for BSD-treated soil inoculated with S. maltophilia as compared to BSD control (Table 2). Meanwhile, a significant increase in fungal diversity, as expressed by S value, was recorded for BSD-treated soil inoculated with A. baumanii compared to BSD control. A significant decrease in fungal biodiversity as expressed by H’ index in BSD-treated soil compared to the control. Similar decrease in fungal H’ index was recorded for BSD-treated soil inoculated with P. putida or S. maltophilia compared to the untreated control (Table 2).
The apparent shift in microbial biodiversity as shown by UPGMA Dendrograms, based on Dice coefficient matrices, for PCR-DGGE banding patterns of Eubacteial 16S rDNA and the fungal SSU rDNA was for P. putida and S. maltophila application after BSD treatment (Fig. 2 & Supp. Figure 2).
Location 2
There was a clear shift in the soil bacteria biodiversity between untreated control, BSD treated, and poor BSD, (Supp. Figure 3 A, B). When P. putida was introduced to BSD-treated soil, a substantial drop in bacterial biodiversity was reported (Table 1). A similar drop in S was caused by S. maltophilia, both biocontrol agents significantly decreased bacterial diversity compared to the untreated control. However, there is no significant difference between untreated controls and poor BSD-treated soil (cabbage-treated soil) (Table 1).
Location 3
UPGMA Dendrograms, based on Dice coefficient matrices, for PCR-DGGE banding patterns of Eubacteial 16 S rDNA, revealed a distinction between BSD treated soil and untreated soil (Supp. 4.).
Location 4
although BSD couldn’t remain for a long period as reported before, a clear shift in bacterial biodiversity was shown between untreated controls, BSD-treated soil and soil amended with different bacterial agents (biocontrol and Azotobacter sp.) (Supp. Figure 5). No significant difference between Species richness (S) and Shannon–Wiener index of eubacterial diversity (H’) calculated from PCR-DGGE banding patterns of Eubacteria extracted from soil after different treatments (Supp. Table 1).
Metagenomic analysis
Location 5
The shift in soil bacterial diversity after different treatments are shown in Figs. 3, 4, 5 and 6 show the shift in soil bacterial species. Manhattan PCA for soil bacterial biodiversity after different treatments (Supp. Figure 6).
Heat map depicting the distribution of the most abundant bacterial families, genera and species in different soil based on ILLUMINA Miseq data (the relative abundance of the bacterial diversity is depicted by the colour intensity), (Location 5). (1) Start of the experiment (infested soil). (2) Soil mixed with 6 weeks of corn leaves (4 kg/ 1m2). (3) Soil after BSD treatment. (4) Final potato soil amended with compost
Bacterial families that were most abundant in soil under various treatments, as determined by analyzing ILLUMINA Miseq data. Start: Start of the experiment (infested soil). Prior BSD: Soil mixed with 6 weeks of corn leaves (4 kg/ 1m2). BSD: Soil after BSD treatment. Potato soil: Final potato soil amended with compost
Bacterial genera that were most abundant in soil under various treatments, as determined by analyzing ILLUMINA Miseq data. Start: Start of the experiment (infested soil). Prior BSD: Soil mixed with 6 weeks of corn leaves (4 kg/ 1m2). BSD: Soil after BSD treatment. Potato soil: Final potato soil amended with compost
Bacterial species that were most abundant in soil under various treatments, as determined by analyzing ILLUMINA Miseq data. Start: Start of the experiment (infested soil). Prior BSD: Soil mixed with 6 weeks of corn leaves (4 kg/ 1m2). BSD: Soil after BSD treatment. Potato soil: Final potato soil amended with compost
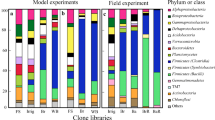
The shift in soil bacterial families (Figs. 3 and 4)
The stacked bar graphs (Fig. 4) and the heatmap (Fig. 3) provide an overview of the changes in the most prevalent bacterial families over the course of the experiment.
