Abstract
In view of the harmful effect of chemical pesticides, alternative approaches like host resistance and biocontrol were evaluated to develop an operative and sustainable option for the management of root-rot disease of tobacco incited by Pythium aphanidermatum. For host resistance, three tobacco cultivars viz., RK-10 P3, RK-18 P8 and RK-12 P3 were evaluated under pot conditions for their relative susceptibility to P. aphanidermatum. The effects of host reaction on total phenol (TP) and salicylic acid (SA) synthesis, as well as their impact on disease aetiology, were investigated. The increase in TP and SA showed negative correlations with the root-rot index. The highest increase in the TP (31%) and SA (20%) contents of the leaf was noted in cv. RK-12 P3, whereas the lowest increase was recorded in cultivar RK-10 P3 (6% TP and 7% SA). Further, the effects of two multi-facial biocontrol agents, Trichoderma harzianum AMUTH-1 and Pseudomonas fluorescens AMUPF-1 against P. aphanidermatum were investigated on all three tobacco cultivars. Soil application of these two biocontrol agents was also compared with the fungicide, carbendazim. Inoculation of P. aphanidermatum caused severe root-rot in cv. RK-10 P3 and cv. RK-18 P8 with a 7–15% decrease in plant growth and 15–20% in chlorophyll and carotenoid contents. However, soil application of BCA and fungicides significantly improved the plant growth variables, chlorophyll and carotenoid contents, and reduced the disease severity. The highest reduction in the root-rot index was recorded with T. harzianum and carbendazim over control. The most significant improvement in the plant growth and biomass production of tobacco was seen in cv. RK-10 P3 with T. harzianum (11–16%), followed by carbendazim (9–14%). The study found that cv. RK-12 P3 expressed resistant reaction to P. aphanidermatum and may be cultivated in the disease prone areas, and T. harzianum may be used in place of fungicide in root-rot-infested fields to increase the tobacco yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tobacco (Nicotiana tabacum L.) is one of the most extensively cultivated non-food crops throughout the world, including India (Rao et al. 2015). India ranks third after China and USA in the production and cultivation of tobacco (Khan and Haque 2013). The crop is highly susceptible to the root-rot pathogen, Pythium aphanidermatum which causes massive damage to the crop (Khan et al. 2012). P. aphanidermatum attacks tobacco plants at different stages, such as pre-and post-emergence damping-off in nursery beds (Subhashini and Padmaja 2009) and root-rot in the main fields (Devaki et al. 2008). The root-rot infection causes significant reduction in the plant growth, and leaves turn fade to light yellow with premature drooping (Khan and Haque 2013).
Given the magnitude of crop losses caused by the root-rot pathogen in tobacco, it is essential to limit crop damage by implementing environmentally friendly and long-term effective management techniques. Generally, Indian farmers widely use chemical pesticides to control the root-rot problem in tobacco because they are highly effective and effortlessly available in the market. However, the application of pesticides does not always prove helpful and economic in diseases management and it also causes a hazardous impact on the ecosystem, environment and human beings (Naher et al. 2014). In this situation, host resistance is one of the best alternatives for chemical pesticides/fungicides. Phenolic chemicals are amongst the most convincing and extensively dispersed secondary products in the plant system, and they operate in the host resistance in a completely different way (Hunt and Ryals 1996). Generally, the phenolic compounds are present in a very small amount in the plant tissue. However, the systemic acquired resistance (SAR) pathway is initiated following infection, resulting in a significant rise in phenol concentrations and salicylic acid in infected plant tissue (Neuenschwander et al. 1996). This causes a significant increase in phenolic chemicals throughout the plant, resulting in broad-spectrum systemic resistance in plants (Dempsey et al. 1999; Sharma and Sain 2005).
Biological control is another eco-friendly and safe disease management approach that is free from residual toxic effects of chemicals. Numerous microbial antagonists are known to antagonize P. aphanidermatum, and their application results in a significant decrease in pathogen suppression (Rao et al. 2015). The microbial antagonists such as Trichoderma spp. (Muthukumar et al. 2011) and Pseudomonas fluorescens (Prabhukarthikeyan and Raguchander 2016; Subhashini and Padmaja 2009) are two of the most often employed biocontrol agents against P. aphanidermatum. Competition for space and resources, release of chitinolytic enzymes, mycoparasitism, and the production of inhibitory chemicals are some of the mechanisms responsible for their biocontrol action (Leelavathi et al. 2014). Trichoderma spp. can suppress the target pathogens through antibiosis by producing fungitoxic metabolites (trichodermin, gliotoxin, viridin etc.), secretes cell wall degrading enzymes (glucanase, cellulase, proteinase and chitinase) and induce host resistance (Howell 2003; Qualhato et al. 2013). Many volatiles and non-volatiles, secondary toxic metabolites are also produced by Trichoderma spp. (Vinale and Sivasithamparam 2020) and these secondary metabolites are commonly related to biocontrol action, plant and root development stimulation, and plant pathogenic fungal suppression (Rao et al. 2015; Saba et al. 2012).
In cultivable soils, Pseudomonas spp. are an essential component of the microbial community and plays a vital role in solubilizing and mobilising essential nutrients for absorption by plant roots (Arora et al. 2013; Verma et al. 2019). Apart from mineral solubilization, these rhizobacteria may colonise the roots of host plants and generate a variety of antimicrobial compounds, which are inhibitory to phytopathogens including P. aphanidermatum (Khan et al. 2020; Weller et al. 2007). The secondary metabolites produced by P. fluorescens viz., 1-carboxylic acid, 2, 4 diacetylphloroglucinol, hydrogen cyanide, kanosamine, pyoluteorin, phenazine, pyocyanin, pyrrolnitrin and viscosinamide are also known to antagonise many soil-borne plant pathogens (Prabhukarthikeyan and Raguchander 2016; Vizcaino et al. 2005).
Since P. aphanidermatum is a serious menace in tobacco cultivation worldwide, independent and special research efforts are essential to devise an ecofriendly and sustainable management option for this problem. With this background, the present study was undertaken to screen three tobacco cultivars viz., RK-10 P3, RK-18 P8 and RK-12 P3 for host resistance against P. aphanidermatum. Further, the effects of two established multi-facial biocontrol agents viz., Trichoderma harzianum AMUTH-1 and Pseudomonas fluorescens AMUPF-1 (Haque and Khan 2021) were investigated against P. aphanidermatum on all three tobacco cultivars. The root-rot index, plant growth, and biochemical characters viz., total phenol (TP), salicylic acid (SA), and leaf pigments (chlorophylls and carotenoids) of tobacco cultivars were assayed to understand tobacco cultivars' response to the biocontrol agents and fungicide (carbendazim).
Materials and methods
Germplasm of tobacco
Ten tobacco cultivars were received from the Central Tobacco Research Institute, Rajamundri, Andhra Pradesh, India to check their relative susceptibility against major diseases and nematode problems of tobacco. Based on their primary screening, three cvs. viz., RK-10 P3, RK-18 P8, and RK-12 P3 were selected for this study.
Inoculum of root-rot pathogen, Pythium aphanidermatum
A pure culture of Pythium aphanidermatum (Edson) Fitzp. was procured from the Indian Type Culture Collection, Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi. The culture was maintained on potato dextrose agar (PDA) in culture tubes and stored in a refrigerator at 5 °C. The pathogen was mass cultured on sorghum seeds. The seeds were soaked overnight in 5% sucrose and 0.03% chloramphenicol solution. The seeds were transferred to conical flasks (500 mL) and autoclaved at 15 kg/cm2 pressure at 121 °C for 15–20 min. Thereafter, the flasks were inoculated with the pure culture of P. aphanidermatum and incubated for 10–15 days in a BOD incubator at 27±2 °C. During incubation, the flasks were shaken manually on daily basis for a few minutes to promote uniform colonization on seeds. To prepare inoculum, a known weight of Pythium-colonized sorghum seeds was mixed with double distilled water (DDW) and ground in an electric grinder. The colony-forming units (CFU) load was calculated and standardised to 2.0 × 106 CFU/g by adding DDW to the ground sterile mixture. The inoculum of 2 g per pot was used in the present study.