Moraxellaceae (G-ve, strict aerobic), Its abundance was 5.4% at the beginning of the experiment and jumped to 30.1% in soil mixed with the fresh OM (SFOM) and sharply declined in BSD-treated soil (0.2%) and was under the detection level in potato soil (treated with compost). Bacillaceae was dominant at the beginning of the experiment (36.4%), 23% in SFOM, 10.1% in BSD soil and only 0.4% in potato soil. Similarly, Paenibacillaceae declined from 5.3% at the beginning to being undetectable in potato soil. In contrast, Enterobacteriaceae was represented by 1.2% at the beginning, which increased to 14.2% in SFOM, sharply declined to 1.9% in BSD soil and declined further to 0.2% in potato soil. Streptomycetaceae was highly abundant 25.5% only in BSD and was undetectable in potato soil. BSD soil characterized also by Sphingomonadaceae (8%), Chitinophagaceae (4.2%), Methylococcaceae (3.6%) Rhodospirillaceae (2.5%), Clostridiaceae (2.5%). Meanwhile, Pseudomonadaceae declined from 3.4% at the beginning to 0.1% in BSD soil and increased again to 7.5% in compost-treated potato soil. Also, many families were predominant in compost-treated soil as follows Flavobacteriaceae (48.7%), Sphingobacteriaceae (9.8%), Cytophagaceae (6.4%), Alcaligenaceae (4.8%), Caulobacteraceae (3.6%), Rhizobiaceae (3.5%), Rhodobacteraceae (2.8%), Sphingomonadaceae (2.8%), Cytophagaceae (2.8%) and Chromatiaceae (2.2%).
The most affected families by BSD treatment are Moraxellaceae, Paenibacillaceae, and Pseudomonadaceae.
The shift in soil bacterial genera (Figs. 3 and 5)
A significant shift in observed genera 52 ± 0.4, 59 ± 0.1 and 46 ± 0.5 (P < 0.01) and in Shannon biodiversity 2.78 ± 0.01, 3.51 ± 0.01, 3.17 ± 0.00 for soil bacterial biodiversity prior BSD, after BSD and in potato soil respectively.
Acinetobacter (G-ve), Its abundance was 6% at the beginning of the experiment and jumped to 48.6% in soil mixed with the fresh OM (SFOM) and sharply declined in BSD-treated soil (0.4%) and was undetectable in potato soil (treated with compost). Bacillus (G-ve), was dominant at the beginning of the experiment (48.9%), 22.4% in SFOM, 2.7% in BSD soil and only 0.2% in potato soil. Similarly, Paenibacillus declined from 7.5% at the beginning to being undetectable in potato soil. Also, Ralstonia and Pantoea declined from the initial abundance (0.8% and 2%, respectively) to be undetectable in potato soil. Streptomyces was highly abundant 47% only in BSD and was undetectable in potato soil. Meanwhile, Pseudomonas declined from 4.3% at the beginning to 0.2% in BSD soil and increased again to 7.6% in compost-treated potato soil. Also, many genera were predominant in this potato soil as follows Chryseobacterium (36.7%), Flavobacterium (19.4%), Pedobacter (5.7%), Sphingobacterium (5.1%), Brevundimonas (3.9%), Rheinheimera (2.8%).
The shift in soil bacterial species (Figs. 3 and 6)
A significant decrease in observed bacterial species from 48 ± 0.3 to 41 ± 0.2 in BSD-treated soil and 44 ± 0.2 in potato soil was recorded (P < 0.01). Meanwhile, a significant increase in bacterial Shannon index from 2.39 ± 0.01 to 2.38 ± 0.01 in BSD soil and 3.67 ± 0.01 in compost amended BSD-treated soil.
Acinetobacter baumannii, (A) calcoaceticus, their abundance were 1.6% and 0.1%, respectively at the beginning of the experiment and jumped to 66.4% and 5.6% in SFOM, sharply declined in BSD-treated soil and were below detection level in potato soil (treated with compost). Bacillus funiculus, (B) koreensis, B. oceanisediminis, Bacillus sp., their abundance in SFOAM were 6.3%, 4%, 1.6%, 1.4% respectively and sharply declined in BSD soil and was represented by 0%, 0.4%, 0%, 0% respectively in potato soil. Meanwhile, Bacillus sp. was predominant in BSD soil (3%) and was undetectable in potato soil. Streptomyces radiopugnans, was highly abundant (67.6%) only in BSD and was undetectable in potato soil. Lysobacter dokdonnsis, was represented by 0.2% in SFOM and increased to 2.7% in BSD and returned back to 0.2% abundance in potato soil.