Mass culture of biocontrol agents
Soil samples were collected from agricultural fields near Aligarh (28° 0ʹ N 79° 06ʹ E), Uttar Pradesh, India and more than 30 microbial isolates were initially isolated by serial dilution plate method (Ben-David and Davidson 2014). Finally, two indigenous isolates, P. fluorescens Threvesan and Migula (isolate AMUPF-1) and Trichoderma harzianum Rifai (isolate AMUTH-1), were chosen for this investigation based on their legitimate identification and established multi-facial biocontrol potentiality (Haque and Khan 2021). The partial gene sequencings of the 16S rRNA fragment for P. fluorescens AMUPF-1 and the 18S rRNA-ITS1 and ITS4 region for T. harzianum AMUTH-1 were carried out and submitted to NCBI Gene Bank with accession codes KY072889 and KM435269, respectively.
The mass culture of P. fluorescens AMUPF-1 was prepared in nutrient broth (NB). The number of CFU was calculated using the serial dilution plate technique (Huong et al. 2009). The final bacterial suspension was 3.0 × 106 CFU mL−1. T. harzianum AMUTH-1 was mass cultured in conical flasks of 250 mL on potato dextrose broth (PDB). The mycelial mats were collected from the flasks and treated separately with 1 L DDW in an electric-grinder to make mycelial suspension. The suspension was then filtered via a 0.15 mm mesh sieve to remove the hyphae (Huong et al. 2009). A haemocytometer was used to count the spores under the microscope and DDW was used to adjust the spore suspension 2.0 × 106 CFU mL−1.
Fungicides
Carbendazim (Bavistin™, 50 WP, Tata Holset, India) were used to compare the effectiveness of biocontrol agents and applied in the soil at 1.6 mg a.i. per pot before the planting of seedlings but after P. aphanidermatum inoculation. The fungicide dose was calculated using the recommended fungicide dose (8 kg a.i. ha-1).
Plant treatments and cultures
Nursery of all three cultivars' of tobacco was raised independently in 25 cm diameter clay pots filled with autoclaved soil. Earthen pots (15 cm diameter) were used for pot experiments and filled with 1 kg autoclaved soil and compost (3:1). Following five treatments were maintained for each cultivar.
Before transplanting tobacco seedlings, P. aphanidermatum (2 g mixed in 10 mL water) was added to pot's topsoil. Three to four leaf stage seedlings (4 weeks old) of tobacco cultivars were placed in the pots (one seedling per pot) on the next day. For each treatment, ten replicates were kept; plants from five replicate pots were removed at 15 days after inoculation for biochemical analysis, and plant growth characteristics were measured at harvest (4 months later). Un-inoculated and inoculated control was maintained. The pots were placed in a completely random pattern. To avert the loss of microbes from the pots, water (200 mL/pot) was cautiously delivered shortly after transplanting without overflooding. Watering was done at 48 h breaks and continued till harvest. Plants were checked regularly for any evident symptoms of the pathogen. Plant length, fresh and dry weight, soil population of root-rot pathogen and biocontrol agents, total phenol, salicylic acid, and leaf pigments were determined. The disease severity in terms of root-rot index was determined at harvest on 0-5 scale as proposed by Ohh et al. (1978). 0 = healthy plants, no infection visible; 1 = slight browning of secondary roots; 2 = severe browning of secondary roots; 3 = firm tap root, secondary roots rotted; 4 = soft tap root, secondary roots completely rotted; 5 = plants dead (decay of roots and cotyledons).
Assay for total phenol, salicylic acid, chlorophyll and carotenoids
The total phenol (Sharma and Sain 2005), salicylic acid (Shane and Kowblansky 1968), Chlorophyll a, chlorophyll b, total chlorophylls, and carotenoid contents of leaves (Arnon 1949; Maclachlan and Zalik 1963) were assayed as per standard protocols.
Soil population of Pythium aphanidermatum and biocontrol agents
The final soil population of P. aphanidermatum and biocontrol agents (T. harzianum and P. fluorescens) were estimated from each inoculated pot near the root zone. The soil sample (1 g) was taken with 10 mL distilled water in a test tube and processed by serial dilution methods. The final soil suspension (1 mL) was taken and spread with a sterilized pipette on solidified potato dextrose agar (for T. harzianum and P. aphanidermatum) or nutrient agar medium (P. fluorescens) in Petri plates and incubated in a BOD at 27 ± 2 °C for 24 h. After incubation for 24 h, the CFU load were determined under a colony counter.
Statistical analysis
The experiment was carried out for 2 years in a row. Because the differences in the data collected across the 2 years of research were not significant (P ≤ 0.05), the data were pooled and subjected to analysis of variance (ANOVA) using SPSS 11.0 for Windows-10. At three probability levels, P ≤ 0.05, 0.01, and 0.001, the least significant differences (LSD), degree of freedom and F-values were determined. The root-rot index values were transformed and single-factor ANOVA was used to examine them. To establish the correlation between the variables, the root-rot index and dry weight of shoots were regressed against data on SA, TP, total chlorophyll and carotenoid contents.
Results
Disease severity and symptoms of Pythium aphanidermatum
Tobacco plant inoculated with 2 g pure culture of P. aphanidermatum showed arrested growth and mild chlorosis in all cultivars except RK-12 P3. The yellowing gradually became pronounced with plant age advancement; at 3 months of age, the entire foliage of plants became discernibly yellowish in cvs. RK-10 P3 and RK-18 P8. The symptoms were considerably more significant in cv. RK-10 P3. When plants were harvested, the roots showed decaying (Supplementary Fig. 1) being highest on cultivar RK-10 P3 (2.66 at 0–5 scale), followed by RK-18 P8 (2.33) and RK-12 P3 (0.33). The cultivar RK-12 P3 exhibit the lowest root-rot index and did not develop any significant root-decaying (P ≤ 0.05; Supplementary Fig. 1).
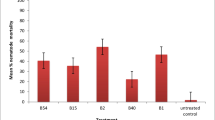
Soil application of biocontrol agents (BCAs) and fungicides controlled the root-rot caused by P. aphanidermatum. The BCAs significantly suppressed the disease severity of root-rot disease as compared to the inoculated control (Fig. 1). The maximum decrease was observed with the treatment of T. harzianum on cv. RK-18 P8 (57%) and RK-10 P3 (45%) in comparison to the inoculated control (Fig. 1). Next in effectiveness, was carbendazim and it also significantly reduced the disease severity of cv. RK-18 P8 (42%) trailed by RK-10 P3 (28%), over inoculated control. Treatment with P. fluorescens was found least effective in decreasing the disease severity (27%) on cv. RK-18 P8 followed by RK-10 P3 (18%) over inoculated control. On cultivar RK-12 P3, BCAs and pesticides' perceptible effect was not recorded as the cultivar was found resistant to the fungus (Fig. 1).
Plant growth and biomass
P. aphanidermatum, the root-rot pathogen, caused a considerable loss in plant length (8–10%), fresh weight (6–14%), and dry weight (6–9%) of shoot and root of tobacco cv. RK-10 P3 when compared to control (Table 1). All plant growth and biomass metrics, such as plant length (8–10%), fresh weight (7–14%), and dry weight (10–13%) of shoot and root, were significantly reduced in the cv. RK-18 P8 compared to the inoculated control. Over the infected control, the cv. RK-12 P3 did not show any significant reduction in plant growth or biomass (1–5%) variables. In cv. RK-10 P3 and RK-18 P8, the percent decrease in the variables evaluated was 7–15% and 6–14%, respectively, over inoculated control (Table 1).
Treatments with biocontrol and fungicides resulted in significant increase in the plant growth and biomass of tobacco cultivars' inoculated with P. aphanidermatum over control (Table 1). Application of T. harzianum and carbendazim resulted in significant enhancement in the plant growth and biomass variable of tobacco ranging from 11–16 and 7–14%, respectively on cv. RK-10 P3 trailed by 5–11 and 4–9%, respectively on cv. RK-18 P8, over inoculated control (Table 1). Treatment with P. fluorescens, a significant increase in plant growth variable in the cv. RK-10 P3 was observed over inoculated control (P ≤ 0.05; Table 1).