Many species were abundant only in compost-treated potato soil, Dyadobacter crusticola (31.1%), Brevundimonas halotolerans 17%), Pedobacter sp. (11%), Pedobacter ginsengisoli (6.2%), Pedobacter koreensis (5.2%), Sphingobacterium nematocida (3.5%), Massilia namucuonensis (3%), Sphingobium vermicomposti (2.9%) and Rhizobium sp., (2.5%) were recorded (Fig. 6).
In general, a sharp decline in bacterial biodiversity in BSD treated soil followed by a significant increase in potato soil treated with compost.
Discussion
Previous studies have shown that BSD can effectively control various phytopathogens, including R. solanacearum, in different parts of the world (Blok et al. 2000; Goud et al. 2004; Messiha et al. 2007b; Shennan et al. 2017). In this study, the efficacy of BSD was evaluated for the first time in Egypt, specifically in Upper Egypt and the Delta region, across different seasons and with various types of fresh organic materials. The results indicated that clover and young maize leaves were more effective in promoting BSD performance than barnyard grass and cabbage leaves, likely due to their higher lignin content and more efficient decomposition rates (Molinuevo-Salces et al. 2013). The study also found that the winter season was more suitable for BSD application in Egypt, as the high temperatures during the summer could cause damage to the plastic sheets and increase oxygen leakage. Additionally, smaller soil areas were found to be less efficient, likely due to the larger edge area in relation to the total surface area, indicating that the procedure was more effective in larger areas.
The efficacy of BSD in controlling soil phytopathogens is believed to be a result of chemical and microbiological mechanisms. Specifically, the accumulation of organic acids like acetic and n-butyric acid has been associated with BSD treatment, leading to a decrease in soil pH and inhibition of bacterial pathogens (Momma et al. 2006). However, in our study conducted in Egypt, we did not observe a decrease in soil pH due to the soil’s buffering capacity towards alkalinity. Instead, pathogen inhibition is likely due to increased levels of ammonia, which can be toxic in alkaline soils (Kissel et al. 1985). The accumulation of these chemicals under anaerobic conditions may also result in an unpleasant odour in BSD-treated soil.
The use of BSD alone may not be adequate for long-term control of soil pathogens and must be used in conjunction with other methods such as crop rotation, compost treatment, and cultivation of resistant cultivars, as noted by Momma (2008). In our study, the application of BSD alone resulted in a significant decrease in potato yield. However, when the BSD-treated soil was inoculated with nitrogen-fixing bacteria (Azotobacter sp.) or compost, it resulted in an increase in yield. In addition, the use of biocontrol agents such as P. putida and P. fluorescens enhanced potato yield in BSD-treated soil. On the other hand, the failure of BSD treatment due to oxygen leakage resulted in a comparable loss in potato yield, and the effectiveness of biocontrol agent application was reduced when the BSD treatment failed.
The decrease in potato yield after BSD and unsuccessful BSD may be due to a reduction in soil nitrogen concentration caused by ammonia production, as well as soil stress. However, the soil is expected to recover after a few crop successions and organic fertilization. P. putida and P. fluorescens are both known as PGRP and biocontrol agents (Meliani et al. 2017), which explains the significant increase in potato yield when they were introduced into the soil after BSD. Additionally, S. maltophilia and P. putida reduced bacterial and/or fungal soil biodiversity in BSD-treated soil, likely due to the production of antifungal and antibacterial compounds (Nakayama et al. 1999; Messiha et al. 2007a). In our study, the effectiveness of biocontrol agents was more apparent when they were inoculated after BSD, as the soil biodiversity was greatly disturbed, allowing for their establishment.
The next-generation sequencing (NGS) analysis of bacterial biodiversity following BSD treatment and plant-animal compost incorporation showed a significant shift towards suppressive bacterial communities.
Moraxellaceae (Acinetobacter baumannii and Acinetobacter calcoaceticus) are gram-negative, strictly aerobic bacteria, which explains their rapid reduction upon BSD. Because the family hosts numerous human diseases, its rapid decrease is beneficial.