Chlorophyll and carotenoid contents of leaf
The total chlorophyll content of leaves decreased by 23% (RK-10 P3), 18% (RK-18 P8), and 10% (RK-12 P3) after being inoculated with P. aphanidermatum (Table 2). In tobacco cultivars, reductions in chlorophylls a and b ranged from 13–16 and 6–29%, respectively. For all cultivars, a drop in carotenoid concentration was substantial (P ≤ 0.05) with the highest decrease in cv. RK-10 P3 compare to the uninoculated control (Table 2). Treatment with BCA and fungicides repressed the negative effect of the root-rot pathogen on leaf pigments, resulting in a significant increase in leaf contents (P ≤ 0.05; Table 2). Among the two BCAs, a greater increase in the chlorophyll and carotenoids was observed with T. harzianum in cvs. RK-10 P3 (12–37%), RK-18 P8 (11–23%) and RK-12 P3 (9–13%) over inoculated control. An increase of 6–37% in the leaf pigments was also noticed with carbendazim treatments in tobacco cultivars over inoculated control (Table 2). The correlation analysis between the root-rot index and leaf pigments (chlorophyll and carotenoid contents) indicated that the decrease in leaf contents was inversely proportional to the root-rot index (Fig. 2). Similarly, a positive correlation was established between the dry weight of shoot and leaf pigments (Fig. 4).
Total phenol and salicylic acid contents of leaf
TP and SA concentration of leaves of P. aphanidermatum inoculated plants (control, without treatment) were amplified by 6 and 7% (cv. RK-10 P3), 17 and 15% (cv. RK-18 P8), 31 and 20% (cv. RK-12 P3), respectively, over un-inoculated control (Fig. 3). Application of BCAs and fungicides induced a much better increase in the TP concentration in the tobacco cv. RK-12 P3 (42–49%), RK-18 P8 (30–44%) and RK-10 P3 (18–22%), over the un-inoculated control (Fig. 3). Treatments with T. harzianum, P. fluorescens and carbendazim resulted in 14, 16 and 13% increase in SA contents of tobacco cv. RK-12 P3; 8, 10 and 9% in cv. RK-18 P8, and 7, 8 and 11% in cv. RK-10 P3, respectively over inoculated control (Fig. 3). A negative correlation was found between the root-rot index and TP or SA (Fig. 2), demonstrated that the increase in TP and SA was considerably low in the cultivars that exhibited higher root-rot. However, the correlation analysis between the decrease in dry weight of shoot and TP or SA showed a positive linear relationship (Fig. 4).
Soil population of Pythium aphanidermatum
The final soil population of P. aphanidermatum was augmented over time, and it was more significant in the cv. RK-10 P3. The population was increased up to 900% (RK-10 P3) over the initial population at harvest (Fig. 5). Overall. the highest population was recorded in RK-10 P3, followed by RK-18 P8 and RK-12 P3. Both the biocontrol and fungicide treatments caused a significant reduction in the soil population of P. aphanidermatum (Fig. 5; P ≤ 0.05). The most significant decline was recorded with carbendazim followed by T. harzianum and P. fluorescens in comparison to inoculated control on cv. RK-10 P3 (93, 85 and 81%), RK-18 P8 (94, 87 and 81%) and RK-12 P3 (100, 99 and 98%; Fig. 5; P ≤ 0.05).
Soil population of biocontrol agents
The rhizosphere population of T. harzianum and P. fluorescens increased by 600–840% and 460–590%, respectively, over the initial population on tobacco cultivars during the experiment (Fig. 5). Among the three cultivars, the highest populations were recorded on the rhizosphere of tobacco cv. RK-10 P3 trailed by RK-18 P8 and RK-12 P3. In the presence of the pathogen, the population of biocontrol agents further increased and was highest with T. harzianum followed by P. fluorescens (Fig. 5; P ≤ 0.05).
Discussion
Inoculation of tobacco plants with P. aphanidermatum (2 g/pot) showed stunted growth and mild leaf yellowing in tobacco cultivars except for cv. RK-12 P3. The leaf yellowing became more prominent as the plants grew older; by 3-months, the whole foliage of the plants had become yellowish, notably in the cv. RK-10 P3. Roots of tobacco cvs. RK-10 P3 and RK-18 P8, for instance, have developed blackened discolourations as a result of P. aphanidermatum infection, as have other tobacco cultivars (Khan and Haque 2013). Root-rot was visible on the cvs. RK-10 P3 and RK-18 P8, indicate an increased vulnerability to the disease (Khan et al. 2012). Soil application of biocontrol agents (BCAs) and fungicide controlled the root-rot disease and significantly reduced the disease severity, as observed in the present study.
Inoculation of P. aphanidermatum caused a significant reduction in the length of shoot and root, fresh and dry weight of shoot and root of tobacco cv. RK-10 P3 and RK-18 P8 in comparison to uninoculated control. Due to the activity of pectolytic and cellulolytic enzymes produced by the pathogen, the afflicted roots, parts, and/or tissues converted to water-soaked, lose cohesiveness, and usually develop wet rot within a few days of inoculation (Goyal and Mattoo 2014). The rotting of primary roots causes disintegration of the interior tissue and affects the capacity of the roots to absorb water and nutrients, resulting in a steady fall in aerial development (Oluma and Oladiran 1993). As a result, the plants growth and biomass are lowered, as found in the present study. A positive linear relationship between disease severity (root-rot index) and percent loss in leaf pigment has also been discovered by the correlation analysis, suggesting that a rise in root rot directly hindered important physiological activities such as water, mineral absorption, and CO2 assimilation, resulting in a decline in dry mass production as seen in the present research.
However, the application of T. harzianum or carbendazim suppressed the pathogenic effect of P. aphanidermatum leading to a significant increase (P ≤ 0.05) in plant growth variables of all three cultivars. Numerous studies under pot and field conditions have confirmed that the soil application of T. harzianum may induce significant enhancement in plant growth and yield of plants infected with root-rot fungi (Muthukumar et al. 2011; Saba et al. 2012). Trichoderma harzianum is a well-established mycoparasite of soil-borne pathogens (Naher et al. 2014) and its application can effectively control the root-rot disease. Similarly, P. fluorescens is known to be an excellent suppressant of plant pathogenic fungi (Weller et al. 2007). The bacterium may antagonize the pathogen through antibiosis, siderophores production etc. Carbendazim belongs to the benzimidazole group which is highly effective against soil-borne fungi (García et al. 2003) and its application provided satisfactory control of the disease.
For chlorophylls and carotenoids, the overall effect of pathogen inoculation and cultivars was substantial. Leaf chlorophylls and carotenoids, are regarded as the fundamental unit for photosynthesis because these pigments absorb light and transport it to cell organelles for CO2 fixation. (Khan and Haque 2013). In the present study, the whole foliage of susceptible cultivars (RK-10 P3 and RK-18 P8) became noticeably yellowish at 3–4 months of age, but the foliage of resistant cultivars (RK-12 P3) remained healthy. The findings show that the chlorophyll pigments are extremely susceptible to P. aphanidermatum-induced changes in host physiology. The water stress resulting due to damage of roots and development of rotting by P. aphanidermatum, chlorophyll and carotenoids molecules get denatured consequently their leaf contents were reduced, subsequently, photosynthetic action of the plant decreased which resulted in lower biomass production (Khan and Haque 2013). Correlation analysis has also shown a positive linear relationship between pigments of leaf and dry weight of shoot where an increase in the chlorophyll or carotenoid contents of leaves resulted in a corresponding increase in dry biomass production. The effect of the treatments used on the leaf pigments was more or less similar to that observed on plant growth parameters. Similarly, soil population and root-rot index greatly decrease with T. harzianum and carbendazim.