Although Streptomycetaceae members are strict aerobic, some members can withstand anaerobic stress for long periods forming spores (Van Keulen et al. 2007). Sphingomonadaceae, and Chitinophagaceae families are facultative anaerobic (Glaeser and Kämpfer 2013, Lee J.C and Whang 2020). The relative abundance of Methylococcaceae may be correlated to the production of methane through OM degradation under anaerobic conditions (Conrad 2020). Most of Rhodospirillaceae members are able to fix nitrogen under anaerobic conditions (Madigan et al. 1984). Clostridiaceae members are dominant in reductive soil disinfestation and are responsible for producing toxic compounds against phytopathogens (Hewavitharana et al. 2021; Huang et al. 2019).
Amendment of compost immediately after removing the plastic terminating the BSD associated with increased abundance of many beneficial bacterial species. For example, Luteolibacter is considered beneficial for the soil (Wu et al. 2021 L). Dyadobacter crusticola and Sphingobium vermicomposti, were abundant only in potato soil treated with compost. Dyadobacter and Sphingobium abundance are correlated to sustainable disease suppression against many pathogens including Ralstonia. (Fu et al. 2017). Flavobacterium possesses an antagonistic potential against many pathogens and considered an indicator of soil quality (Kolton et al. 2016). Flavobacterium anhuiense strain STDF27859 (55) isolated from potato soil treated with compost showed strong antagonistic potential against R. solanacearum in vitro (Messiha et al. unpublished).Also, Pedobacter, Family: Sphingobacteriaceae is a PGRP (Yang et al. 2021). Meanwhile, Pseudomonas sharply declined after BSD but it was restored in compost-treated soil.
Conclusion
The method is considered expensive due to the costs of the plastic sheets as well as the fresh organic materials. However, the plastic can be reused if rupturing is avoided. Large exporters may afford the practice in an emergency, i.e. when infestation inside the pest-free zone (PFA) is reported. Under Egyptian conditions (or similar climate), it is best to use the method in the winter or early spring to escape hot weather conditions that can destroy the plastic sheets. Clover and young maize leaves are the most useful fresh organic matter, whereas barnyard grass and cabbage leaves were less efficient . Enriching the soil with suppressive compost would provide long-term disease suppression by introducing SOM and beneficial microorganisms that would occupy the niche, making it difficult for the pathogen to re-invade.
Data Availability
The data and material used during the current study are available from the author on reasonable request.
Change history
23 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42360-023-00679-w
Abbreviations
- BSD:
-
Biological soil disinfestation
- Poor BSD:
-
Soil Oxygen concentration > 1
- PD:
-
Plantenziektenkundige Dienst (Plant protection Service, Wageningen, the NL)
- S :
-
Species richness
- H’:
-
Shannon index
- DGGE:
-
Denaturing gradient gel electrophoresis
- NGS:
-
Next generation sequencing
- CMC:
-
Carboxymethyl cellulose
- ARC:
-
Agricultural Research Center
- FITC:
-
Fluorescein Isothiocyanate
- OM:
-
Organic matter
- SFOM:
-
Soil mixed with the fresh OM
References
Angelopoulou DJ, Naska EJ, Paplomatas EJ, Tjamos SE (2014) Biological control agents (BCAs) of verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol 63:1062–1069. https://doi.org/10.1111/ppa.12198
Anonymous (1998) Council Directive 98/57/EC of 20 July 1998 on the control of Ralstonia solanacearum (Smith) Yabuuchi et al. Publication 97/647/EC Official Journal European Communities L235:8–39
AOAC (1990) Official methods of analysis. Association of Analytical Chemists, Virginia, USA. APHA, 1998. Standard methods for the examination of waters and wastewaters. APHAAWWA-WEF, Washington, DC
Athira S, Anith KN (2020) Plant growth promotion and suppression of bacterial wilt incidence in tomato by rhizobacteria, bacterial endophytes and the root endophytic fungus Piriformospora indica. Indian Phytopathol 73:629–642. https://doi.org/10.1007/s42360-020-00283-2