The total phenol content of leaves, inoculated with the P. aphanidermatum, increased in all three cultivars, although the rise was not uniform. The increase in the total phenol was lowest in the cvs. RK-10 P3 and RK-18 P8 which were found highly and moderately susceptible to P. aphanidermatum, respectively whereas the increase was greatest in cv. RK-12 P3 which expressed resistant reaction to P. aphanidermatum. This is also discernable from the correlation analysis done between disease (root-rot) and phenolic contents. This suggests that phenolic chemicals help plants to defend themselves against infection by phytopathogens (Nicholson and Hammerschmidt 1992; Hammond-Kosack and Jones 1996). These chemicals, which operate as phytoalexins, are found in extremely low amounts in healthy plants. However, as observed in this study, when plants are infected with the pathogen, their concentration rises drastically. Salicylic acid (SA) has also been linked to a crucial component in the signal transduction pathway that leads to disease resistance in plants (Ryals et al. 1996; Sharma and Sain 2005). The highest SA concentration was found in cvs. RK-12 P3, which did not demonstrate root-rot or reduced plant growth, whereas the lowest SA rise was found in cvs. RK-10 P3 and RK-18 P8, which were susceptible to P. aphanidermatum. The tolerance and susceptible reactions of tobacco cultivars against root-rot pathogen were apparently due to varied synthesis of SA as shown by a positive linear relationship. It has been found that increased SA accumulation in tobacco leaves leads to a considerable reduction in disease symptoms produced by the fungus Cercospora nicotianae, Peronospora tabacina and Phytophthora parasitica (Ryals et al. 1996). Salicylic acid had a substantially higher F-value than total phenols, indicating that the former plays a bigger role in disease tolerance.
The present study demonstrated that the tobacco cultivars viz., RK-10 P3, RK-18 P8 and RK-12 P3 screened for host resistance against P. aphanidermatum showed a varying degree of susceptibility. Based on the morphological (root-rot index and plant growth parameters) and biochemical host reactions (total phenol, salicylic acid, chlorophyll and carotenoids), the cv. RK-12 P3 revealed resistance/tolerance response and can be economically exploited for P. aphanidermatum resistance, while the cv. RK-10 P3 expressed a highly susceptible reaction and should be avoided in disease-prone fields. Further, the soil treatment of two multi-facial biocontrol agents, T. harzianum AMUTH-1 and P. fluorescens AMUPF-1 adequately controlled P. aphanidermatum and considerably enhanced the plant growth and biomass production of all three tobacco cultivars. However, the relative effectiveness of T. harzianum AMUTH-1 was higher than P. fluorescens AMUPF-1 and carbendazim. Hence, to increase tobacco yield in root rot-infested fields, soil treatment with T. harzianum may prove better than a fungicide.
References
Arnon D (1949) Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol 24:15
Arora NK, Tewari S, Singh R (2013) Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In: Arora N (ed) Plant microbe symbiosis: fundamentals and advances. Springer, New Delhi, pp 411–449
Ben-David A, Davidson CE (2014) Estimation method for serial dilution experiments. J Microbiol Methods 107:214–221
Dempsey DMA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. CRC Crit Rev Plant Sci 18(4):547–575
Devaki NS, Shankarabhat S, Bhat G, Manjunath KR (2008) Antagonistic activities of Trichoderma harzianum against Pythium aphanidermatum and Pythium myriotylum on tobacco. J Phytopathol 136:82–87
García PC, Rivero RM, Ruiz JM, Romero L (2003) The role of fungicides in the physiology of higher plants: implications for defense responses. Bot Rev 69(2):162–172
Goyal RK, Mattoo AK (2014) Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress. Plant Sci 228:135–149
Hammond Kosack KE, Jones JG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8:1773–1791
Haque Z, Khan MR (2021) Identification of multi-facial microbial isolates from the rice rhizosphere and their biocontrol activity against Rhizoctonia solani AG1-IA. Biol Cont. https://doi.org/10.1016/j.biocontrol.2021.104640
Howell CR (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis 87:4–10
Hunt M, Ryals J (1996) Systemic acquired resistance signal transduction. Crit Rev Plant Sci 15:583–606
Khan MR, Haque Z (2013) Morphological and biochemical responses of five tobacco cultivars to simultaneous infection with Pythium aphanidermatum and Meloidogyne incognita. Phytopathol Medit 52(1):98–109
Khan MR, Haque Z, Anwer MA (2012) Morphological and biochemical response of selected germplasm of tobacco to soil inoculation with Pythium aphanidermatum. Arch Phytopathol Plant Prot 45(1):99–109
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y (2020) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 8(6):817
Le Huong TT, Padgham JL, Sikora RA (2009) Biological control of rice root knot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. Int J Pest Manag 55:31–36
Leelavathi MS, Vani L, Reena P (2014) Antimicrobial activity of Trichoderma harzianum against bacteria and fungi. Int J Curr Microbiol Appl Sci 3(1):96–103
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration and free amino acid composition of chlorophyll mutant of barley. Can J Bot 41:1053–1062
Muthukumar A, Eswaran A, Sanjeevkumas K (2011) Exploitation of Trichoderma species on the growth of Pythium aphanidermatum in chilli. Braz J Microbiol 42(4):1598–1607
Naher L, Yusuf UK, Ismail A, Hossain K (2014) Trichoderma spp.: a biocontrol agent for sustainable management of plant diseases. Pak J Bot 46(4):1489–1493
Neuenschwander U, Lawton K, Ryals J (1996) Systemic acquired resistance. In: Stacey G, Keen NT (eds) Plant–microbe interactions, vol 1. Chapman and Hall, New York, pp 81–106
Nicholson RL, Hammersehmid R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Ohh SH, King TH, Kommedahl T (1978) Evaluating peas for resistance to damping-off and root rot caused by Pythium ultimum. Resistance 68:1644–1649
Oluma HOA, Oladiran AO (1993) Pythium aphanidermatum root rot of pawpaw (Carica papaya L.) in Nigeria. Mycopathologia 123:111–115
Prabhukarthikeyan SR, Raguchander T (2016) Antifungal metabolites of Pseudomonas fluorescens against Pythium aphanidermatum. J Pure Appl Microbiol 10(1):579–584
Qualhato TF, Lopes FAC, Steindorff AS, Brandao RS, Jesuino RSA, Ulhoa CJ (2013) Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: evaluation of antagonism and hydrolytic enzyme production. Biotechnol Lett 35(9):461–1468
Rao KM, Raju KS, Ravisankar H (2015) Antifungal properties of native Trichoderma isolates against Sclerotium rolfsii and Pythium aphanidermatum infecting tobacco. J Environ Biol 36(6):1349
Ryals JK, Neuenschwander UH, Willits MG, Molina A, Steiner H, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Saba H, Vibhash D, Manisha M, Prashant KS, Farhan H, Tauseef A (2012) Trichoderma—a promising plant growth stimulator and biocontrol agent. Mycosphere 3(4):524–531
Shane N, Kowblansky M (1968) Determination of acetyl salicylic acid, salicyclamide, acetominophone and caffeine in tablet or powder by independent methods. J Pharm Sci 57(7):1218–1223
Sharma P, Sain SK (2005) Use of biotic compounds against damping off of cauliflower by Pythium aphanidermatum. Indian Phytopath 58(4):395–401
Subhashini DV, Padmaja K (2009) Exploitation of Pseudomonas fluorescens for the management of damping-off disease of tobacco in seed beds. Indian J Plant Prot 37(1/2):147–150
Verma DK, Pandey AK, Mohapatra B, Srivastava S, Kumar V, Talukdar D, Yulianto R, Zuan ATK, Jobanputra AH, Asthir B (2019) Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agriculture and improved crop production. In: Verma DK (ed) Microbiology for sustainable agriculture, soil health, and environmental protection apple. Academic Press, New York, pp 3–80
Vinale F, Sivasithamparam K (2020) Beneficial effects of Trichoderma secondary metabolites on crops. Phytother Res 34(11):2835–2842
Vizcaino JA, Sanz L, Cardoza RE, Monte E, Gutierrez S (2005) Detection of putative peptide synthetase genes in Trichoderma species. Application of this method to the cloning of a gene from T. harzianum CECT 2413. FEMS Microbiol Lett 244:139–148
Weller DM, Landa BB, Mavrodi OV, Schroeder KL, De La Fuente L, Blouin Bankhead S, Allende Molar R, Bonsall RF, Mavrodi DV, Thomashow LS (2007) Role of 2, 4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol 9(1):4–20
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haque, Z., Khan, M.R. Host resistance and bio-management of tobacco root-rot caused by Pythium aphanidermatum. Indian Phytopathology 75, 703–712 (2022). https://doi.org/10.1007/s42360-022-00491-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-022-00491-y