Biswal G, Singh D, Dhal NK (2018) Synergistic effect of Bacillus subtilis and boric acid on management of bacterial wilt disease of potato caused by Ralstonia solanacearum in coastal plains of Odisha under field conditions. Indian Phytopathol 71:431–434. https://doi.org/10.1007/s42360-018-0053-8
Blok WJ, Lamers JG, Termorshuizen AJ, Bollen GJ (2000) Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathol 90:253–259.https://doi.org/10.1094/PHYTO.2000.90.3.253
Conrad R (2020) Methane Production in Soil Environments-Anaerobic Biogeochemistry and Microbial Life between Flooding and Desiccation. Microorganisms. 2020;8(6):881. https://doi.org/10.3390/microorganisms8060881
Eichner CA, Erb RW, Timmis KN, Wagner-Döbler I (1999) Thermal gradient gel electrophoresis analysis of bioprotection from pollutant shocks in the activated sludge microbial community. Appl Environ Microbiol 65:102–109. https://doi.org/10.1128/aem.65.1.102-109.1999
Elphinstone JG, Stanford HM, Stead DE (1998) Detection of Ralstonia solanacearum in potato tubers, Solanum dulcamara, and associated irrigation water. In: Prior P, Allen C, Elphinstone JG (eds) Bacterial Wilt Disease: molecular and ecological aspects. Springer, Berlin, Germany, pp 133–139. https://doi.org/10.1007/978-3-662-03592-4_19
Farag NS, Eweda WE, Mostafa MI, Balabel NM (2004) Preliminary observations on the bacteriology and pathology of Ralstonia solanacearum. Egypt J Agric Res 82:1519–1523
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem 104:39–48. https://doi.org/10.1016/j.soilbio.2016.10.008
Gamliel A, Austerweil M, Kritzman G (2000) Non Chemical Approach to soilborne pest management - Organic amendments. Crop Prot 19:847–853
García R, García A, Delgado L (1999) Distribution, incidence and variability of Ralstonia solanacearum, causal agent of bacterial wilt of potato, in Mérida state. Venezuela Bioagro 11(1):12–23
Glaeser S, Kämpfer P (2013) The Family Sphingomonadaceae. The Prokaryotes: Alphaproteobacteria and Betaproteobacteria 641–707. https://doi.org/10.1007/978-3-642-30197-1_302
Goud JC, Termorshuizen AJ, Blok WJ, van Bruggen AHC (2004) Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis 88:688–694. https://doi.org/10.1094/PDIS.2004.88.7.688
Graham J, Jones DA, Lloyd AB (1979) Survival of Pseudomonas solanacearum race 3 in plant debris and latently infected potato tubers. Phytopathol 69:1100–1103
Heuer H, Smalla K (1997) Application of denaturing gradient gel electrophoresis for studying soil microbial communities. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil Microbiology. Marcel Dekker Inc, New York, pp 353–373
Hewavitharana SS, Klarer E, Reed AJ, Leisso R, Poirier B, Honaas L, Rudell DR, Mazzola M (2019) Temporal Dynamics of the Soil Metabolome and Microbiome during simulated anaerobic soil Disinfestation. Front Microbiol 10:2365. https://doi.org/10.3389/fmicb.2019.0236
Hewavitharana SS, Klarer E, Muramoto J, Shennan C, Mazzola M (2021) Analysis of environmental variables and Carbon Input on Soil Microbiome, Metabolome and Disease Control Efficacy in Strawberry Attributable to Anaerobic Soil Disinfestation. Microorganisms 9:1638. https://doi.org/10.3390/microorganisms9081638
Huang XQ, Liu LL, Zhao J, Zhang JB, Cai ZC (2019) The families Ruminococcaceae, Lachnospiraceae, and Clostridiaceae are the dominant bacterial groups during reductive soil disinfestation with incorporated plant residues. Appl Soil Ecol 135:65–72
ICARDA (2013) Methods of Soil, Plant, and Water Analysis: A manual for the West Asia and North Africa region. Eds. Estefan G, Sommer R, and Ryan J (Third Edition) 243pp
Janse JD (1988) A detection method for Pseudomonas solanacearum in symptomless potato tubers and some data on its sensitivity and specificity. Bull OEPP/EPPO Bull 18:343–351
Kabir Z, Fennimore SA, Duniway JM, Martin FN, Browne GT, Winterbottom CQ, Ajwa HA, Westerdahl BB, Goodhue RE, Haar MJ (2005) Alternatives to methyl bromide for strawberry runner plant production. HortScience 40:1709–1715. https://doi.org/10.21273/HORTSCI.40.6.1709
Katan J (2000) Physical and culture methods for the management of soil borne pathogens. Crop Prot 19:725–731
Kawicha P, Laopha A, Chamnansing W et al (2020) Biocontrol and plant growth-promoting properties of Streptomyces isolated from vermicompost soil. Indian Phytopathol 73:655–666. https://doi.org/10.1007/s42360-020-00267-2
Kissel DE, Sander DH, Ellis R (1985) Fertilizer-plant interaction in alkaline soils. In: Engelstad OP (ed) Fertilizer Technology and Use. Soil Science Society of America, Madison, WI, pp 153–196
Kolton M, Erlacher A, Berg G, Cytryn E (2016) The flavobacterium genus in the plant holobiont: ecological, physiological, and applicative insights. Microb Model Environ Ind Sustain 189–207. https://doi.org/10.1007/978-981-10-2555-6_9
Kumar R, Tiwari RK, Jeevalatha A et al (2021) Potato apical leaf curl disease: current status and perspectives on a disease caused by tomato leaf curl New Delhi virus. J Plant Dis Prot 128:897–911. https://doi.org/10.1007/s41348-021-00463-w
Kumar S, Biswas SK, Kumar A et al (2023) Effect of Integrated Disease Management (IDM) Practices on Disease Severity and Incidence of Common Scab of Potato. Potato Res (2023). https://doi.org/10.1007/s11540-023-09629-5
Lee J-C, Whang K-S (2020) Agriterribacter humi gen. nov., sp. nov., a novel bacterium of the family Chitinophagaceae isolated from soil. Int J Syst Evol Microbiol 70(9):5123–5130. https://doi.org/10.1099/ijsem.0.004397
Madigan MT, Cox SS, Stegeman RA (1984) Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae. J Bacteriol 157:73–78. https://doi.org/10.1128/JB.157.1.73-78.1984
Meliani A, Bensoltane A, Benidire L, Oufdou K (2017) Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res Reviews: J Bot Sci 6(2):16–24
Messiha NAS, van Diepeningen AD, Farag NS, Abdallah SA, Janse JD, van Bruggen AHC (2007a) Stenotrophomonas maltophilia: a new potential biocontrol agent of Ralstonia solanacearum, the causal agent of potato brown rot. Eur J Plant Pathol 118:211–225. https://doi.org/10.1007/s10658-007-9136-6
Messiha NAS, van Diepeningen AD, Wenneker M, van Beuningen AAR, Janse JD, Coenen TGC, Termorshuizen AJ, van Bruggen AHC, Blok WJ (2007b) Biological Soil Disinfestation, a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur J Plant Pathol 117:403–415.https://doi.org/10.1007/s10658-007-9109-9
Messiha NAS, Elhalag KMA, Balabel NM, Farag SMA, Matar HA, Hagag MH, Khairy AM, Abd El-Aliem MM, Ali EE, Saleh OE et al (2019) Microbial Biodiversity as related to Crop Succession and Potato Intercropping for Management of Brown Rot Disease. Egypt J Biol Pest Co 29:84.https://doi.org/10.1186/s41938-019-0185-x
Messiha NAS, Elhalag KMA, Balabel NM, Matar HA, Farag SMA, Hagag MH, Khairy AM, Abd El-Aliem MM, Hanafy MS, Farag NS (2021) Efficiency of organic manuring and mineral fertilization regimes in potato brown rot suppression and soil microbial biodiversity under field conditions. Arch Phytopathol Plant Prot 54(9–10):534–556. https://doi.org/10.1080/03235408.2020.1844523
Molinuevo-Salces B, Gómez X, Morán A, García-González MC (2013) Anaerobic co-digestion of livestock and vegetable processing wastes: fibre degradation and digestate stability. Waste Manag 33:1332–1338. https://doi.org/10.1016/j.wasman.2013.02.021
Momma N (2008) Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jarq-Jpn Agric Res 42:7–12. https://doi.org/10.6090/jarq.42.7
Momma N, Yamamoto K, Simandi P, Shishido M (2006) Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J Gen Plant Pathol 72: 247-252. https://doi.org/10.1007/s10327-006-0274-z
Nakayama T, Homma Y, Hashidoko Y, Mizutani J, Tahara S (1999) Possible ole of xanthobaccins produced by Stenotrophomonas sp. Strain SB-K88 in suppression of sugar beet damping-off disease. Appl Environ Microbiol 65:4334–4339. https://doi.org/10.1128/AEM.65.10.4334-4339.1999
PM 7/21 (3) (2022) Ralstoniasolanacearum, R. pseudosolanacearum and R. syzygii (Ralstonia solanacearum species complex). EPPO Bull 52: 225– 261.https://doi.org/10.1111/epp.12837
Rahman M, Borah SM, Borah PK, Bora P, Sarmah BK, Lal MK, Tiwari RK, Kumar R (2023) Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front Plant Sci 14:2023. https://doi.org/10.3389/fpls.2023.1141506
Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T (2017) Disease suppressive soils: new insights from the soil microbiome. Phytopath 107:1284–1297.https://doi.org/10.1094/PHYTO-03-17-0111-RVW
Sharma P (2023) Biocontrol strategies – retrospect and prospects. Indian Phytopathol 76:47–59. https://doi.org/10.1007/s42360-023-00601-4
Shennan C, Muramoto J, Baird G, Daugovish O, Koike S, Fenimore S, Bolda M, Rosskoph E, Butler D, Kokalis Burrelle N et al (2017) Anaerobic soil disinfestation is a potential alternative to fumigation for control of some soil-borne pathogens in strawberry production. Plant Pathol 67:51–66
Shinmura A (2004) Principle and effect of soil sterilization method by reducing redox potential of soil (in japanese). PSJ Soilborne Dis Workshop Rep 22:2–12
Stremińska MA, Runia WT, Termorshuizen AJ, Feil H, Van Der Wurff AWG (2014) Anaerobic soil disinfestation in microcosms of two sandy soils. Commun Agric Appl Biol Sci 79(2):15–19 PMID: 26084078
Takehara T (2004) Principles and effects of soil disinfestation with hot water. PSJ Soilborne Disease Workshop Report 22:22–37
Takeuchi S (2004) Control of soil-borne diseases by steam sterilization in Kochi Prefecture. PSJ Soilborne Disease Workshop Report 22:49–60
van Diepeningen AD, de Vos OJ, Korthals GW, van Bruggen AHC (2006) Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl Soil Ecol 31(1–2):120–135. https://doi.org/10.1016/j.apsoil.2005.03.003
van Keulen G, Alderson J, White J, Sawers RG (2007) The obligate aerobic actinomycete Streptomyces coelicolor A3(2) survives extended periods of anaerobic stress. Environ Microbiol 9:3143–3149
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE (2000) Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl Environ Microbiol 66(7):2853–2858. https://doi.org/10.1128/AEM.66.7.2853-2858.2000
Wu T, Qin Y, Li M (2021) Intercropping of tea (Camellia sinensis L.) and chinese Chestnut: variation in the structure of Rhizosphere Bacterial Communities. J Soil Sci Plant Nutr 21:2178–2190. https://doi.org/10.1007/s42729-021-00513-0
Yang T, Lupwayi N, Marc S-A, Siddique KHM, Bainard LD (2021) Anthropogenic drivers of soil microbial communities and impacts on soil biological functions in agroecosystems. Glob Ecol Conserv 27: e01521.https://doi.org/10.1016/j.gecco.2021.e01521
Acknowledgements
This study was conducted as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research. The author is deeply appreciative of the financial support and collaborative efforts.
Professor Nabil S. Farag, Emeritus Professor at the Plant Pathology Research Institute (PPATHRI), ARC, Egypt, is acknowledged for his invaluable review of the work and insightful feedback. Special thanks are extended to Professor Ahmed A. Gomah, Emeritus Professor at PPATHRI, ARC, for his valuable contributions to field trials and his expert advice on experiments in the field.
Funding
The study was carried out as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, which was funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research.
Author information
Authors and Affiliations
Contributions
Single author.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate are not required for this manuscript.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised:
The Funding section was missing from this article and should have read:
Acknowledgements
This study was conducted as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research. The author is deeply appreciative of the financial support and collaborative efforts.
Professor Nabil S. Farag, Emeritus Professor at the Plant Pathology Research Institute (PPATHRI), ARC, Egypt, is acknowledged for his invaluable review of the work and insightful feedback. Special thanks are extended to Professor Ahmed A. Gomah, Emeritus Professor at PPATHRI, ARC, for his valuable contributions to field trials and his expert advice on experiments in the field.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Messiha, N.A.S. The impact of biological soil disinfestation in conjunction with antagonists and organic materials on potato brown rot control. Indian Phytopathology 76, 1001–1014 (2023). https://doi.org/10.1007/s42360-023-00668-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00668-z